Abstract
The pH of the surface ocean is changing as a result of increases in atmospheric carbon dioxide (CO2), and there are concerns about potential impacts of lower pH and associated alterations in seawater carbonate chemistry on the biogeochemical processes in the ocean. However, it is important to place these changes within the context of pH in the present-day ocean, which is not constant; it varies systematically with season, depth and along productivity gradients. Yet this natural variability in pH has rarely been considered in assessments of the effect of ocean acidification on marine microbes. Surface pH can change as a consequence of microbial utilization and production of carbon dioxide, and to a lesser extent other microbially mediated processes such as nitrification. Useful comparisons can be made with microbes in other aquatic environments that readily accommodate very large and rapid pH change. For example, in many freshwater lakes, pH changes that are orders of magnitude greater than those projected for the twenty second century oceans can occur over periods of hours. Marine and freshwater assemblages have always experienced variable pH conditions. Therefore, an appropriate null hypothesis may be, until evidence is obtained to the contrary, that major biogeochemical processes in the oceans other than calcification will not be fundamentally different under future higher CO2/lower pH conditions.
Keywords: ocean acidification, rapid pH change, biogeochemical processes
The pH of the oceans will change in the coming decades as the result of anthropogenic emission of carbon dioxide (CO2); changes to ocean chemistry are incontrovertible (Doney et al., 2009). The chemistry is straightforward but not simple; as anthropogenic CO2 increases in the atmosphere, it dissolves in the surface ocean and aqueous carbon dioxide (CO2 (aq)) reacts with water to form carbonic acid (H2CO3)—a weak acid:
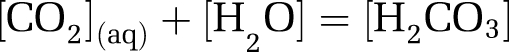 |
Carbonic acid dissociates to hydrogen ions (H+) and bicarbonate ions (HCO3−):
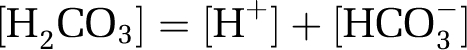 |
A large fraction of the additional hydrogen ions combines with carbonate ions (CO32−) to form bicarbonate ions:
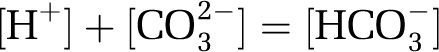 |
Therefore, the consequence of dissolving CO2 in seawater is an increase in the concentrations of aqueous carbon dioxide, carbonic acid, hydrogen ions and bicarbonate ions, and a decrease in carbonate ions. As pH is defined by the negative logarithm of the activity of hydrogen ions, it is clear that an increase in H+ must result in a decrease in pH. It is inevitable that the pH of the surface oceans will change with increasing anthropogenic CO2.
Caldeira and Wickett (2003), in their seminal paper, modelled the possible future changes in pH in the oceans and suggested that surface seawater pH would reduce by 0.77 units by the year 2300 if we burned the bulk of available fossil fuels (mostly coal). They also pointed out that, if CO2 emissions continued at the present rate, ocean pH would show larger changes, and faster rates of change, than are detected in the geological record with the possible exception of a few past catastrophic events (Kump et al., 2009). Pearson and Palmer (2000) suggested that the pH of seawater has varied by less than 0.1 units in the past 25 million years. As a result of these and other studies, there has been increasing concern that ‘ocean acidification' could have severe consequences for marine biota (http://www.ocean-acidification.net). Calcifying organisms, such as corals, molluscs and coccolithophores, will undoubtedly be particularly vulnerable to higher CO2 conditions, as under lower pH the saturation levels decline for calcium carbonate minerals, leading to increased difficulty in maintaining calcite and aragonite shells and skeletons. However, there is little firm evidence for how many other organisms will respond.
All microbes have complex proton pumps that are involved in bioenergetics, but it is not clear how microbes might respond to changes in environmental proton balance. The situation is complicated by the fact that the partial pressure of dissolved CO2 (pCO2) will increase in the future ocean, as well as pH decreasing. Some laboratory studies have shown that higher pCO2 has a fertilizer effect for some phytoplankton species under certain conditions. There is even less information about how heterotrophic microbes might respond to the future, coupled pCO2-pH change in the surface ocean—but there is a great deal of relevant information from other environments that can be evaluated. As ocean warming and increased stratification of the upper water column will also lead to decreases in dissolved oxygen (O2) concentrations (Keeling et al., 2010), changes in the O2/CO2 ratios may impose thermodynamic limitations to aerobic microbial life, especially in regions of low ambient O2 (Brewer and Peltzer, 2009).
Surface ocean pH is variable
Discussions of ocean pH are often made in the context of geological time scales (Pearson and Palmer, 2000; Caldeira and Wickett, 2003), and they imply a constancy and spatial uniformity of pH for the present-day oceans that is inconsistent with field observations. Over timescales of decades or longer, it may be appropriate to consider average annual values. But in reality, pH of the oceans is not constant and there are considerable seasonal, depth and regional variations. Therefore, when considering the overall consequences of ocean acidification for biogeochemical processes, it is necessary to understand that microbes in the present-day ocean experience variable pH; indeed, most of that variability is a consequence of microbial activity. For example, phytoplankton blooms can rapidly reduce pCO2, with a concomitant increase in pH. Although this is the opposite sign in pH change to that expected in the future ocean, it demonstrates that pH is naturally variable and that marine organisms—particularly microbes—must already be capable of adapting to rapid and sometimes large changes in pH. Nevertheless, it is important to know if the average surface water decline of ∼0.3 pH units that is expected by the current century end will present particular challenges for marine microbes or the elemental cycles that they sustain.
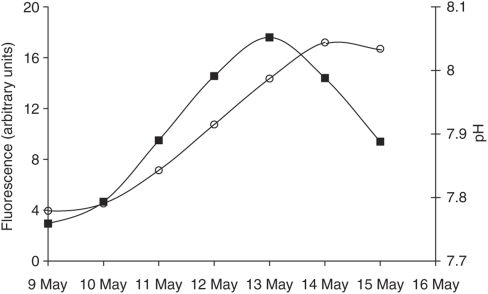
Effects of biological activity on ocean pH were recognized in some of the earliest studies of marine chemistry. Atkins (1922) reported that the pH of seawater in laboratory reservoirs was highly variable and that in tanks containing a decaying shrimp, pH decreased from 8.27 to 7.2 over a period of 16 days. Although there may be doubts about the accuracy and precision of these early measurements, it is clear that these changes were a likely consequence of microbial respiration increasing CO2. Rapid changes in CO2, and hence pH, also occur in the present-day ocean. For example, a detailed study in Antarctic coastal water involving daily measurements of pH, clearly demonstrated how pH in the sea is altered by biological activity (Shibca et al., 1977). For most of a 10-month period, pH was 7.9, but in situ acid-base chemistry changed rapidly during a phytoplankton bloom to pH 8.8. Shibca et al. (1977) calculated that this represented pCO2 saturation of only 15%, at a time when the measured O2 saturation was 120%—clear evidence that phytoplankton photosynthesis results in large and rapid changes in CO2 and hence in pH. The potential speed of pH change in a phytoplankton bloom is illustrated in Figure 1. In a mesocosm experiment to investigate ocean acidification (Gilbert et al., 2008), nutrient additions initiated a phytoplankton bloom in an enclosed seawater sample (11 000 l) that had been adjusted to pH 7.8 by bubbling with CO2-enriched air (750 μatm). Within 4 days, through the utilization of CO2, the actively growing phytoplankton assemblage had returned pH to present-day conditions—a change of 0.3 pH units.
Figure 1.
Rapid change in pH (○) as phytoplankton biomass (▪) increased during a bloom in a mesocosm experiment (Gilbert et al., 2008). Water had been bubbled with CO2-enriched air (750 μatm) and the bloom was initiated by the addition of nitrate and phosphate. The mesocosm was covered and there was no exchange of CO2 with the atmosphere; the observed changes in pH were the result to the utilization of dissolved CO2 by the actively growing phytoplankton assemblage.
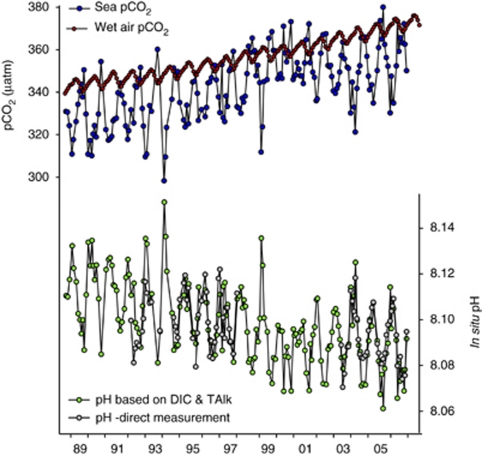
There are other examples of the importance of biology in changing the surface water pH. Figure 2 compares CO2 at station ALOHA, off the Hawaiian Islands (Dore et al., 2009), with the atmospheric record from Mauna Loa on the Island of Hawaii. Seasonal variations in the surface water are greater than those in the atmosphere because of biological and physical processes (mostly temperature). The pH can change by up to 0.06 pH units during the year even in the oligotrophic Central Pacific, which does not experience the dramatic phytoplankton blooms of temperate oceans.
Figure 2.
Long-term trends in surface ocean pH and CO2 at station ALOHA in the Central Pacific, along with atmospheric CO2 from nearby Mauna Loa. Net CO2 flux was into the ocean, adding carbonic acid and lowering pH. The long-term declining pH trend was highly significant, but is overlain by substantial seasonal variability (Dore et al., 2009). Direct pH measurements agreed well with calculated values based on measurements of total dissolved inorganic carbon (DIC) and alkalinity Surface ocean pH declined by 0.032 pH units from 1989–2006, an approximate 7% increase in H+ concentration.
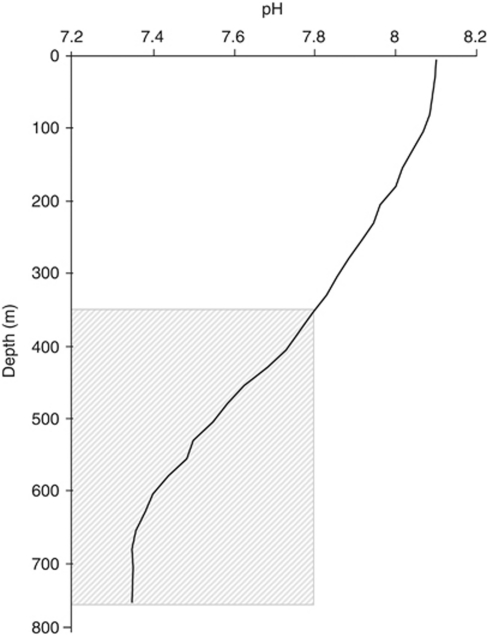
In many environments, bacteria are already experiencing pH as low (or lower) as that projected for the end of the century in the surface ocean. For example, the thermocline at station ALOHA is a region where respiration exceeds photosynthesis and where sinking organic matter decomposition by aerobic respiration results in the release of CO2 and a reduction in pH (Figure 3). Water below 350 m has a pH less than 7.8, which is projected to be the pH of the surface ocean by the year 2100, yet microbial processes continue. Even in regions of the surface ocean, high respiration:photosynthesis ratios (for example, high latitude regions in winter; residual signal in newly upwelled waters) could result in periodic reduced pH, if dissolved CO2 accumulates. Increases in pH with depth were also noted by Atkins (1922) as a feature of one of the earliest studies of pH in the ocean. Some regions, particularly estuaries and coastal regions, show large seasonal and spatial variations in pH (Salisbury et al., 2008); indeed, they have been among the first regions to demonstrate significant ecological change as a result of a steep decline in seawater pH over time (for example, study of benthic algal and invertebrate community by Wootton et al., 2008).
Figure 3.
Depth profile of pH at station ALOHA in the central Pacific. The shaded area indicates water that is more acidic than that projected for surface waters at the end of the twenty first century.
Given their vital function in marine productivity and planetary habitability, it is imperative to know if microbial assemblages will continue to function at the lower pH values that are projected for the near future. That is, do present-day populations have the metabolic and genetic plasticity to compensate for lower pH conditions? Or are marine microbes less able to acclimate because the average pH of the oceans has changed more slowly over geological time periods, a factor of 30–100 times slower than projected rates for this century, and perhaps has not varied by more than 0.6 pH units for 350 million years (Kump et al., 2009)?
One clue might come from a comparison with freshwater lakes. These have much lower buffering capacity than the oceans, so significant daily variations in pH occur as a result of normal temporal phasing of net photosynthesis and net respiration. For example, Maberly (1996) showed that diel variations in a lake can be as much as 2–3 pH units. In contrast, the pH change in the ocean is expected to be ∼0.3 pH units over the next 100 years. Variations in pH also occur over very small distances. Talling (2006) showed that in some English lakes, pH could change by >2.5 pH units over a depth of only 14 m in the water column. Yet phytoplankton, bacteria, archaea and metazoans are all present in lakes, and appear to be able to accommodate large daily and seasonal changes in pH. Will marine microbes be different, with a lower capacity to acclimate and adapt than freshwater microbes? Is it possible that long-term exposure to much smaller variations in pH means that marine microbes are less able to accommodate pH change than freshwater microbes?
This seems unlikely from the evidence available to date, at least from distribution studies of marine heterotrophic bacteria. For example, a genomic study at station ALOHA (DeLong et al., 2006) investigated the vertical distribution of bacteria. Although there were large differences with depth, particularly, in cyanobacteria and numbers of sequences retrieved, a number of taxa were found throughout the water column to a depth of 4000 m. These included representatives of the alphaproteobacteria and the gammaproteobacteria with 16S sequences from SAR202, SAR324 and SAR406 detected at every depth sampled, including within the core of the acidified zone (200–4000 m). Although it is likely that there were different phenotypes and genetic sub-populations with depth, the 16S sequences were identical (as demonstrated for SAR11 by Field et al., 1997). These data demonstrate that diverse bacterial assemblages, composed of similar lineages, are present over a range of pH (as well as different temperatures and pressures). Important questions that could be readily addressed by sampling a depth profile, or comparing marine, coastal and freshwater systems is: Do marine phytoplankton, bacteria and archaea have the genetic flexibility for homeostasis of intracellular pH (Booth, 1985) under higher CO2/lower pH (that is, they can acclimate)? Or will genetic change be required, involving adaptation by the acquisition of genes through lateral gene transfer or selection of beneficial mutations? Or will small variations in external pH affect chemical equilibria and kinetics at the cell surface and with membrane transporters, leading to consequences for microbial physiology? We urgently need answers to these questions.
Microbially mediated processes—will they be sensitive to ocean acidification?
Table 1 is a brief summary of current knowledge of how key biogeochemical processes may respond in a high CO2 ocean. Experiments to date do not provide a clear indication of how pH change might affect marine microbes and results are inconsistent and at times conflicting. For example, a number of studies have investigated the consequences of reduced pH for calcifying phytoplankton (Table 1). Results by a number of laboratories suggest negative effects of higher CO2/lower pH on coccolithophore cultures (Riebesell et al., 2000, 2007) but at least two studies indicate enhanced calcification under elevated CO2 (Langer et al., 2006; Iglesias-Rodriguez et al., 2008). Ridgwell et al. (2009) have suggested that some of these differences may be due to strain variations in the cultures used for these experiments; or there may be experimental design consequences resulting from the procedures used to adjust the pH (Rost et al., 2008). Clearly, further experiments are required to clarify the effect of ocean acidification on this important group of phytoplankton.
Table 1. Current state of knowledge on microbially mediated process that may be susceptible to ocean acidification.
| Process | Significance | State of knowledge |
|---|---|---|
| Primary productivity | Major influence on global carbon cycle, as ocean productivity is equivalent to terrestrial primary production. | Laboratory and field incubations showing both little change or enhanced productivity with elevated CO2. No reliable estimates of how global ocean productivity will change in relation to higher pCO2/lower pH. |
| Dominant phytoplankton species | Size of phytoplankton is important, influencing food availability (different grazing efficiencies) and potentially changing population structure of herbivores and carbon export. | Little information available; Kim et al. (2006) found only small changes in diatoms populations, whereas Tortell et al. (2008) report increase in chain-forming diatoms. |
| Phytoplankton biochemical composition | Food quality may change (for example, protein, lipid and carbohydrate content). | Changes in C/N ratio, with higher C content reported in some studies (Riebesell et al., 2007) but not others (Hutchins et al., 2009); experimental conditions (light and nutrients) are important (Leonardos and Geider, 2005). |
| Calcification and carbonate dissolution | Calcifying phytoplankton and zooplankton produce particulate inorganic carbon that can alter particle sinking and export flux (ballast hypothesis) and influence bio-optical properties; balance between calcification and carbonate dissolution influences large-scale inorganic carbon cycle and geochemical processes. | Conflicting data on effect of high pCO2 on coccolithophore calcification with reports of both reduced (Riebesell et al., 2000, 2007) and enhanced (Iglesias-Rodriguez et al., 2008) rates. Ridgwell et al. (2009) emphasize importance of strain differences in culture experiments. There is clearer evidence of reduced calcification for foramaniferia and pteropods (Fabry et al., 2008). |
| Particle sinking and export of organic carbon | The biological carbon pump controls carbon sequestration in the deep ocean (linked to species composition, as small cells do not sink) and “ballast”—the organic carbon associated with sinking inorganic particles. | Delille et al. (2005) suggested vertical flux will increase in higher pCO2. |
| Bacterial respiration and remineralization | The vast majority of organic carbon in the surface oceans is utilized by heterotrophic bacteria; changes in bacterial activity and growth efficiency could profoundly affect the oceanic carbon balance. | Grossart et al. (2006) found changes in bacterial metabolism in high pCO2, but it was difficult to distinguish direct effects on bacteria from changes in phytoplankton assemblages at high pCO2. |
| Nitrogen cycle | Microbes control the cycling of nutrients, particularly, biologically available nitrogen which limits primary productivity in many oceanic provinces. Key processes are nitrogen fixation, denitrification and nitrification. | Hutchins et al. (2007, 2009) and Levitan et al. (2007) found increased rates of nitrogen fixation by Trichodesmium at high pCO2. Huesemann et al. (2002) and Beman et al. (2009) report reductions in nitrification under elevated CO2. The impact of realistic CO2 variations on denitrification has not been examined (Hutchins et al., 2009). |
| Trace gas production | Microbes drive the production and consumption of many other potent climate-active gases (for example, methane, nitrous oxide) and alter atmospheric chemistry (dimethylsulphide and organohalides). | Hopkins et al. (2010) found decreased production of dimethylsulphide and volatile iodocarbon compounds under high pCO2 conditions. |
| Trace metal availability | Altered pH may change trace metal availability, for through changes in pH- and CO2-dependent chemical speciation and dust dissolution. | Shi et al. (2010) report a reduction in iron bioavailability and phytoplankton uptake under elevated CO2. |
Neither are there robust, consistent results on the effects of pH on non-calcifying phytoplankton. Increasing photosynthesis with elevated CO2 is observed for some cyanobacteria (Synechococcus) but not others (Prochlorococcus) (Fu et al., 2007), and many eukaryotic phytoplankton species, most notably diatoms, have carbon concentrating mechanisms that diminish almost entirely the sensitivity of photosynthesis to CO2 variations (Tortell et al., 1997). Bottle incubations and mesocosm experiments with natural plankton communities indicate only a weak sensitivity of primary production to CO2 (Tortell et al., 2000), although limited CO2 fertilization is observed in some cases (Tortell et al., 2008; Egge et al., 2009). There are suggestions that the carbon content of phytoplankton cells may increase under high CO2 conditions (Riebesell et al., 2007), but any physiological changes appear to be quite subtle, and there is conflicting evidence from different studies on how plankton carbon/nitrogen stoichiometry varies with CO2. In terms of all of the major biogeochemical processes listed in Table 1, there is still a state of considerable ignorance about how the ocean system will respond to higher CO2/lower pH.
Future priorities
A number of experimental approaches could be taken that will lead to better understanding. Many of these have been reviewed in the report of a recent expert group of microbial oceanographers that met to consider the consequences of ocean acidification for marine microbes (http://cmore.soest.hawaii.edu/oceanacidification/) and will be only briefly summarized here. A combination of approaches varying greatly in scope and scale was recommended. These range from autecological studies on model marine microbes to investigate intracellular pH regulation, to microcosm and mesocosm scale experiments, and possibly open-ocean, mesoscale perturbation experiments. Comparative studies of similar marine, coastal and freshwater microbial communities (such as those dominated by diatoms or dinoflagellates) could help to answer questions about whether there are intrinsic differences in the response to rapid (that is, hourly or daily) pH change, as occurs in lakes (and to some degree in coastal waters) but not the same extent in open-ocean waters. Much of the present literature on microbe responses to pH/CO2 perturbations is phenomenological, and more detailed studies are required to assess mechanisms at biochemical and cellular levels. Advances in high-throughput sequencing technology now offers opportunities to define how complex microbial communities might respond to CO2/lower pH—for example using 16S rRNA-tag pyrosequencing of the V6 region (Sogin et al., 2006) or similar approaches. Metagenomics and metatranscriptomics technology has now developed sufficiently to study how whole communities might respond to CO2 perturbation (Gilbert et al., 2008). Some existing projects to sample long ocean transects, such as the Atlantic Meridional Transect (Robinson et al. (2006), offer opportunities to test how the increasingly well-defined microbial assemblages in different ocean provinces (Schattenhofer et al., 2009) might vary with pH. Large-scale surveys and time-series efforts are also crucial for scaling up physiological and genomic studies to ecosystems, with the ultimate goal of understanding impacts on biogeochemical cycling and ecosystem services (Doney et al., 2004).
Laboratory experiments with cultured organisms are invaluable for exploring plankton physiological responses to perturbations in pH and CO2. However, it should be recognized and appreciated that most plankton cultures have been maintained in the laboratory for many generations and may not be appropriate model organisms for these investigations on the effects of pH change. Most growth media do not adequately control pH and, at the high cell densities that are common in the maintenance of stock cultures, cells may well have been growing at >pH 9 for phytoplankton, and <pH 6 for heterotrophic bacteria, for many decades in some cases. Existing culture collections may have unwittingly selected for high or low pH conditions, respectively, or for organisms less sensitive to rapid pH variations with time. It may be necessary to conduct experiments using fresh isolates, rather than relying on the more convenient established cultures. Change and selection in laboratory cultures is poorly documented but could be readily investigated. Whole genome sequences are available for an increasing number of phytoplankton and bacterial species. It would be very interesting to compare the genomes of fresh isolates with long established cultures. For example, the common laboratory diatom, Phaeodactylum, was isolated from the English Channel in 1910 (Allen and Nelson, 1910) and has been maintained in culture collections for 100 years—although under poorly defined conditions. How would the genome of this long-term culture compare with a fresh isolate from the English Channel?
Long-term experiments lasting many generations may be necessary to establish how individual organisms might respond to higher CO2/lower pH. Yet these are very challenging experiments. Collins and Bell (2004) maintained Chlamydomonas for 1000 generations under high CO2 (1050 μatm) but failed to find evidence for adaptive, evolutionary change. Long-term adaptation experiments may need to be maintained for decades—much longer than is possible with current funding models (Boyd et al., 2008). And there is no guarantee that adaptation/evolution would be detected at the end of the experiment, so there is a large element of risk in the design and conduct of these studies.
Finally, ocean acidification is only one aspect of habitat variability and climate change. The temperature of the oceans will also increase as a consequence of higher CO2 concentrations in the atmosphere, but little is known about how natural assemblages will respond to higher temperatures. Higher temperatures will also lead to increased stratification that will alter the flux of nutrients from below and the mean light levels experienced by microbes throughout the euphotic zone (Steinacher et al., 2010). Given that ocean acidification will occur at the same time as temperature increases, nutrient decreases and light flux alterations, to name a few habitat variables, there is a strong case for multifactorial experiments to examine the possible synergistic or antagonistic effects of multiple stressors on microbial assemblage diversity and function.
In conclusion, CO2 and pH in the surface ocean are not, and never have been, constant. Microbes in some parts of the present-day ocean already experience average surface ocean pH that will occur by the end of the century. And freshwater and coastal microbes experience short-term and seasonal changes that are many times greater than those that will occur in the open-ocean. Given these facts, perhaps the most appropriate null hypothesis to test is that marine microbes possess the flexibility to accommodate pH change and there will be no catastrophic changes in marine biogeochemical processes that are driven by phytoplankton, bacteria and archaea. Clearly, calcifying organisms are a special case as carbonate minerals will be less saturated—and for the case of aragonite, undersaturated in surface waters in a high-CO2 ocean. Photosynthetic organisms may also be influenced and it is even possible that higher CO2 may be beneficial. But the rest of the microbial community should not be assumed to be at risk until evidence to the contrary is obtained.
Acknowledgments
We acknowledge stimulating discussions with members of the expert group of microbial oceanographers that met to consider the consequences of ocean acidification for marine microbes (http://cmore.soest.hawaii.edu/oceanacidification/). Funding from the Gordon and Betty Moore Foundation, and logistical support from the Plymouth Marine Laboratory and the Center for Microbial Oceanography: Research and Education (National Science Foundation grant EF-0424599) are gratefully acknowledged.
References
- Allen EJ, Nelson EW. On the artificial culture of marine plankton organisms. J Mar Biol Assoc UK. 1910;8:421–474. [Google Scholar]
- Atkins WRG. The hydrogen ion concentration of sea water in its biological relation. J Mar Biol Assoc UK. 1922;12:717–771. [Google Scholar]
- Beman JM, Chow CE, Popp BN, Fuhrman JA, Feng Y, Hutchins DA.2009Alteration of oceanic nitrification under elevated carbon dioxide concentrationsPaper abstract, ASLO Aquatic Sciences Meeting, Nice, France, January 25–30, 2009 (http://www.aslo.org/meetings/nice2009/files.html).
- Booth IR. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985;49:359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd PW, Doney SC, Strzepek R, Dusenberry J, Lindsay K, Fung I. Climate-mediated changes to mixed-layer properties in the Southern Ocean: assessing the phytoplankton response. Biogeosciences. 2008;5:847–864. [Google Scholar]
- Brewer PG, Peltzer ET. Limits to marine life. Science. 2009;324:347–348. doi: 10.1126/science.1170756. [DOI] [PubMed] [Google Scholar]
- Caldeira K, Wickett ME. Anthropogenic carbon and ocean pH. Nature. 2003;425:365. doi: 10.1038/425365a. [DOI] [PubMed] [Google Scholar]
- Collins S, Bell G. Phenotypic consequences of 1000 generations of selection at elevated CO2 in a green alga. Nature. 2004;431:566–569. doi: 10.1038/nature02945. [DOI] [PubMed] [Google Scholar]
- Delille B, Harlay J, Zondervan I, Jacquet S, Chou L, Wollast R, et al. Response of primary production and calcification to changes of CO2 during experimental blooms of the coccolithophorid Emiliania huxleyi. Global Biogeochem Cycles. 2005;19:GB2023. [Google Scholar]
- DeLong EF, Preston CM, Mincer T, Rich V, Hallam SJ, Frigaard NU, et al. Community genomics among stratified microbial assemblages in the ocean's interior. Science. 2006;311:296–503. doi: 10.1126/science.1120250. [DOI] [PubMed] [Google Scholar]
- Doney SC, Abbott MR, Cullen JJ, Karl DM, Rothstein L. From genes to ecosystems: the ocean's new frontier. Frontiers Ecol Environ. 2004;2:457–466. [Google Scholar]
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: the other CO2 problem. Annu Rev Mar Sci. 2009;1:169–192. doi: 10.1146/annurev.marine.010908.163834. [DOI] [PubMed] [Google Scholar]
- Dore JE, Lukas R, Sadler DW, Church MJ, Karl DM. Physical and biogeochemical modulation of ocean acidification in the central North Pacific. Proc Natl Acad Sci. 2009;106:12235–12240. doi: 10.1073/pnas.0906044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egge JK, Thingstad TF, Larsen A, Engel A, Wohlers J, Bellerby RGJ, et al. Primary production during nutrient-induced blooms at elevated CO2 concentrations. Biogeosciences. 2009;6:877–885. [Google Scholar]
- Fabry VJ, Seibel BA, Feely RA, Orr JC. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci. 2008;65:414–432. [Google Scholar]
- Field KG, Gordon D, Wright T, Rapp M, Urbach E, Vergin K, et al. Diversity and depth-specific distribution of SAR11 cluster rRNA genes from marine planktonic bacteria. Appl Environ Microbiol. 1997;63:63–70. doi: 10.1128/aem.63.1.63-70.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu F-X, Warner ME, Zhang Y, Feng Y, Hutchins DA. Effects of increased temperature and CO2 on photosynthesis, growth and elemental ratios of marine Synechococcus and Prochlorococcus (cyanobacteria) J Phycol. 2007;43:485–496. [Google Scholar]
- Gilbert JA, Field D, Huang Y, Edwards R, Li W, Gilna P, et al. Detection of large numbers of novel sequences in the metatranscriptomes of complex marine microbial communities. PLoS One. 2008;3:e3042. doi: 10.1371/journal.pone.0003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossart H-P, Allgaier M, Passow U, Riebesell U. Testing the effect of CO2 concentration on the dynamics of marine heterotrophic bacterioplankton. Limnol Oceanogr. 2006;51:1–11. [Google Scholar]
- Hopkins FE, Turner SM, Nightingale PD, Steinke M, Bakker D, Liss PS. Ocean acidification and marine trace gas emissions. Proc Natl Acad Sci. 2010;107:760–765. doi: 10.1073/pnas.0907163107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesemann MH, Skillman AD, Crecelius EA. The inhibition of marine nitrification by ocean disposal of carbon dioxide. Marine Pollution Bulletin. 2002;44:142–148. doi: 10.1016/s0025-326x(01)00194-1. [DOI] [PubMed] [Google Scholar]
- Hutchins DA, Fu F-X, Zhang Y, Warner ME, Feng Y, Portune K, et al. CO2 control of Trichodesmium N2 fixation, photosynthesis, growth rates, and elemental ratios: implications for past, present, and future ocean biogeochemistry. Limnol Oceanogr. 2007;52:1293–1304. [Google Scholar]
- Hutchins DA, Mulholland MR, Fu FX. Nutrient cycles and marine microbes in a CO2-enriched ocean. Oceanography. 2009;22:128–145. [Google Scholar]
- Iglesias-Rodriguez MD, Halloran PR, Rickaby REM, Hall IR, Colmenero-Hidalgo E, Gittins JR, et al. Phytoplankton calcification in a high-CO2 world. Science. 2008;320:336–340. doi: 10.1126/science.1154122. [DOI] [PubMed] [Google Scholar]
- Keeling RF, Kortzinger A, Gruber N. Ocean deoxygenation in a warming world. Ann Rev Mar Sci. 2010;2:199–229. doi: 10.1146/annurev.marine.010908.163855. [DOI] [PubMed] [Google Scholar]
- Kim J-M, Lee K, Shin K, Kang J-H, Lee H-W, Kim M, et al. The effect of seawater CO2 concentration on growth of a natural phytoplankton assemblage in a controlled mesocosm experiment. Limnol Oceanogr. 2006;51:1629–1636. [Google Scholar]
- Kump LR, Bralower TJ, Ridgwell A. Ocean acidification in deep time. Oceanography. 2009;22:94–107. [Google Scholar]
- Langer MR, Geisen M, Baumann K-H, Klas J, Riebesell U, Thoms S, et al. Species-specific responses of calcifying algae to changing seawater carbonate chemistry. Geochem Geophys Geosys. 2006;7:Q09006. [Google Scholar]
- Leonardos N, Geider RJ. Elevated atmospheric carbon dioxide increases organic carbon fixation by Emiliania huxleyi (Haptophyta), under nutrient-limited, high-light conditions. J Phycol. 2005;41:1196–1203. [Google Scholar]
- Levitan O, Rosenberg G, Setlik I, Selikova E, Grigel J, Klepetar J, et al. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Global Change Biol. 2007;13:531–538. [Google Scholar]
- Maberly SC. Diel, episodic and seasonal changes in pH and concentrations of inorganic carbon in a productive lake. Freshwater Biol. 1996;35:579–598. [Google Scholar]
- Pearson PN, Palmer MR. Atmospheric carbon dioxide concentrations over the past 60 million years. Nature. 2000;406:695–699. doi: 10.1038/35021000. [DOI] [PubMed] [Google Scholar]
- Riebesell U, Schulz KG, Bellerby RGJ, Botros M, Fritsche P, Meyeröfer M, et al. Enhanced biological carbon consumption in a high CO2 ocean. Nature. 2007;450:545–548. doi: 10.1038/nature06267. [DOI] [PubMed] [Google Scholar]
- Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe RE, Morel FMM. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature. 2000;407:364–367. doi: 10.1038/35030078. [DOI] [PubMed] [Google Scholar]
- Ridgwell A, Schmidt DN, Turley C, Brownlee C, Maldonado M, Tortell P, et al. From laboratory manipulations to Earth system models: scaling calcification impacts of ocean acidification. Biogeosciences. 2009;6:2611–2623. [Google Scholar]
- Robinson C, Poulton AJ, Holligan PM, Baker AR, Forster G, Gist N, et al. The Atlantic Meridional Transect (AMT) programme: a contextual view 1995–2005. Deep-Sea Res Part II: Top Stud Oceanogr. 2006;53:1649–1665. [Google Scholar]
- Rost B, Zondervan I, Wolf-Gladrow D. Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: current knowledge, contradictions and research directions. Mar Ecol Prog Ser. 2008;373:227–237. [Google Scholar]
- Salisbury J, Green M, Hunt C, Campbell J. Coastal acidification by rivers: a threat to shellfish. Eos. 2008;89:513–528. [Google Scholar]
- Schattenhofer M, Fuchs BM, Amann R, Zubkov MV, Tarran GA, Pernthaler J. Latitudinal distribution of prokaryotic picoplankton populations in the Atlantic Ocean. Environ Microbiol. 2009;11:2078–2093. doi: 10.1111/j.1462-2920.2009.01929.x. [DOI] [PubMed] [Google Scholar]
- Shibca SV, Hedgpeth DW, Park PK.1977Dissolved oxygen and pH increases by primary production in the surface water of Arthur Harbor, Antarctica 1970–71In: Llano, GA (ed).Adaptations within Antarctic Ecosystems: Proceedings of the Third SCAR Symposium on Antarctic Biology Smithsonian Institution: Washington; 83–97. [Google Scholar]
- Shi DL, Xu Y, Hopkinson BM, Morel FMM. Effect of ocean acidification on iron availability to marine phytoplankton. Science. 2010;327:676–679. doi: 10.1126/science.1183517. [DOI] [PubMed] [Google Scholar]
- Sogin ML, Morrison HG, Huber JA, Mark Welch D, Huse SM, Neal PR, et al. Microbial diversity in the deep sea and the underexplored ‘rare biosphere'. Proc Natl Acad Sci. 2006;103:12115–12120. doi: 10.1073/pnas.0605127103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinacher M, Joos F, Frölicher TL, Bopp L, Cadule P, Cocco V, et al. Projected 21st century decrease in marine productivity: a multi-model analysis. Biogeosciences. 2010;7:979–1005. [Google Scholar]
- Talling JF. Interrelated seasonal shifts in acid-base and oxidation-reduction systems that determine chemical stratification in three dissimilar English lake basins. Hydrobiol. 2006;568:275–286. [Google Scholar]
- Tortell PD, Rau GH, Morel FMM. Inorganic carbon acquisition in coastal Pacific phytoplankton communities. Limnol Oceanogr. 2000;45:1485–1500. [Google Scholar]
- Tortell PD, Reinfelder JR, Morel FMM.1997Active uptake of bicarbonate by diatoms Nature 390243–244.9384376 [Google Scholar]
- Tortell PD, Payne CD, Li YY, Trimborn S, Rost B, Smith WO, et al. CO2 sensitivity of Southern Ocean phytoplankton. Geophys Res Lett. 2008;35:L04605. [Google Scholar]
- Wootton JT, Pfister CA, Forester JD. Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc Natl Acad Sci. 2008;105:18848–18853. doi: 10.1073/pnas.0810079105. [DOI] [PMC free article] [PubMed] [Google Scholar]