Abstract
Alemtuzumab serum levels and clinical response after subcutaneous administration (10 mg 3 times/week for six weeks) have been explored in 29 chronic lymphocytic leukemia patients receiving the monoclonal antibody as consolidation. Serum concentrations after each administration gradually increased during the first week and more markedly during weeks 2 and 3, approaching the steady-state at week 6. Absorption continued slowly through the tissues for about 2–3 weeks after the last administration, starting to decrease thereafter. Difference between Responders and Non-responders was statistically significant: maximal concentration (Cmax) was 1.69 μg/mL vs. 0.44 μg/mL; concentration before subcutaneous administration (Cpre-dose) on day 15 was 0.7 vs. 0.21 μg/mL, area under curve (AUC0−12h) was 11.09 vs. 2.26 μg x h/mL for Responders and Non-responders, respectively. Higher systemic exposure to alemtuzumab correlated with a better clinical response and minimal residual disease. Results suggest that an adjusted schedule according to serum level could improve clinical outcome of patients receiving subcutaneous alemtuzumab.
Keywords: alemtuzumab, pharmacokinetics, chronic lymphocytic leukemia
Introduction
Alemtuzumab (Campath, Mab Campath®) is a humanized IgG1 monoclonal antibody (MoAb) consisting of a murine FAB segment conjugated to a human Fc fragment.1
This antibody binds CD52, an antigen abundantly expressed on the surface of T- and B-cell lymphocytes, monocytes/macrophages and eosinophils, but is not expressed on hematopoietic stem cells.2–4
Alemtuzumab was initially approved for the treatment of chronic lymphocytic leukemia (CLL) in patients who have been previously treated with an alkylating agent and who have failed fludarabine therapy.5
Its use as a single agent has been shown to be effective when used as first-line therapy or in patients with relapsed or refractory disease. Alemtuzumab, administered alone or in combination, was shown to induce minimal residual disease (MRD)-negative responses.6–10
Alemtuzumab is usually administered intravenously (IV) at the dose of 30mg, 3 times a week. The subcutaneous (sc) route, at the same IV administration dose, has been studied in an attempt to reduce side-effects and make the treatment more manageable.11
Hale et al.12 reported alemtuzumab concentrations in patients with relapsed CLL after subcutaneous administration at a dose of 30mg, for up to 18 weeks, while Rebello and Hale13 reported only Cmax values in patients with CLL who received subcutaneous alemtuzumab at a dose of 30 mg (3 times/week) for up to 12 weeks.
Montillo et al.14 in one clinical study, evaluated preliminary pharmacokinetic data in 16 CLL patients who were receiving the monoclonal antibody subcutaneously at a lower dose (10 mg 3 times/week for six weeks). Patients who responded to fludarabine-chemotherapy as induction showing persistence of minimal residual disease received alemtuzumab as consolidation.
The aim of the present study was to describe the pharmacokinetic properties of alemtuzumab in a larger patient population made up of 29 patients receiving as consolidation therapy 10 mg of the anti CD52 MoAb, 3 times/week for six weeks by subcutaneous administration and evaluate the possible association between serum levels and clinical response. These data should improve our understanding of the alemtuzumab therapeutic concentration and consequently of the pharmacokinetic profile, and may lead to more rapid and effective treatment regimens.
Design and Methods
This clinical study was carried out with the approval of the Ethical Committees of the respective hospitals.
Patients’ characteristics are shown in Table 1.
Table 1.
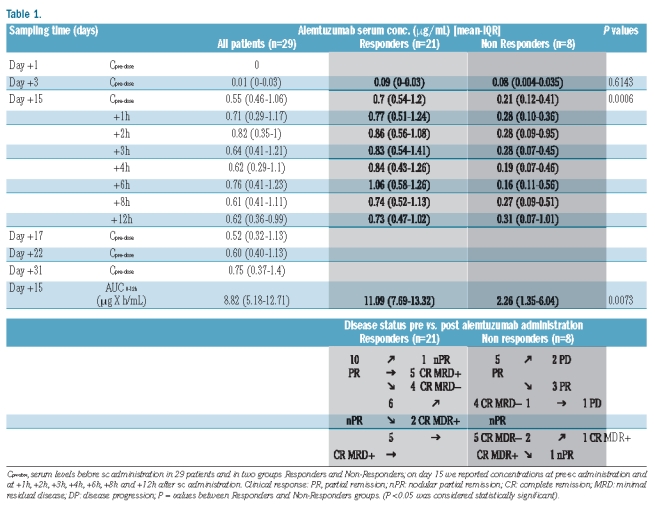
Twenty-nine alemtuzumab naïve patients under the age of 65 years who responded to a fludarabine-based induction therapy received consolidation therapy with alemtuzumab as previously reported.14
Patients showing improvement following immunotherapy were defined as Responders while patients with stable or progressive disease were defined as Non-responders. Twenty-one of 29 patients responded to therapy. Serum samples were collected from the patients on days 1, 3, 5, 15, 17, 22 and 31, immediately before subcutaneous administration. On day 15, serum samples were drawn also at 1, 2, 3, 4, 6, 8 and 12 h after alemtuzumab administration. After the last dose on day 40, samples were collected according to these time points, and whenever possible for logistical reasons, up to day 101. Serum samples collected by the treatment schedule reported previously14 from all time points were kept frozen at −20°C until analysis.
Serum concentrations were evaluated using an extremely sensitive enzyme-linked immunoassay (ELISA) developed and validated in our laboratory.15
Pharmacokinetic and statistical analysis
Alemtuzumab concentration-time data were analyzed using the statistical pharmacokinetic population program P-Pharma (Version 3, Simed, Creteil, France).
For each kinetic profile, the Cpre-dose (concentration before the following subcutaneous administration), Tmax (time to reach maximum concentration, over 101 days), Cmax (maximum serum concentration), and AUC0−12h (area under the concentration-time curve from 0 to 12 h on day 15) were reported.
As the quantitative variables were not normally distributed (Shapiro-Wilk’s test), these were summarized using the median and interquartile range (IQR); the non-parametric Mann-Whitney U test was used to compare Responders and Non-responders. P<0.05 was considered statistically significant and all tests were two-sided. Data analysis was performed with the STATA statistical package (version 9; Stata Corporation, College Station, 2008, Texas, USA).
Results and Discussion
Median serum concentrations of alemtuzumab were reported before subcutaneous administration for the first 31 days of the multiple dosing regimens and at follow up after the last dose in all patients. These data are summarized in Table 1.
A statistical evalution was not made of the follow-up samples due to the small number of samples.
The median Tmax during the 101 day period was at 29 days (IQR 18–45 days) and the median Cmax was 1.20 μg/mL (IQR 0.61–2.47 μg/mL).
Only 14 of our 29 patients (48%) reached 1.0 μg/mL after the median cumulative dose of 49 mg (range 44–74 mg) at a median of 16 days (range 13–22 days).
Pharmacokinetic analysis and parameters were evaluated by subdividing all patients into two groups, Responders and Non-Responders, according to whether an improvement was seen after immunotherapy.
The difference between the two groups was statistically significant: Cmax was 1.69 μg/mL and 0.44 μg/mL (P=0.0002) and Tmax occurred at 31 days and 22 days; the median Cpre-dose before subcutaneous administration on day 15 was 0.7 vs. 0.21 μg/mL (P=0.0006) and the median total systemic exposure to alemtuzumab (AUC0−12h) was 11.09 vs. 2.26 μg x h/mL (P=0.0073) for Responders and Non-Responders, respectively.
Only in the accumulation phase, on day 3, was the median Cpre-dose before subcutaneous administration not statistically significant (P=0.6143).
After the last dose on day 40 median concentrations were: 1.14 μg/mL on day 43 (three days after the last dose); 1.17 μg/ml on day 52 (12 days after the last dose); 1.19 μg/mL on day 72 (32 days after the last dose) and 0.19 μg/mL for only one patient on day 101 (61 days after the last dose). Again, a statistical evaluation was not made due the small number of samples during follow up.
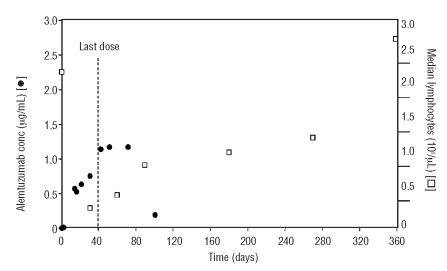
We followed the levels of lymphocytes and antibody concentration during the administration and the follow-up period. No relationship between the number of lymphocytes and level of anti-CD52 MoAb in the sera was detected.
The lymphocyte values decreased sharply during the administration of alemtuzumab (0.29×109/μL on day 30) then slowly increased after the last dose (day 40), although antibody plasma levels remained high until approximately three months of therapy.
Lymphocyte values returned to normal after 9–12 months following the start of therapy (1.3–2.7×109/μL, respectively) (Figure 1).
Figure 1.
Alemtuzumab serum concentration-time profile in relation to the mean lymphocyte counts in patients treated with alemtuzumab. [□] Indicates median lymphocyte values (109/μL) reported during the 12 months after start of therapy; [●], alemtuzumab concentration (μg/mL) reported during the first 100 days after start of therapy.
The safety profile of alemtuzumab has been well characterized. In particular, intravenous administration of alemtuzumab has been frequently associated with infusion reactions (e.g. rigor, fever, nausea, etc.), whereas subcutaneous administration reduces or eliminates the occurrence of such reactions.
For this reason, some clinicians chose the subcutaneous route of administration with the aim of reducing side effects while maintaining the same efficacy. However, the pharmacokinetics of a subcutaneous MoAb is slower than with intravenous administration: the absorption of antibodies at the site of administration may continue for hours or days and may require prolonged dosing to obtain the same clinical effect.16–18
In our study, alemtuzumab serum concentrations quantified after each subcutaneous administration gradually increased during the first week and became more marked during weeks 2 and 3. Subsequently, drug accumulation followed the pattern expected for a drug with an elimination half-life of about 12 days, approaching the steady-state at week 6.19
The initial slower accumulation of the antibody in the blood may be due to a less favorable biodistribution, or more effective binding to tumor cells, or the slower dose escalation used in the subcutaneous administration compared with intravenous administration.
Following subcutaneous injection drugs reach the bloodstream via the capillaries or the lymphatic system depending on the molecular size of the therapeutic agents. There is a linear relationship between the molecular weight (a surrogate of molecular size) of an injected protein and the portion of the dose absorbed into the peripheral lymphatic system at the subcutaneous injection site. Increasing molecular size appears to enhance access to the lymph, even though larger molecules may eventually restrict lymphatic drainage. This explains the slow absorption of alemtuzumab through the tissues for about 2–3 weeks after the last administration, providing steady state plasma levels which thereafter start to decrease.
The available information regarding the pharmacokinetics of alemtuzumab mainly derive from intravenous administration in patients undergoing stem cell transplantation and with no substantial leukemic burden. Rebello and co-workers20 evaluated two different intravenous alemtuzumab dosing schedules as a preparative regimen for unrelated stem cell transplant. Morris et al.21 evaluated alemtuzumab concentrations in a conditioning regimen prior to stem cell transplantation in patients with a variety of hematologic malignancies, without conducting a pharmacokinetic study.
Our study is the first to investigate the pharmacokinetic profile of alemtuzumab in CLL patients receiving low-dose subcutaneous administration as consolidation treatment. In the accumulation phase, only 14 of 29 patients (48%) reached 1.0 μg/mL after the median cumulative dose of 49 mg (range 44–74 mg), corresponding to a median of 16 days (range 13–22 days).
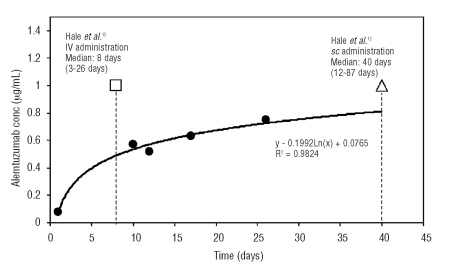
In a dose comparison study, Hale et al.18 reported that patients achieved a 1.0 μg/mL concentration after intravenous alemtuzumab and subcutaneous administration of 30 mg/3 times/week. In the intravenous administration group, the mean cumulative dose was 90.4 mg (median 73 mg, range 13–316 mg) corresponding to a median of eight days (range 3–26 days). In the subcutaneous administration group the mean cumulative dose was 551 mg (median 503 mg, range 146–1,106 mg), corresponding to a median of 40 days (range 12–87 days). A comparison of our data with that of Hale et al.12 is shown in Figure 2.
Figure 2.
Alemtuzumab concentration levels in the accumulation phase from this study compared with data reported by Hale.12 Serum levels from this study were compared with alemtuzumab levels after IV administration and after sc administration, reported by Hale:12 (●) indicates concentration from this study during sc administration; (□), concentration of 1.0 μg/mL reached after a median of eight days, after IV administration reported by Hale;12 (ρ), concentration of 1.0 μg/mL reached after a median of 40 days, after sc administration reported by Hale.12
In this study, after the last dose, the alemtuzumab levels exceeded 1.0 μg/mL (1.14 μg/mL) but not before day 43 (2 patient samples only) and remained at similar levels up to day 72 (1.19 μg/mL, 2 patient samples only).
We also observed a strong correlation between the concentration and clinical improvement or negativity of minimal residual disease (MRD).
In our study, all Responder values were significantly higher than those of Non-responders. The lack of a significant difference between the two groups (P=0.6143) was only seen at the first week, on day 3, when levels were low (around 0). This is in agreement with the data reported by Elter et al.22,23 in an intravenous study, which found a correlation between higher Cmax and improved clinical response, and is further confirmed by our preliminary data,14 indicating a correlation between clinical response and AUC0−12h in 16 patients.
In our study, AUC0−12h levels in Responders were significantly higher than in Non-responders. Higher AUC0−12h values significantly correlated with better clinical response: 90.5% of 21 Responders had over 5 μg x h/mL, while only 37.5% of 8 Non-responders had over 5 μg x h/mL.
Since we observed that the drug accumulates in the first 2–3 weeks, achieving a steady-state thereafter, and that there is a correlation between antibody plasma levels and clinical response, we recommend that pharmacokinetic monitoring be introduced in future studies. Additional dose finding studies are warranted. When AUC0−12h values after the 7th dose (on day 15) are below 5 μg x h/mL, the dose could be increased to improve the likelihood of obtaining a response.
As reported in previous studies, the interpatient variability in pharmacokinetic results for alemtuzumab administration is large, probably reflecting differences in CLL tumor burden.23,24 This observation does not apply to the consolidation setting where we assume a significantly lower tumor burden shortly after induction treatment. For this reason, a reduced alemtuzumab dose has been proposed. However, the smaller amount of anti-CD 52 MoAb administered in the present trial resulted to be effective, thus confirming the positive correlation between plasma alemtuzumab level and patient response.
Now that there is a wider clinical use of alemtuzumab, we need to clearly define the most effective dosage and route of administration, the role of alemtuzumab in combination chemotherapy, and the possibility of developing a customized approach. A better understanding of alemtuzumab therapeutic concentration and pharmacokinetic profile may lead to a more rapid and effective treatment regimen. Pharmacokinetic analysis is a valid tool to help improve our understanding and plays a pivotal role in optimizing dosing regimens and the design of clinical trials.
Supplementary Material
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Dyer MJ, Hale G, Hayhoe FG, Waldmann H. Effects of Campath-1 antibodies in vivo in patients with lymphoid malignancies: influence of antibody isotope. Blood. 1989;73(6):1431–9. [PubMed] [Google Scholar]
- 2.Treumann A, Lifely MR, Schneider P, Ferguson MAJ. Primary structure of CD52. J Biol Chem. 1995;270(11):6088–99. doi: 10.1074/jbc.270.11.6088. [DOI] [PubMed] [Google Scholar]
- 3.Hale G, Xia MQ, Tighe HP, Dyer MJS, Waldmann H. The Campath-1 antigen (CDw52) Tissue Antigens. 1990;35(3):118–27. doi: 10.1111/j.1399-0039.1990.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 4.Gilleece MH, Dexter TM. Effect of Campath-1H antibody on human hematopoietic progenitors in vitro. Blood. 1993;82(3):807–12. [PubMed] [Google Scholar]
- 5.Montillo M, Schinkoethe T, Elter T. Eradication of minimal residual disease with Alemtuzumab in B-cell chronic lymphocytic leukaemia (B-CCL) patients: the need for a standard method of detection and the potential impact of bone marrow clearance on disease outcome. Cancer Invest. 2005;23(6):488–96. doi: 10.1080/07357900500201418. [DOI] [PubMed] [Google Scholar]
- 6.Lundin J, Kimby E, Bjorkholm M, Broliden PA, Celsing F, Hjalmar V, et al. Phase II trial of subcutaneous anti-CD 52 monoclonal antibody Alemtuzumab (Campath-1H) as first line treatment for patients with B-cell chronic lymphocityc leukaemia (B-CCL) Blood. 2002;100(3):768–73. doi: 10.1182/blood-2002-01-0159. [DOI] [PubMed] [Google Scholar]
- 7.Moreton P, Kennedy B, Lucas G, Leach M, Rassam SMB, Haynes A, et al. Eradication of minimal residual disease in B-cell chronic lymphocytic leukaemia after Alemtuzumab therapy is associated with prolonged survival. J Clin Oncol. 2005;23(13):2971–9. doi: 10.1200/JCO.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, et al. Therapeutic role of Alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99(5):3554–61. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy B, Rawstron A, Carter C, Mary Ryan M, Speed K, Lucas G, et al. Campath-1H and fludarabine in combination are highly active in refractory chronic lymphocytic leukaemia. Blood. 2002;99(6):2245–7. doi: 10.1182/blood.v99.6.2245. [DOI] [PubMed] [Google Scholar]
- 10.Elter T, Borchmann P, Schulz H, Reiser M, Trelle S, Schnell R, et al. Fludarabine in combination with alemtuzumab is effective and feasible in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: results of a phase II trial. J Clin Oncol. 2005;23(28):7024–31. doi: 10.1200/JCO.2005.01.9950. [DOI] [PubMed] [Google Scholar]
- 11.Österborg A, Fassas AS, Anagnostopoulos A, Dyer MJS, Catovsky D, Mellstedt H. Humanized CD52 monoclonal antibody campath-1H as first-line treatment in chronic lymphocytic leukaemia. Br J Haematol. 1996;93(1):151–3. doi: 10.1046/j.1365-2141.1996.450989.x. [DOI] [PubMed] [Google Scholar]
- 12.Hale G, Rebello P, Brettman LR, Fegan C, Kennedy B, Kimby E, et al. Blood concentrations of alemtuzumab and antiglobulin responses in patients with chronic lymphocytic leukemia following intravenous or subcutaneous routes of administration. Blood. 2004;104(4):948–55. doi: 10.1182/blood-2004-02-0593. [DOI] [PubMed] [Google Scholar]
- 13.Rebello P, Hale G. Pharmacokinetics of Campath-1H: assay development and validation. J Immunol Methods. 2002;260(1–2):285–302. doi: 10.1016/s0022-1759(01)00556-7. [DOI] [PubMed] [Google Scholar]
- 14.Montillo M, Tedeschi A, Miqueleiz S, Veronese S, Cairoli R, Intropido L, et al. Alemtuzumab as consolidation after a response to Fludarabine is effective in purging residual disease in patients with chronic lymphocytic leukaemia. J Clin Oncol. 2006;24(15):2337–42. doi: 10.1200/JCO.2005.04.6037. [DOI] [PubMed] [Google Scholar]
- 15.Montagna M, Avanzini MA, Visai L, Locatelli F, Montillo M, Morra E, et al. A new sensitive enzyme-linked immunosorbent assay (ELISA) for Alemtuzumab determination: development, validation and application. Int J Immunopathol Pharmacol. 2007;20(2):363–71. doi: 10.1177/039463200702000217. [DOI] [PubMed] [Google Scholar]
- 16.Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93(11):2645–68. doi: 10.1002/jps.20178. [DOI] [PubMed] [Google Scholar]
- 17.Guidelines on the Clinical Investigation of the pharmacokinetics of therapeutic protein. EMEA. 2006 [Google Scholar]
- 18.Mascelli MA, Zhou H, Getsy J, Davis HM, Gitrham M, Abernethy D. Molecular, biologic and pharmacokinetic properties of monoclonal antibodies: impact of these parameters on early clinical development. J Clin Pharmacol. 2007;47(5):553–65. doi: 10.1177/0091270006298360. [DOI] [PubMed] [Google Scholar]
- 19.Frampton JE, Wagstaff AJ. Alemtzumab. Drugs. 2003;63(12):1229–43. doi: 10.2165/00003495-200363120-00003. [DOI] [PubMed] [Google Scholar]
- 20.Rebello P, Cwynarsky K, Varughese M, Eades A, Apperley JF, Hale G. Pharmacokinetics of Campath-1H in BMT patients. Cytotherapy. 2001;3(4):261–7. doi: 10.1080/146532401317070899. [DOI] [PubMed] [Google Scholar]
- 21.Morris EC, Rebello P, Thomson KJ, Peggs KS, Kyriakou C, Goldostone AH, et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood. 2003;102(1):404–6. doi: 10.1182/blood-2002-09-2687. [DOI] [PubMed] [Google Scholar]
- 22.Elter T, Molnar I, Kuhlmann J, Hallek M, Wendtner C. Pharmacokinetics of alemtuzumab and the relevance in clinical practice. Leuk Lymphoma. 2008;49(12):2256–62. doi: 10.1080/10428190802475303. [DOI] [PubMed] [Google Scholar]
- 23.Elter T, Klip J, Borchmann P, Schulz H, Hallek M, Engert A. Pharmacokinetics of alemtuzumab in combination with fludarabine in patients with relapsed or refractory B-cell chronic lymphocytic leukaemia. Haematologica. 2009;94(1):150–2. doi: 10.3324/haematol.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mould DR, Baumann A, Kuhlmann J, Keating MJ, Weitman S, Hillmen P, et al. Population pharmacokinetics–pharmacodynamics of alemtuzumab (Campath®) in patients with chronic lymphocytic leukaemia and its link to treatment response. Br J Haematol. 2007;64(3):278–91. doi: 10.1111/j.1365-2125.2007.02914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.