Abstract
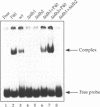
We have previously described the characterisation of an abundant mitochondrial protein (p40) that binds specifically to 5'-untranslated leaders of mitochondrial mRNAs in yeast. p40 consists of two polypeptides with M(r) of 40 and 39 kDa. Limited sequence analysis of p40 identifies it as the Krebs cycle enzyme NAD(+)-dependent isocitrate dehydrogenase (Idh). Both enzyme and RNA-binding activities are specifically lost in cells containing disruptions in either IDH1 or IDH2, the nuclear genes encoding the two subunits of the enzyme, thus conclusively identifying p40 as Idh and showing that both activities are dependent on the simultaneous presence of both subunits. Although we still must ascertain whether and how either function of Idh is regulated and whether the two functions are compatible or mutually exclusive, this combination of dehydrogenase activity and RNA-binding in a single protein may be part of a general regulatory circuit linking the need for mitochondrial function to mitochondrial biogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G. Animal mitochondrial DNA: an extreme example of genetic economy. Int Rev Cytol. 1985;93:93–145. doi: 10.1016/s0074-7696(08)61373-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braun H. P., Emmermann M., Kruft V., Schmitz U. K. The general mitochondrial processing peptidase from potato is an integral part of cytochrome c reductase of the respiratory chain. EMBO J. 1992 Sep;11(9):3219–3227. doi: 10.1002/j.1460-2075.1992.tb05399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E., Voeller D., Koeller D. M., Drake J. C., Takimoto C. H., Maley G. F., Maley F., Allegra C. J. Identification of an RNA binding site for human thymidylate synthase. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):517–521. doi: 10.1073/pnas.90.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo M. C., Fox T. D. Control of mitochondrial gene expression in Saccharomyces cerevisiae. Annu Rev Genet. 1990;24:91–113. doi: 10.1146/annurev.ge.24.120190.000515. [DOI] [PubMed] [Google Scholar]
- Costanzo M. C., Fox T. D. Specific translational activation by nuclear gene products occurs in the 5' untranslated leader of a yeast mitochondrial mRNA. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2677–2681. doi: 10.1073/pnas.85.8.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupp J. R., McAlister-Henn L. Cloning and characterization of the gene encoding the IDH1 subunit of NAD(+)-dependent isocitrate dehydrogenase from Saccharomyces cerevisiae. J Biol Chem. 1992 Aug 15;267(23):16417–16423. [PubMed] [Google Scholar]
- Cupp J. R., McAlister-Henn L. NAD(+)-dependent isocitrate dehydrogenase. Cloning, nucleotide sequence, and disruption of the IDH2 gene from Saccharomyces cerevisiae. J Biol Chem. 1991 Nov 25;266(33):22199–22205. [PubMed] [Google Scholar]
- Dekker P. J., Papadopoulou B., Grivell L. A. Properties of an abundant RNA-binding protein in yeast mitochondria. Biochimie. 1991 Dec;73(12):1487–1492. doi: 10.1016/0300-9084(91)90182-z. [DOI] [PubMed] [Google Scholar]
- Dekker P. J., Stuurman J., van Oosterum K., Grivell L. A. Determinants for binding of a 40 kDa protein to the leaders of yeast mitochondrial mRNAs. Nucleic Acids Res. 1992 Jun 11;20(11):2647–2655. doi: 10.1093/nar/20.11.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denslow N. D., Michaels G. S., Montoya J., Attardi G., O'Brien T. W. Mechanism of mRNA binding to bovine mitochondrial ribosomes. J Biol Chem. 1989 May 15;264(14):8328–8338. [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989 Feb;108(2):229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelmeier T. M., Dieckmann C. L. CBP1 function is required for stability of a hybrid cob-oli1 transcript in yeast mitochondria. Curr Genet. 1990 Dec;18(5):421–428. doi: 10.1007/BF00309911. [DOI] [PubMed] [Google Scholar]
- Myers A. M., Pape L. K., Tzagoloff A. Mitochondrial protein synthesis is required for maintenance of intact mitochondrial genomes in Saccharomyces cerevisiae. EMBO J. 1985 Aug;4(8):2087–2092. doi: 10.1002/j.1460-2075.1985.tb03896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi B. G., Lukins H. B., Linnane A. W., Nagley P. Biogenesis of mitochondria: a mutation in the 5'-untranslated region of yeast mitochondrial oli1 mRNA leading to impairment in translation of subunit 9 of the mitochondrial ATPase complex. Nucleic Acids Res. 1987 Mar 11;15(5):1965–1977. doi: 10.1093/nar/15.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou B., Dekker P., Blom J., Grivell L. A. A 40 kd protein binds specifically to the 5'-untranslated regions of yeast mitochondrial mRNAs. EMBO J. 1990 Dec;9(12):4135–4143. doi: 10.1002/j.1460-2075.1990.tb07636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploug M., Jensen A. L., Barkholt V. Determination of amino acid compositions and NH2-terminal sequences of peptides electroblotted onto PVDF membranes from tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis: application to peptide mapping of human complement component C3. Anal Biochem. 1989 Aug 15;181(1):33–39. doi: 10.1016/0003-2697(89)90390-4. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Stout C. D., Kaptain S., Harford J. B., Klausner R. D. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991 Mar 8;64(5):881–883. doi: 10.1016/0092-8674(91)90312-m. [DOI] [PubMed] [Google Scholar]
- Schulte U., Arretz M., Schneider H., Tropschug M., Wachter E., Neupert W., Weiss H. A family of mitochondrial proteins involved in bioenergetICS and biogenesis. Nature. 1989 May 11;339(6220):147–149. doi: 10.1038/339147a0. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Singh R., Green M. R. Sequence-specific binding of transfer RNA by glyceraldehyde-3-phosphate dehydrogenase. Science. 1993 Jan 15;259(5093):365–368. doi: 10.1126/science.8420004. [DOI] [PubMed] [Google Scholar]
- Van Loon A. P., Van Eijk E., Grivell L. A. Biosynthesis of the ubiquinol-cytochrome c reductase complex in yeast. Discoordinate synthesis of the 11-kd subunit in response to increased gene copy number. EMBO J. 1983;2(10):1765–1770. doi: 10.1002/j.1460-2075.1983.tb01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]