The published manuscript by Ammatuna et al.1 reported features and treatment outcome of patients with therapy-related acute promyelocytic leukemia (tAPL) presenting after multiple sclerosis (MS). We would like to point out a possible increased incidence of central nervous system (CNS) bleeding events in this patient cohort by describing a female patient with multiple sclerosis and tAPL who presented with a subarachnoid hemorrhage. Although the occurrence of coagulopathy and associated complications such as bleeding disorders are typical symptoms in patients with APL, the incidence and mortality of cerebral hemorrhage are still unknown.2
We report on a 59-year-old woman who initially presented in a poor performance status with headache, dyspnea, fever, petechia and mucosal bleeding. The bone marrow aspirate led to the diagnosis of an APL with 77% blasts. Cytogenetic and molecular analyses revealed a t(15;17) and a PML-Rarα bcr1 isoform, respectively and a FLT3-ITD mutation. A cranial computerized tomography (CT) showed a small subarachnoid hemorrhage in the right frontal lobe (Figure 1) which did not require surgical intervention. Despite fast initiation of specific therapy and improvement of coagulation parameters through substitution and transfusion, the patient developed aphasia several hours later. However, another CT scan did not show progressive CNS hemorrhage. The patient’s case history showed a multiple sclerosis categorized as primary progressive disease and treated with 6 cycles of mitoxantrone (MTZ) with a cumulative dose of 64 mg/m2. While there was no evidence of an acute episode she presented with paraparesis and a dysfunctional bladder. The latency period between the first dose of MTZ and the development of the tAPL as secondary malignancy due to the exposure to this topoismerase-II inhibitor was 47 months. The APL treatment started immediately with all-trans retinoic acid (ATRA) and idarubicin as recommended.2,3 While the day 28 control bone marrow aspirate showed persistent 7% blasts, on day 47 a complete remission could be observed. Currently, after 3 cycles of consolidation therapy with idarubicin, mitoxantrone and ATRA the patient is in complete cytogenetic and molecular remission and receives maintenance therapy.
Figure 1.
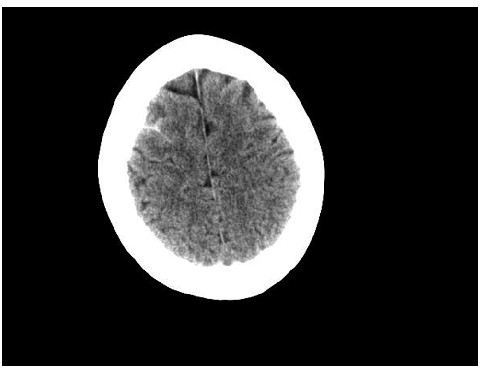
Cranial computerized tomography showing a subarachnoid hemorrhage as a white area in the right frontal lobe.
Ammatuna et al. described in their paper that 6% of patients with t-APL died of cerebral hemorrhage.1 Another study by Hasan et al. reported 14% of patients with tAPL after MS experiencing lethal cerebral hemorrhage.4 However, with regards to the data of the US Intergroup and the PETHEMA group showing 5% of all APL-patients who could not even start induction therapy because of cerebral and pulmonary hemorrhage,5 a subclinical higher fraction cannot be excluded. Given these data and the case report of our patient, one could speculate that patients with antecedent MS who present with tAPL are at an even higher risk for CNS hemorrhages at diagnosis. Whether this is due to previous cytotoxic therapy with MTZ, repetitively performed lumbar punctures due to MS diagnostics with subsequently disturbed blood-brain barriers or because of the pathophysiological mechanisms of inflammation, elevated cytokines and breakdown of tight junctions,6 remains speculative.
Recently, an increasing fraction of secondary APL due to an advanced application of topoisomerase-II inhibitors like MTZ could be observed.7 The European APL group estimates 22% of all APLs to be therapy-related.8
To summarize, the known frequent coagulopathy in APL patients, the increasing number of secondary APLs and the observed elevated risk of CNS bleeding events in patients with APL after MS necessitate both rapid diagnosis and careful management. Any neurological symptom in this patient cohort requires immediate imaging procedures. Furthermore, joint European or International studies are needed to analyze an adequate number of MS-APL patients to identify the true incidence of CNS hemorrhage.
Supplementary Material
Footnotes
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


