Abstract
A distinct subgroup of euglenozoans, referred to as the ‘Symbiontida,' has been described from oxygen-depleted and sulfidic marine environments. By definition, all members of this group carry epibionts that are intimately associated with underlying mitochondrion-derived organelles beneath the surface of the hosts. We have used molecular phylogenetic and ultrastructural evidence to identify the rod-shaped epibionts of the two members of this group, Calkinsia aureus and B.bacati, hand-picked from the sediments of two separate oxygen-depleted, sulfidic environments. We identify their epibionts as closely related sulfur or sulfide-oxidizing members of the epsilon proteobacteria. The epsilon proteobacteria generally have a significant role in deep-sea habitats as primary colonizers, primary producers and/or in symbiotic associations. The epibionts likely fulfill a role in detoxifying the immediate surrounding environment for these two different hosts. The nearly identical rod-shaped epibionts on these two symbiontid hosts provides evidence for a co-evolutionary history between these two sets of partners. This hypothesis is supported by congruent tree topologies inferred from 18S and 16S rDNA from the hosts and bacterial epibionts, respectively. The eukaryotic hosts likely serve as a motile substrate that delivers the epibionts to the ideal locations with respect to the oxic/anoxic interface, whereby their growth rates can be maximized, perhaps also allowing the host to cultivate a food source. Because symbiontid isolates and additional small subunit rDNA gene sequences from this clade have now been recovered from many locations worldwide, the Symbiontida are likely more widespread and diverse than presently known.
Keywords: Symbiontida, epibionts, epsilon proteobacteria, Calkinsia, Bihospites, co-evolution, sulfide
Introduction
Examples of symbiotic relationships between prokaryotes and eukaryotes in deep sea oxygen-depleted marine environments have been well documented for some groups, starting with the discovery of associations between metazoa and bacteria at hydrothermal vents (Cavanaugh et al., 1981), cold seeps (Barry et al., 1996) and the edges of silled basins (for example, Distel and Felbeck, 1988). Chemosynthetic autotrophy supports many of these associations and involves the oxidation of hydrogen sulfide or methane by endosymbiotic bacteria within the animal hosts. Similar associations have also been observed between prokaryotes and marine protists. These include a wide range of metabolic relationships observed in shallow marine, primarily reducing environments, including endosymbiotic methanogens in ciliates to epibiotic hydrogen sulfide oxidizers on euglenids (Fenchel et al., 1995; Ott, 1996; Epstein et al., 1998). The first observations of episymbiotic relationships between protists and prokaryotes in the deep sea were documented in cold seeps of Monterey Bay, CA, USA (Buck and Barry, 1998; Buck et al., 2000) and in the oxygen-depleted Santa Barbara Basin, CA, USA (Bernhard et al., 2000, 2010). Both sites were at water depths greater than 500 m, and had high concentrations of mat-forming chemoautotrophic bacteria. In Santa Barbara Basin bottom water, oxygen concentration rarely exceeds 5 μmol l−1 (∼0.1 ml l−1) (Kuwabara et al., 1999) and sulfide concentration can exceed 50 μ between 0.5–1.0 cm depth (Bernhard, 2003; Bernhard et al., 2003). In this environment, euglenozoan flagellates (including Calkinsia aureus) were numerically the most abundant group and most eukaryotic taxa harbored bacterial epibionts and/or endosymbionts (Bernhard et al., 2000).
The Euglenozoa comprises a large group of flagellates with diverse nutritional modes, and consists of four distinct subgroups: euglenids, kinetoplastids, diplonemids and symbiontids. Calkinsia aureus, B. bacati and Postgaardi mariagerensis have been isolated from oxygen-depleted marine environments; each one of them is covered with rod-shaped epibiotic bacteria (Simpson et al., 1997; Bernhard et al., 2000; Yubuki et al., 2009; Breglia et al., 2010). All three of these species have been characterized at the ultrastructural level, and C. aureus and B. bacati have also been characterized at the molecular phylogenetic level using small subunit rDNA sequences (Breglia et al., 2010; Simpson et al., 1997; Yubuki et al., 2009). The data from C. aureus and B. bacati showed a distinct subgroup of euglenozoans from oxygen-depleted environments (including seven environmental DNA sequences from Northern Europe and South America) referred to as the ‘Symbiontida.' Symbiontid isolates and additional small subunit rDNA sequence representatives of the clade have now been recovered from seafloor sediments of Santa Barbara Basin, CA, USA coastal sediments of British Columbia, Canada, Northern Germany and anoxic and sulfidic waters in Venezuela, Denmark and Norway (Yubuki et al., 2009; Breglia et al., 2010).
The rod-shaped epibionts of C. aureus are 3–5 μm long and 0.350-μm wide and form a tightly-packed coat over the entire surface of the host cell, with at least 128 bacterial cells observed in a transverse section through C. aureus (Yubuki et al., 2009). Moreover, the distinctively orange color of C. aureus is attributable to a complex extracellular matrix. The prolate-shaped cells of C. aureus are around 48 μm (42.6–71.3 μm) long and around 17 μm (14.2–19.5 μm) wide (for a detailed ultrastructure analysis see Yubuki et al. (2009). Unlike most of their euglenozoan relatives (for example, kinetoplastids and euglenids), C. aureus lacks recognizable mitochondria with cristae, and instead possesses superficially arranged double-membrane bound organelles nearly identical in morphology to the well-described hydrogenosomes found in flagellates from other anoxic environments (Fenchel and Finlay, 1995). Hydrogenosomes function to produce molecular hydrogen, acetate, CO2 and adenosine-5′-triphosphate in anoxic environments (Barbera et al., 2007).
B. bacati, which was recovered from oxygen-depleted sandy sediments in a shallow tidal flat in South-western British Columbia, Canada, is 40–120 μm long and 15–30 μm wide and the cell surface is covered with two different morphotypes of epibionts: (1) spherical-shaped bacteria about 0.6 μm in diameter with an extrusive apparatus and (2) rod-shaped bacteria 3–5 μm long and arranged in bands along the longitudinal axis of the host (Breglia et al., 2010). Longitudinal bands of rod-shaped bacteria were separated by single or double rows of spherical-shaped bacteria. Molecular phylogenetic analyses of small subunit rRNA gene sequences showed that to date B. bacati is positioned as the earliest diverging known representative of the Symbiontida, a position consistent with comparative ultrastructure. Several morphological features of B. bacati are transitional between those found in C. aureus and those found in phagotrophic euglenids. Although these ultrastructural data suggest that the Symbiontida is nested within the Euglenida, current molecular phylogenetic data do not shed any light on this hypothesis (Breglia et al., 2010). C. aureus and B. bacati, share the feature of a coupling of rod-shaped epibionts with a superficial layer of hydrogenosome-like, mitochondrion-derived organelles having reduced or absent cristae. This seems to be a unifying characteristic of the Symbiontida, and suggests a mutualistic relationship that has enabled symbiontids to diversify within oxygen-depleted environments.
In addition to C. aureus and B. bacati, other euglenozoans from oxygen-depleted environments have been identified with epibiotic bacteria, including Postgaardi mariagerensis (Fenchel et al., 1995; Simpson et al., 1997), Euglena helicoideus (Leander and Farmer, 2000), Dylakosoma pelophilum (Wolowski, 1995) and five unidentified euglenozoans (Bernhard et al., 2000; Buck et al., 2000; Buck and Bernhard, 2002). Hypotheses for the biological role(s) of rod-shaped epibionts of eukaryotic hosts usually involve commensalism, with the bacteria benefiting from metabolic byproducts secreted by the host (Fenchel et al., 1995; Simpson et al., 1997; Leander and Keeling, 2004). It has also been hypothesized that the epibionts might be chemoautotrophic sulfur- or methane-oxidizers that form a mutualistic relationship with the host, whereby the host provides a substrate for the bacteria and the bacteria detoxify the immediate environment for the host (Bernhard, 2003; Bernhard et al., 2003, 2010). Under certain conditions, the epibiotic bacteria may serve as food for the host (Breglia et al., 2010). To better understand the evolutionary history of symbiotic relationships within the Symbiontida, we generated molecular data from the rod-shaped epibionts on both C. aureus and B. bacati. The resulting molecular data enabled us to more rigorously identify the epibionts, to infer co-evolutionary relationships between the bacteria and the symbiotid hosts, and to begin to ascertain biogeographical patterns of the group as a whole.
Materials and methods
Sample collection
The Santa Barbara Basin, which is located off California (USA), has a maximum depth of ∼600 m and sill depth of ∼475 m. The Santa Barbara samples used for this study were collected using a Soutar box corer or an MC800 multicorer from sea floor sediments (580–592 m depth) in September 2007, June 2008, October 2008 and June 2009 using the RV Robert Gordon Sproul. Samples bearing Calkinsia aureus were collected along a north–south trending transect along 120°02′W, from 34°17.6′N to 34°13.0′N. Our samples were collected from box cores that exhibited a surface covering of sulfide-oxidizing bacteria (either Thioploca or Beggiatoa, both of which require sulfide and little or no oxygen). Surface ∼0–2 cm sediments were transferred to 100–250 ml high density polyethylene bottles with an overlayer of bottom water, and were stored at ∼7 °C. Oxygen concentrations of overlying bottom water on top of Soutar box cores were determined using the microwinkler method (Browenkow and Cline, 1969).
Individual cells of B. bacati were isolated by micropipetting from sediment samples collected in Boundary Bay, British Columbia (location details in Breglia et al. (2010)) using a Leica DM IL inverted microscope (Meyer Instruments, Houston, TX, USA). The samples were taken 30–50 m from the coastline (that is, the high tide boundary) and at a depth of about 3 cm below the sediment surface, within a conspicuous layer of black sand.
Light microscopy
Light micrographs of over 20 Calkinsia aureus living cells and fluorescence images of minimum of 60 fixed cells were taken using a Zeiss Axioplan 2 imaging microscope equipped with a Zeiss AxioCam camera (Carl Zeiss Microimaging, Inc., Thornwood, NY, USA). Confocal microscope images were taken with an Olympus Fluoview 300 Confocal Laser Scanning Microscope equipped with an Argon laser for FITC/Alexa488. Images of living cells of B. bacati were taken with a Zeiss Axioplan Microscope connected to a Leica DC500 color camera.
Electron microscopy
Cells of C. aureus were prepared for scanning electron micrograph (SEM) by mixing an equal volume of fixative solution containing 4% (v/v) glutaraldehyde in 0.2 sodium cacodylate buffer (SCB) (pH 7.2) at room temperature. The fixed cells were mounted on glass plates coated with poly--lysine at room temperature for 1 h. The cells were rinsed with 0.1 SCB and fixed in 1% osmium tetroxide for 30 min as described in Yubuki et al. (2009). The cells of B. bacati were prepared for SEM by using 4% osmium tetroxide vapor for half an hour, before adding drops of osmium 4% for around half an hour. The cells were then transferred onto a 10-μm polycarbonate membrane filter and dehydrated with a graded ethanol series as described in Breglia et al. (2010). Cells of C. aureus prepared for transmission electron micrograph (TEM) were rinsed with 0.2 SCB (pH 7.2) three times and then fixed in 1% (w/v) osmium tetroxide in 0.2 SCB (pH 7.2) at room temperature for 1 h as described in Yubuki et al. (2009). Cells of B. bacati were prepared for TEM using 4% (v/v) glutaraldehyde in 0.2 SCB (pH 7.2) with the addition of 0.3 sorbitol as described in Breglia et al. (2010).
DNA extraction, PCR amplification, alignment and phylogenetic analysis
The two eukaryotic hosts (and their epibionts) were independently collected from different geographic locations (Santa Barbara Basin, CA, USA vs Boundary Bay, British Columbia), in different oceanographic realms (600 m depth vs near-surface beach sand), and at different times. Cell isolation, cell washing, DNA extractions, polymerase chain reaction (PCR), cloning and sequencing were performed at different times in different laboratories on opposite sides of North America (that is, DNA sequences were acquired from the epibionts of C. aureus at the Wood Hole Oceanographic Institution, MA, USA; DNA sequences were acquired from the epibionts of B. bacati at the University of British Columbia, BC, Canada). Collectively, these factors make it nearly impossible that there could be cross-contamination of samples at any stage.
Single cells of Calkinsia aureus were picked from whole sediment samples under a dissecting microscope. To greatly minimize contamination, cells were rinsed three times in sterile seawater before being placed into 2.0-ml microfuge tubes and frozen at –20 °C for DNA extraction. Individuals were then divided into two groups: single individuals/PCR tube for direct PCR amplification or pools of ∼30 individuals for DNA extraction. DNA was extracted from these pools using the Masterpure Complete DNA and RNA Purification Kit (Epicenter Biotechnologies, WI, USA) following the manufacturer's recommendations. Both bacteria- and archaea-specific primers were tested for positive amplification. Bacterial primers were Bact8F (Amman et al., 1995) or Bact341F (Muyzer and Smalla, 1998) paired with U1492R (Longnecker and Reysenbach, 2001). PCR amplification for the 8F/1492R primer pair and the Arch25F/1492R pair was: 95 °C for 5 min, followed by 35 cycles of 95 °C for 1 min, 45 °C for 1 min, 72 °C for 1.5 min and a final cycle of 72 °C for 7 min. For the 341F/1492R primer pair amplification was: 95 °C for 5 min followed by 35 cycles of 95 °C for 1 min, 50 °C for 1 min and 72 °C for 2.5 min, and a final cycle of 72 °C for 10 min. Archaeal amplification was tested with Arch25F (Urbach et al., 2001) paired with U1492R. Amplified DNA was checked for quality by agarose gel electrophoresis, bands were gel purified using the Qiaquick Gel Extraction Kit (Qiagen, Valencia, CA, USA), and cloned into the vector pCR4-TOPO using the TOPO TA Cloning Kit (Invitrogen, CA, USA) following the manufacturer's instructions. Plasmid DNA from 20 clones was prepared using a MWG Biotech RoboPrep2500 (MWG Biotech, Ebersberg, Germany), and inserts were sequenced bi-directionally using the universal M13 primers and an Applied Biosystems 3730XL capillary sequencer at the Keck Facility at the Josephine Bay Paul Center at the Marine Biological Laboratory, Woods Hole, MA, USA. Processing of sequence data used PHRED, PHRAP (Ewing and Green, 1998; Ewing et al., 1998) and a pipeline script. The sequences were checked for chimeras using the Bellerophon Chimera Check and the Check_Chimera utilities (Ribosomal Database Project) (Cole et al., 2003).
Genomic DNA from B. bacati, and its epibionts, was extracted using the MasterPure Complete DNA and RNA purification Kit (Epicenter Biotechnologies) from 30 cells that were individually isolated and washed three times in sterile seawater. PCR reactions were performed using PuRe Taq Ready-To-Go PCR beads kit (GE Healthcare, Buckinghamshire, UK). Nearly the entire 16S rDNA gene was amplified from each isolate using the following primers: API F1 (5′-GTGCCAGCAGCMGCGGTAATAC-3′) and API R1 (5′-TACGGYTACCTTGTTACGACTTC-3′) (Lang-Unnasch et al., 1998). PCR amplifications consisted of an initial denaturing period (95 °C for 3 min), 35 cycles of denaturing (93 °C for 45 s), annealing (5 cycles at 45 °C and 30 cycles at 55 °C, for 45 s), extension (72 °C for 2 min) and a final extension period (72 °C for 5 min). The amplified DNA fragments were purified from agarose gels using UltraClean 15 DNA Purification Kit (MO Bio, CA, USA), and subsequently cloned into the TOPO TA Cloning Kit (Invitrogen). Eight clones of the 16S rRNA gene were sequenced with the ABI Big-Dye reaction mix using the vector primers oriented in both directions.
For phylogenetic analyses, we aligned the clone sequences from the symbionts of both symbiontid species to 16S rRNA sequences available in the ARB package (Ludwig et al., 2004) (http://www.arb-home.de). The rRNA alignment was corrected manually according to secondary structure information. Only unambiguously aligned positions (1389 bp) were used to construct phylogenetic trees. To this alignment, we added the closest relatives of our original sequences retrieved from Genbank using BLASTn. Bootstrapping and determination of the best estimate of the ML tree topology for these data sets were conducted with the Rapid Bootstrapping algorithm of RAxML (Stamatakis, 2006; Stamatakis et al., 2008) version 7.0 under the GTR+I model (selected by ModelTest (Posada and Crandall, 1998)) running on the CIPRES portal (www.phylo.org).
Catalyzed Reporter Deposition-Fluorescence In Situ Hybridization (CARD-FISH)
CARD-FISH was performed with only minor modifications to the methods of Pernthaler et al. (2002). Individual cells were hand picked and rinsed in sterile seawater and fixed in 2% (final concentration) paraformaldehyde for 1 h, then rinsed three times with 5 ml sterile phosphate-buffered saline (PBS) by filtration onto a 0.2 μm pore size, 25 mm Isopore GTTP filter (Millipore, Billerica, MA, USA). After air-drying, the filters were overlaid with 37 °C 0.2% (w/v) MetaPhor agarose and filters were dried at 50 °C. To inactivate endogenous peroxidases, filter sections were incubated in 10 ml of 0.01 HCl for 10 min at room temperature. Filters were washed in 50 ml 1 × PBS, then in 50 ml of distilled, deionized water (ddH20). The epibiont cells were permeabilized by incubating the individual filter pieces in 2.0-ml Eppendorf microfuge tubes for 60 min at 37 °C in a lysozyme solution (0.05 EDTA, pH 8.0; 0.1 Tris HCL, pH 8.0; 10 mg ml−1 lysozyme). The filters were washed in 50 ml ddH20 for 2 min, followed by 50 ml of absolute ethanol (96%) and air-dried. Hybridization buffer and probe were mixed 300:1 in 2.0-ml Eppendorf tubes (probe at 50 ng per microliter). For 50 ml of hybridization buffer we mixed 3.6 ml 5 NaCl, 0.4 ml 1 Tris HCl and ddH20 depending on formamide concentration for each probe used (see Table 1). A total of 2 g of dextran sulfate was added and the mixture was heated (40–60 °C) and shaken until the dextran sulfate was dissolved. After cooling, formamide was added (% formamide noted for each probe used in Table 1), 2.0 ml blocking reagent was added (50 ml of 100 m maleic acid in ddH20 combined with 50 ml of 150 m NaCl and pH adjusted to 7.5 with NaOH, plus 10 g Roche Blocking Reagent (Roche Diagnostics GmbH, Roche Applied Science, Mannheim, Germany), and volume adjusted to 20 ml with ddH20. Hybridization was performed at 46 °C for 2 h. Filters were washed for 5 min by placing them in 50 ml tubes of wash buffer (0.5 ml 0.5 EDTA, 1.0 ml 1 Tris HCl plus volume of 5 NaCl depending on probe used (see Table 1) and ddH20 to make 50 ml). After washing, filters were transferred to 50 ml 1 × PBS (pH 7.6) for 15 min at room temperature. A total volume of 1000 μl of amplification buffer (4 ml 10 × PBS, 16 ml 5 NaCl and sterile ddH20 were mixed to a volume of 35 ml, then 4 g dextran sulfate (Sigma-Aldrich, St. Louis, MO, USA) were added and mixture was heated to 40–60 °C until dextran sulfate was dissolved. After cooling, 0.4 ml Blocking Reagent (see above) was added and water to a final volume of 40 ml, and the solution was filtered through a 0.2-μm filter unit. This solution was mixed with 10 μl of 100 × H2O2 stock (199 μl of 1 × PBS plus 1 μl 30% H2O2). Filter pieces were transferred to 2.0-ml Eppendorf tubes containing amplification buffer plus 2 μl of fluorescently labeled tyramide (Alexa488-labeled from Biomers.net GmbH, Germany) and incubated at 37 °C for 15 min in the dark on a rotary shaker. Filter pieces were washed in 50 ml 1 × PBS for 15 min at room temperature, then 50 ml ddH20, followed by 96% ethanol and air-dried, all in the dark. Filters were mounted in Citifluor (Citifluor Ltd., London, UK)/Vectashield (Vector Laboratories, Burlington, CA) mounting solution (5.5 parts Citifluor, 1 part Vectashield, 0.5 parts 1 × PBS) with 1 μg ml−1 final concentration of DAPI, and stored at –20 °C until microscopy was performed. The probes used include EUB338 I-III (Daims et al., 2001), NON338 (Wallner et al., 1993), Arch915 (Stahl and Amann, 1991), Alf968 (Neef, 1997), Gam42a (Manz et al., 1992) and Gam42a competitor (Yeates et al., 2003), BET42a (Manz et al., 1992) and BET42a competitor (Yeates et al., 2003), DELTA495a, b and c and the corresponding competitor probes for each, cDELTA495a, b and c (Lucker et al., 2007), EPS549 (Lin et al., 2006) and Arcobacter probe ARC94 (Snaidr et al., 1997).
Table 1. CARD-FISH probes used in this study. Percent formamide in hybridization buffer and NaCl concentration in wash buffer are noted for each probe.
| Probe | Specificity | % FA | Concentration NaCl in wash buffer in Mol |
|---|---|---|---|
| EUB338-I-III | Most bacteria | 35 | 0.080 |
| NON 338 | Background control | 35 | 0.080 |
| ARCH915 | Most archaea | 35 | 0.080 |
| ALF968 | 81% Alpha- proteobacteria | 35 | 0.080 |
| GAM42a | Most gamma- proteobactera | 35 | 0.080 |
| GAM42a competitor | 35 | 0.080 | |
| BET42a | Most beta- proteobactera | 35 | 0.080 |
| BET42a competitor | 35 | 0.080 | |
| DELTA495a, b and c | Most delta- proteobacteria | 35 | 0.080 |
| cDELTA495a, b and c | 35 | 0.080 | |
| EPS549 | Most epsilon- proteobacteria | 55 | 0.020 |
| ARC94 | Arcobacter | 20 | 0.225 |
Abbreviations: CARD-FISH, Catalyzed Reporter Deposition-Fluorescence In Situ Hybridization; FA, formamide.
Results
Porewater oxygen concentrations
Dissolved oxygen concentrations in sediments used for the recovery of C. aureus, analyzed for CARD-FISH, ranged from 0.2–0.5 μ. Concentrations <1 μ are typical for these sites (Bernhard et al., 2000, 2003). It should be noted that bottom water oxygen and sulfide concentrations vary considerably in Santa Barbara Basin (for example, Bernhard et al., 2003; Kuwabara et al., 1999; Reimers et al., 1990; Reimers et al., 1996).
Light, fluorescence and electron microscopy
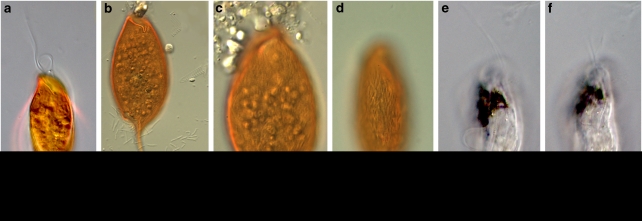
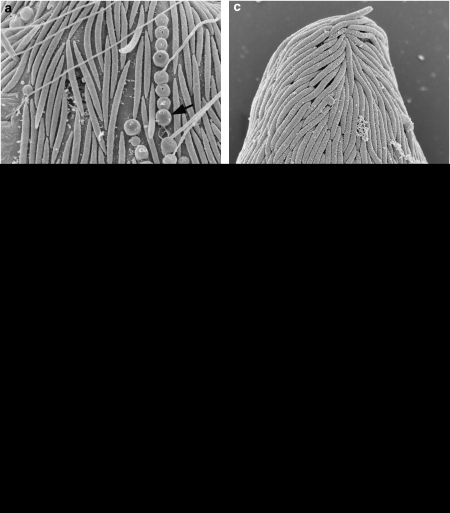
Figure 1 presents light micrographs of C. aureus and B. bacati. In agreement with Yubuki et al. (2009) microscopic analysis revealed C. aureus to be on average 48.6 μm long and 16.7 μm wide, and Figures 1a–d shows that the oval-shaped C. aureus cells were distinctively orange in color, dorsoventrally compressed with a tapered tail that is about 10 μm long and covered in rod-shaped epibiotic bacteria. Those bacteria were attached to a robust extracellular matrix that contains a uniform distribution of conduits that join the glycocalyx beneath the epibionts to the plasma membrane in both hosts (Figures 2c and d). Rod-shaped bacteria similar to the epibionts on B. bacati were observed within the host cells during TEM, but at this point we have no further evidence that these are the same cells as the epibionts. Longitudinally arranged fibrous material was present within the epibiotic bacteria of C. aureus (Figures 2e and f). In agreement with Breglia et al. (2010), B. bacati ranged from 40 to 120 μm long and 15 to 30 μm wide. Figures 1e and f show that unlike the orange color of C. aureus, B. bacati was colorless with distinctive black inclusions within the anterior half of the cell. The cell surface of B. bacati was covered with rod-shaped epibiotic bacteria that were connected to the plasma membrane of B. bacati by a glycocalyx (Figures 2a and b). Spherical-shaped, extrusive epibionts were also observed on the surface (Figure 2a).
Figure 1.
Differential interference contrast (DIC) light microscope images of B. bacati and Calkinsia aureus. (a–d) C. aureus showing the epibionts that disassociate with the host almost immediately upon stress. Cells can be seen floating free of host cell in (b) and (c). (e and f) B. bacati showing black inclusions within the anterior part of the cell. Scale bar=20 μm except panel (c), which is 10 μm.
Figure 2.
Electron micrographs of the epibionts on Calkinsia aureus and B.bacati. (a) SEM of B. bacati showing rod-shaped epibiotic bacteria and spherical-shaped, extrusive epibiotic bacteria (arrow). Note that this image is the same magnification as C. (b) TEM of the peripheral area of B. bacati showing epibionts connected to the plasma membrane of B. bacati by a glycocalyx. Hydrogenosome-like organelles (H) were located under the plasma membrane. Note that this image is the same magnification as D. (c) SEM of Calkinsia aureus showing the rod-shaped epibionts on the cell surface. (d). TEM of the peripheral area of C. aureus showing epibionts connected to a robust extracellular matrix (Ex) through a glycocalyx. Arrow indicates conduits in the extracellular matrix. Hydrogenosome-like organelles (H) were located under the plasma membrane (arrow). (e) A longitudinal section of the epibionts of C. aureus showing fibrous material (arrowhead). (f) The transverse section of the epibionts of C. aureus showing fibrous material (arrowhead). Scale bar=2 μm in (a and c), 300 nm in (b, d and f) and 500 nm in (e).
The rod-shaped epibionts of both C. aureus and B. bacati quickly became disassociated with the host cell during light microscopy. The rod-shaped bacteria can be seen floating free of the host cell in Figures 1b and c. The CARD-FISH protocol also resulted in partial to significant loss of epibionts in spite of the protective agarose over-layer. Most of this cell loss probably occurred before the application of the agarose over-layer.
Sequencing
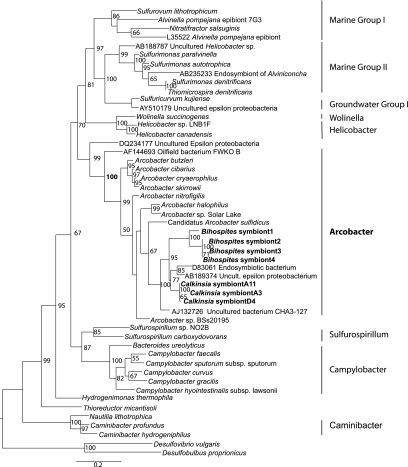
Of the 16S clones obtained from whole DNA extracts from single cells of Calkinsia aureus and from pools of ∼30 cells 92% were associated with Arcobacter; however, a few clones were affiliated with Desulfobacterium and uncultured alpha-proteobacteria. Sequencing of 16S ribosomal RNA genes amplified using whole DNA extracts from B. bacati revealed four sequence types, all of which cluster together (100% bootstrap support under maximum likelihood) as a clade within the Arcobacter group of the epsilon proteobacteria (Figure 3).
Figure 3.
Phylogenetic analysis of 16S rDNA genes from the epibionts of B.bacati and Calkinsia aureus. Tree is based on an alignment of 1389 nucleotides. Bootstrapping and determination of the test estimate of the ML tree topology for these data sets were conducted with the Rapid Bootstrapping algorithm of RAxML version 7.0 under the GTR+I model running on the CIPRES portal (Stamatakis, 2006; Stamatakis et al., 2008) (www.phylo.org).
The C. aureus epibiont sequences clustered together (bootstrap support 100% under maximum likelihood) within the Arcobacter group. The C. aureus epibionts together with two sequences from uncultured epsilon proteobacteria, formed the sister group to the epibionts of B. bacati (bootstrap support 95% under maximum likelihood). The topology and the branch lengths on the separate phylogenetic trees for the hosts (as inferred from 18S rDNA) and the epibionts (as inferred from 16S rDNA) were very similar (compare the phylogeny of the epibionts shown in Figure 3 with the phylogeny of the eukaryotic hosts presented by Breglia et al. (2010)). Figure 4 shows a general comparison of tree topologies and branch lengths for the epibionts and their hosts. In specific, the number of substitutions per site from the nearest common ancestor was 0.143 for B. bacati (Breglia et al., 2010) and 0.145 for the B. bacati epibionts (Figure 3); the number of substitutions per site from the nearest common ancestor was 0.060 for C. aureus (Breglia et al., 2010) and 0.053 for the C. aureus epibionts (Figure 3).
Figure 4.
General comparison of tree topologies and branch lengths in phylogenetic analyses of host and epibiont small subunit rDNA sequences.
CARD-FISH
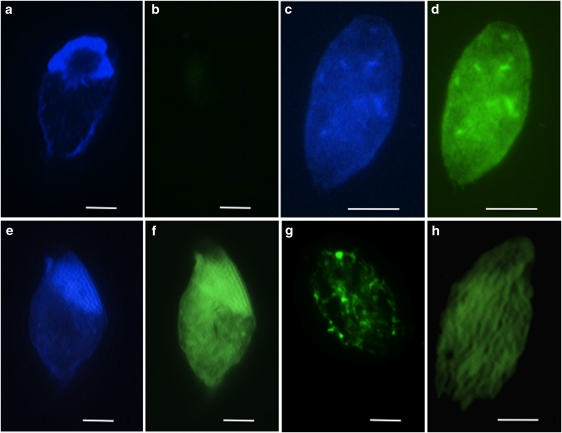
DAPI staining reveals the surface epibionts on C. aureus, and hybridization with the Alexa488-labeled NON338 probe produced virtually no signal (Figures 5a and b). Hybridization of CARD-FISH probes to Alpha-, Beta-, Delta- and Gamma-proteobacterial groups were negative on the surface of the eukaryotic host cells (data not shown). A positive hybridization was observed with the Alexa488-labeled EUB338 probe to bacteria (DAPI Figure 5c, and Alexa488 Figure 5d). No hybridization was observed with the archaeal probe ARCH915 (data not shown). A strong signal was observed with the epsilon-proteobacterial probe EPS549 (DAPI Figure 5e, Alexa488 Figure 5f and Alexa488 with confocal microscopy Figure 5h). A strong signal was also observed with the Arcobacter-specific probe ARC94 (Alexa488 Figure 5g).
Figure 5.
CARD-FISH. (a) Non-probe DAPI, (b) non-probe Alexa488, (c) EUB338-probe DAPI, (d) EUB338-probe Alexa488, (e) epsilon-proteobacterial probe EPS549 DAPI, (f) epsilon-proteobacterial probe EPS549 Alexa488, (g) Arcobacter-specific probe ARC94 Alexa488, (h) epsilon-Proteobacterial probe EPS549 confocal fluorescein isothiocyanate (FITC). Scale bars=10 μm.
Discussion
Sulfidic and oxygen-depleted marine habitats can occur anywhere, sulfate-reducing bacteria degrade abundant organic matter and thereby, produce hydrogen sulfide, such as oxygen minimum zones along open ocean margins, hydrothermal-vent sediments, soft-sediment-associated cold seeps, fjords and silled basins. Recent studies have revealed abundant protist populations in these environments (for example, Bernhard et al., 2000; Edgcomb et al., 2002; Behnke et al., 2006; Massana et al., 2006; Stoeck et al., 2006, 2009; Kolodziej and Stoeck, 2007; Not et al., 2008). In laboratory cultures, certain flagellates have been shown to tolerate high concentrations of hydrogen sulfide (up to 30 m (Atkins et al., 2002)), which together with the recent diversity data supports the idea that protists form important components of microbial communities in anoxic, oxygen-depleted and/or sulfidic marine environments. What is intriguing is that, hydrogen sulfide at micromolar concentrations is known to inhibit respiration and, therefore, the otherwise aerobic eukaryotes in these environments must have physiological adaptations that allow them to survive. The observation that the majority of observed protists in sulfidic environments have prokaryotic epibionts and/or endobionts (for example, Fenchel and Finlay, 1995; Bernhard et al., 2000; Ott et al., 2008) suggests that symbiosis may be one of the most significant adaptations. In the water column of the Cariaco Basin, Edgcomb et al. (unpublished data) observed that in contrast to ciliates in the oxic upper water column where epibionts on ciliates were rarely observed, over 90% of the ciliates on SEM filters from the anoxic and sulfidic (up to 36.8 μ) deeper waters (900 m) had visible prokaryotic epibionts.
Although most of the observed taxa in the dysoxic and sulfidic sediments of Santa Barbara Basin harbored bacterial epibionts and/or endobionts (Bernhard et al., 2000), certain taxa consistently did not, which supports the notion that the bacteria observed on the surfaces of most protists are not merely using the protists as a substrate (Bernhard et al., 2000). The consistent observation of a highly specific ultrastructural affinity between B. bacati and C. aureus with their bacterial epibionts leads us to infer a symbiotic relationship. The environmental characteristics of Santa Barbara Basin and Boundary Bay, together with information on the identity and close phylogenetic relationships of the epibionts of these two members of the Symbiontida, allows us to more confidently infer that this symbiosis is a mutualism and reflects a co-evolutionary history between the two symbiontid hosts isolated from different habitats. In contrast to the deeper (∼600 m depth) sediments of Santa Barbara Basin, Boundary Bay is a vast tidal flat. The sediments we sampled were covered with water during high tides and exposed during low tides. A common factor between the two sites is that the near-surface horizons from which the flagellates were isolated were reduced, as evidenced by a conspicuous blackish layer of sediment/sand. Phylogenetic analyses indicate that the epibionts of C. aureus and B. bacati are affiliated with the nitrate-reducing, sulfide-oxidizing autotrophic Arcobacter, with the most closely related named species, Candidatus Arcobacter sulfidicus (Wirsen et al., 2002) (Figure 3). Affiliation with Arcobacter is also confirmed by a positive CARD-FISH hybridization with both the general probe for epsilon proteobacteria and the Arcobacter-specific probe to the epibionts of C. aureus (Figures 5f–h).
As indicated in the Materials and methods section, there are five factors that collectively make it very unlikely that the epibiont sequences from either host represent contamination from non-epibiont bacteria or cross-contamination of samples of C. aureus and B. bacati during molecular procedures. First, both hosts and their symbionts were collected by different researchers from different geographic locations and depths, at different times, and their DNA was isolated, PCR amplified, cloned and sequenced by two different laboratories on opposite coasts of North America. Second, laboratories followed stringent measures to minimize contamination from other environmental bacteria and archaea by washing individually hand picked flagellate cells several times in sterile seawater before DNA preparation. Third, molecular phylogenetic analyses show that the vast majority (92% for C. aureus and 100% for B. bacti) of the 16S rDNA gene clones from the independent isolates of C. aureus and B. bacati are very closely related to one another and to the Arcobacter within the epsilon proteobacteria. Fourth, the phylogenetic topology inferred from 16S rDNA mirrors the topology for the host organisms as inferred from 18S rDNA (Breglia et al., 2010), both in terms of branching order and relative branch lengths (Figure 4). Fifth, 16S rDNA gene probes applied with CARD-FISH confirm that the epibionts on C. aureus are affiliated with the Arcobacter.
Arcobacter sp. have been isolated from oil-field waters (Gevertz et al., 2000), activated sludge (Snaidr et al., 1997), human and veterinary sources (for example, Van Dreiessche et al., 2004), a variety of retail meats (Houf et al., 2003), North Sea bacterioplankton (Eilers et al., 2000), salt marsh sediments (McClung et al., 1983), in association with deep-sea hydrothermal vent vestimentieran tube worms and chimney material (Naguanuma et al., 1997; Cilia and Prieur, unpublished data, accession number AJ132728uncultured eubacterium CHA3-437), Wadden Sea sediments (llobet-Brossa et al., 1998), Cariaco Basin, Venezuela (Madrid et al., 2001) and hypersaline cyanobacterial mats from Solar Lake (Sinai) (Teske et al., 1996). Arcobacter sp. have an optimum growth temperature around 30 °C, use H2, formate and sulfide as electron donors, and nitrate (reduced to NO2−), oxygen (microaerobic) and elemental sulfur (reduced to H2S) as electron acceptors (Campbell et al., 2006). The most closely related sequences to the epibiont sequences from both hosts were a sequence (D83061) isolated from the endosymbiont of a vestimentiferan tubeworm and a sequence (AB189374) from Japan Trench cold seep sediments (clade held together by 95% bootstrap support under maximum likelihood). Without any further information about the organisms from which these Arcobacter sequences came, we look at the closest cultured representative for insight into the metabolism of our epibionts, however, it should be noted that the bootstrap support holding our epibiont sequences together with Candidatus Arcobacter sulfidicus is modest (67% under maximum likelihood) and that it is, therefore, not possible to conclude that these epibionts have an identical metabolism. Candidatus A. sulfidicus is a chemoautotroph that uses sulfide (400–1200 μ tested in laboratory cultures) as electron donor and is capable of fixing nitrogen (Wirsen et al., 2002). Arcobacter sp. includes chemoautotrophs and chemoorganotrophs.
Although showing metabolic exchange between the host and epibionts was outside the scope of this project, we speculate that through the consumption of sulfide, the epibionts may detoxify the immediate environment surrounding the host cell membrane and provide the host with metabolic byproducts (Bernhard et al., 2003). Polz et al. (Polz et al., 2000) provide additional examples of animals and protists with sulfur-oxidizing chemoautotrophs growing on their surfaces in sulfide-enriched environments (nematodes, shrimp and colonial ciliates that grow in the interstitial pores of marine sediments, at hydrothermal vents and on decaying plant material in mangrove forests, respectively). Those authors state that the driving force behind those observed symbioses is a nutritional interaction, whereby the bacterial symbionts exploit sulfide and oxygen gradients and provide the host with a constant supply of food on its body. Although the proposed role of detoxification is logical given the environment in which these symbiontid hosts live, it should be noted that some eukaryotes inhabiting sulfide-enriched habitats lacking symbionts have other adaptations to sulfide exposure. For example, in some animals, sulfide oxidation occurs in mitochondria (for example, Parrino et al., 2000). Because C. aureus and B. bacati lack canonical mitochondria, we doubt that possibility in this study. In some metazoa, hemoglobin binds sulfide as a protective mechanism, and a sulfur-dependent anaerobic energy metabolism can be invoked (for details see reviews by Childress et al., 1991; Grieshaber and Voelkel, 1998; Hagerman, 1998; Somero et al., 1989; Vismann, 1991).
The epibionts, which have adapted to achieve high packing density on the surface of these flagellates (Figures 1 and 2), might benefit from the eukaryotic association in several possible ways. C. aureus and B. bacati are heterotrophs and they release metabolic byproducts that may be used by the epibionts as carbon substrates for chemoorganotrophy. The hosts also provide a motile substrate on which the epibionts possibly establish themselves relatively free of competition, where they are delivered to oxic/anoxic interface environments possibly favored by the hosts for grazing activities, and ideal for sulfide oxidation activities of the epibionts. Hans et al. (2009) showed that the sulfide-oxidizing epibionts on a filter-feeding peritrich ciliate were able to sustain 100-times the sulfide uptake rates of bacteria on flat surfaces, such as microbial mats. Because sulfide and oxygen are usually mutually exclusive, they typically are found in close proximity at oxic/anoxic interfaces. As Polz et al. (2000) note, these transition zones can be quite variable in time and space, and as a result, free-living chemoautotrophic bacteria rarely live at optimal conditions. As a moveable substrate, the protist host allows for a more continuous supply of sulfide and oxygen and hence, a competitive advantage. Possible advantages to the host in these oxygen-depleted and sulfidic environments include detoxification of the immediate surroundings and the ability to farm their own food source.
Conclusion
Using both molecular phylogenetic and ultrastructural evidence, we have identified the rod-shaped epibionts of C. aureus and B. bacati as closely related sulfur- or sulfide-oxidizing members of the epsilon proteobacteria, which generally have a significant role in deep-sea habitats as primary colonizers, primary producers and in symbiotic associations (Campbell et al., 2006). Because there is an intimate connection between these bacterial epibionts and the underlying organelles beneath the surface of the hosts that are likely mitochondrion-derived (the best unifying feature of the Symbiontida as a whole), the epibionts of C. aureus and B. bacati likely fulfill a role in detoxifying the immediate surroundings for hosts that live in oxygen-depleted and sulfidic environments. Moreover, the eukaryotic hosts serve as a transport vehicle bringing the epibionts to the ideal locations along oxic/anoxic interfaces, whereby their growth rates can be maximized. The nearly identical episymbiotic rod-shaped bacteria on the closely related symbiontid hosts provide evidence for a co-evolutionary history between the two sets of partners. In fact, the phylogenetic tree topologies inferred from 18S rDNA from the hosts and 16S rDNA from the bacterial epibionts are essentially identical. With such a wide geographic distribution of symbiontid isolates and additional small subunit rDNA sequence representatives of the clade, it is clear that members of the Symbiontida are likely more widespread and diverse than currently known.
Acknowledgments
We thank the captain and crew of the R/V Robert Gordon Sproul and Richard Sperduto who helped with sampling, Hilary Morrison and Rich Fox (MBL) for the use of their pipeline scripts for sequence data processing, and Matt First who helped with confocal microscopy. VE would like to thank Dagmar Woebkin for helpful discussions about CARD-FISH approaches. This research was supported by a grant from NSF (MCB-0604084) to VE and JMB and by grants from the Tula Foundation (Centre for Microbial Diversity and Evolution) and the National Science and Engineering Research Council of Canada (NSERC 283091-09) to BSL.
References
- Amman RI, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins MS, Hanna MA, Kupetsky EA, Saito MA, Taylor CD, Wirsen CO. Tolerance of flagellated protists to high sulfide and metal concentrations potentially encountered at deep-sea hydrothermal vents. Mar Ecol Prog Ser. 2002;226:63–75. [Google Scholar]
- Barbera MJ, Ruiz-Trillo I, Leigh J, Hug LA, Roger AJ.2007The Diversity of Mitochondrion-Related Organelles Amongst Eukaryotic Microbes In Origin of mitochondria and hydrogenosomesMartin WF, Muller, M Eds., Springer, Heidelberg: Germany; 239–275. [Google Scholar]
- Barry JP, Greene HG, Orange DL, Baxter CH, Robinson BH, Kochevar RE, et al. Biologic and geologic characteristics of cold seeps in Monterey Bay, California. Deep-Sea Res. 1996;43:1739–1762. [Google Scholar]
- Behnke A, Bunge J, Barger K, Breiner HW, Alla V, Stoeck T. Microeukaryote community patterns along an O2/H2S gradient in a supersulfidic anoxic Fjord (Framvaren, Norway) Appl Environ Microbiol. 2006;72:3626–3636. doi: 10.1128/AEM.72.5.3626-3636.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard JM. Potential symbionts in bathyal foraminfera. Science. 2003;299:861. doi: 10.1126/science.1077314. [DOI] [PubMed] [Google Scholar]
- Bernhard JM, Buck KR, Farmer MA, Bowser SS. The Santa Barbara Basin is a symbiosis oasis. Nature. 2000;403:77–80. doi: 10.1038/47476. [DOI] [PubMed] [Google Scholar]
- Bernhard JM, Goldstein ST, Bowser SS.2010An ectobiont-bearing foraminiferan, Bolivina pacifica, that inhabits micro-oxic pore waters: Cell biological and paleoceanographic insights Environ MicrobiolIn press, DOI: 10.1111/j.1462-2920.2009.02073.x. [DOI] [PubMed]
- Bernhard JM, Visscher PT, Bowser SS. Submillimeter life positions of bacteria, protists, and metazoans in laminated sediments of the Santa Barbara Basin. Limnol Oceanogr. 2003;45:813–828. [Google Scholar]
- Breglia SA, Yubuki N, Hoppenrath M, Leander BS. Ultrastructure and molecular phylogenetic position of a novel euglenozoan with extrusive episymbiotic bacteria: Bihospites bacati n. gen. et sp. (Symbiontida) BMC Microbiol. 2010;10:145. doi: 10.1186/1471-2180-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browenkow WW, Cline JD. Colorimetric determination of dissolved oxygen at low concentrations. Limnol Oceanogr. 1969;14:450–454. [Google Scholar]
- Buck KR, Barry JP. Monterey Bay cold seep infauna: quantitative comparison of bacterial mat meiofauna with non-seep control sites. Cah Biol Mar. 1998;39:333–335. [Google Scholar]
- Buck KR, Barry JP, Simpson AGB. Monterey Bay cold seep biota: Euglenozoa with chemoautotrophic bacterial epibionts. Eur J Protistol. 2000;36:117–126. [Google Scholar]
- Buck KR, Bernhard JM.(eds). (2002Protistan-Prokaryotic Symbioses in Deep-Sea Sulfidic Sediments Kluwer Academic Publishers: Dordrecht; 507–517. [Google Scholar]
- Campbell BJ, Engel AS, Porter ML, Takai K. The versatile epsilon-proteobacteria: key players in sulphidic habitats. Nat Rev Microbiol. 2006;4:458–468. doi: 10.1038/nrmicro1414. [DOI] [PubMed] [Google Scholar]
- Cavanaugh CM, Gardiner SL, Jones ML, Jannasch HW, Waterbury JB. Prokaryotic cells in the hydrothermal vent tube worm Riftia pachyptila Jones: possible chemoautotrophic symbionts. Science. 1981;213:340–341. doi: 10.1126/science.213.4505.340. [DOI] [PubMed] [Google Scholar]
- Childress JJ, Fisher CR, Favuzzi JA, Kochevar RE, Sanders NK, Alayse AM. Sulfide-driven autotrophic balance in the bacterial symbiont-containing hydrothermal vent tubeworm, Riftia pachyptila Jones. Biol Bull. 1991;180:135–153. doi: 10.2307/1542437. [DOI] [PubMed] [Google Scholar]
- Cole JR, Chai B, Marsh TL, Farris RJ, Wang Q, Kulam SA, et al. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 2003;31:442–443. doi: 10.1093/nar/gkg039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daims H, Ramsing NB, Schleifer K-H, Wagner M. Cultivation-independent, semiautomatic determination of absolute bacterial cell numbers in environmental samples by fluorescence in situ hybridization. Appl Environ Microbiol. 2001;67:5810–5818. doi: 10.1128/AEM.67.12.5810-5818.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel DL, Felbeck H. Pathways of inorganic carbon fixation in the endosymbiont-bearing lucinid clam Lucinoma aequizonata. I. Purification and characterization of endosymbiotic bacteria. J Exp Zool. 1988;247:1–10. [Google Scholar]
- Edgcomb VP, Kysela DT, Teske A, Gomez AD, Sogin ML. Benthic eukaryotic diversity in the Guaymas Basin hydrothermal vent environment. Proc Natl Sci USA. 2002;99:7658–7662. doi: 10.1073/pnas.062186399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers H, Pernthaler J, Glockner FO, Amann R. Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl Environ Microbiol. 2000;66:3044–3051. doi: 10.1128/aem.66.7.3044-3051.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein S, Fowle W, Bazylinski D. Epibiotic bacteria on several ciliates from marine sediments. J Euk Micro. 1998;45:64–70. [Google Scholar]
- Ewing B, Green P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998;8:186–194. [PubMed] [Google Scholar]
- Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res. 1998;8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- Fenchel T, Bernard C, Esteban G, Finlay BJ, Hansen PJ, Iversen N. Microbial diversity and activity in a Danish fjord with anoxic deep water. Ophelia. 1995;43:45–100. [Google Scholar]
- Fenchel T, Finlay BJ. Ecology and Evolution in Anoxic Worlds. Oxford University Press: Oxford; 1995. [Google Scholar]
- Gevertz DA, Telang G, Voordouw G, Jenneman GE. Isolation and characterization of strains CVO an FWDO B, two novel nitrate-reducing, sulfide-oxidizing bacteria isolated from oil field brine. Appl Environ Microbiol. 2000;66:2491–2501. doi: 10.1128/aem.66.6.2491-2501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieshaber MK, Voelkel S. Animal adaptations for tolerance and exploitation of poisonous sulfide. Ann Rev Physiol. 1998;60:30–53. doi: 10.1146/annurev.physiol.60.1.33. [DOI] [PubMed] [Google Scholar]
- Hagerman L. Physiological flexibility; a necessity for life in anoxic and sulphidic habitats. Hydrobiologia. 1998;376:241–254. [Google Scholar]
- Hans R, Vopel K, Huettel M, Jorgensen BB. Sulfide assimilation by ectosymbionts of the sessile ciliate, Zoothamnium niveum. Mar Biol. 2009;156:669–677. doi: 10.1007/s00227-008-1117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houf K, De Zutter L, Verbeke B, Van Hoof J, Vandamme P. Molecular characterization of Arcobacter isolates collected in a poultry slaughterhouse. J Food Prot. 2003;66:364–369. doi: 10.4315/0362-028x-66.3.364. [DOI] [PubMed] [Google Scholar]
- Kolodziej K, Stoeck T. Cellular identity of a novel uncultured MAST-12 lineage and phylogeny of the uncultured marine stramenopile sequence clade MAST-12. Appl Environ Microbiol. 2007;73:2718–2726. doi: 10.1128/AEM.02158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara JS, van Geen A, McCorkle DC, Bernhard JM. Dissolved sulfide distributions in the water column and sediment pore waters of the Santa Barbara Basin. Geochim Cosmochim Acta. 1999;63:2199–2209. [Google Scholar]
- Lang-Unnasch N, Reith ME, Munholland J, Barta JR. Plastids are widespread and ancient in parasites of the phylum Apicomplexa. Int J Parasitol. 1998;28:1743–1754. doi: 10.1016/s0020-7519(98)00136-2. [DOI] [PubMed] [Google Scholar]
- Leander BS, Farmer MA. Epibiotic bacteria and a novel pattern of strip reduction on the pellicle of Euglena helicoideus (Bernhard) Lemmermann. Eur J Protistol. 2000;36:405–413. [Google Scholar]
- Leander BS, Keeling PJ. Symbiotic innovation in the oxymonad Streblomastix strix. J Eukaryot Microbiol. 2004;51:291–300. doi: 10.1111/j.1550-7408.2004.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Lin X, Wakeham SG, Putnam IF, Astor YM, Scranton MI, Chistoserdov AY, et al. Comparison of vertical distributions of prokaryotic assemblages in the anoxic Cariaco Basin and Black Sea by use of fluorescence in situ hybridization. Appl Environ Microbiol. 2006;72:2679–2690. doi: 10.1128/AEM.72.4.2679-2690.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- llobet-Brossa E, Rossello-Mora R, Amann R. Microbial community composition of Wadden Sea sediments as revealed by fluorescence in situ hybridization. Appl Environ Microbiol. 1998;64:2691–2696. doi: 10.1128/aem.64.7.2691-2696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker K, Reysenbach AL. Expansion of the geographic distribution of a novel lineage of epsilon-Proteobacteria to a hydrothermal vent site on the Southern East Pacific Rise. FEMS Microbiol Ecol. 2001;35:287–293. doi: 10.1111/j.1574-6941.2001.tb00814.x. [DOI] [PubMed] [Google Scholar]
- Lucker S, Steger D, Kjeldsen KU, MacGregor BJ, Wagner M, Loy A. Improved 16S rRNA-targeted probe set for analysis of sulfate-reducing bacteria by fluorescence in situ hybridization. J Microbiol Meth. 2007;69:523–528. doi: 10.1016/j.mimet.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, et al. ARB: a software environment for sequence data. Nucleic Acids Res. 2004;32:1363–1371. doi: 10.1093/nar/gkh293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid VM, Taylor GT, Scranton MI, Chistoserdov AY. Phylogenetic diversity of bacterial and archaeal communities in the anoxic zone of the Cariaco Basin. Appl Environ Microbiol. 2001;67:1663–1674. doi: 10.1128/AEM.67.4.1663-1674.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz W, Amann R, Ludwig W, Wagner M, Schleifer K-H. Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria—problems and solutions. Syst Appl Microbiol. 1992;15:539–600. [Google Scholar]
- Massana R, Terrado R, Forn I, Lovejoy C, Pedros-Alio C. Distribution and abundance of uncultured heterotrophic flagellates in the world oceans. Environ Microbiol. 2006;8:1515–1522. doi: 10.1111/j.1462-2920.2006.01042.x. [DOI] [PubMed] [Google Scholar]
- McClung CR, Patriquin DG, Davis RE. Campylobacter nitrofigilis sp. nov., a nitrogen-fixing bacterium associated with roots of Spartina alterniflora. Int J Syst Bacteriol. 1983;33:605–612. [Google Scholar]
- Muyzer G, Smalla K. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie van Leeuwenhoek. 1998;73:127–141. doi: 10.1023/a:1000669317571. [DOI] [PubMed] [Google Scholar]
- Naguanuma T, Kato C, Hirayama H, Moriyama N, Hashimoto J, Horikoshi K. Intracellular occurrence of epsilon-proteobacterial 16S rDNA sequences in the vestimentiferan trophosome. J Oceanogr. 1997;53:193–197. [Google Scholar]
- Neef A.1997. Vol. PhD thesisTU: Munchen, Germany [Google Scholar]
- Not F, Latasa M, Scharek R, Viprey M, Karleskind P, Balague V, et al. Protistan assemblages across the Indian Ocean, with a specific emphasis on the picoeukaryotes. Deep-Sea Res I. 2008;55:1456–1473. [Google Scholar]
- Ott JA. Sulphide ectosymbioses in shallow marine habitats. Mar Ecol Prog Ser. 1996;11:369–382. [Google Scholar]
- Ott JA, Novak R, Schiemer F, Hentschel U, Nebelsick M, Polz M. Tackling the sulfide gradient: a novel strategy involving marine nematodes and chemoautotrophic ectosymbionts. Mar Ecol. 2008;12:261–279. [Google Scholar]
- Parrino V, Kraus D, Doeller J. ATP production from the oxidation of sulfide in gill mitochondria of the ribbed mussel Geukensia demissa. J Exp Biol. 2000;203:2209–2218. doi: 10.1242/jeb.203.14.2209. [DOI] [PubMed] [Google Scholar]
- Pernthaler A, Pernthaler J, Amann R. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl Env Microbol. 2002;68:3094–3101. doi: 10.1128/AEM.68.6.3094-3101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polz M, Ott JA, Bright M, Cavanaugh CM. Associations between sulfur-oxidizing bacteria and eukaryotes represent spectacular adaptations to environmental gradients. ASM News. 2000;66:531–539. [Google Scholar]
- Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Reimers CE, Lange CB, Tabak M, Bernhard JM. Seasonal spillover and varve formation in the Santa Barbara Basin, California. Limnol Oceanog. 1990;35:1577–1585. [Google Scholar]
- Reimers CE, Ruttenberg KC, Canfield DE, Christiansen MB, Martin JB. Pore water pH and authigenic phases formed in the uppermost sediments of the Santa Barbara Basin. Geochim Cosmochim Acta. 1996;60:4037–4057. [Google Scholar]
- Simpson AGB, van den Hoff J, Bernard C, Burton H, Patterson DJ. The ultrastructure and systematic position of the Euglenozoan Postgaardi mariagerensis, Fenchel et al. Arch Protistenkd. 1997;147:213–225. [Google Scholar]
- Snaidr J, Amann R, Huber I, Ludwig W, Schleifer K-H. Phylogenetic analysis and in situ identification of bacteria in activated sludge. Appl Environ Microbiol. 1997;63:2884–2896. doi: 10.1128/aem.63.7.2884-2896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somero GN, Childress JJ, Anderson AE. Transport, metabolism, and detoxification of hydrogen sulfide in animals from sulfide-rich marine environments. Rev Aquatic Sci. 1989;1:591–614. [Google Scholar]
- Stahl DA, Amann R.(eds). (1991Development and Application of Nucleic Acid Probes in Bacterial Systematics Wiley & Sons Ltd.: Chichester, England; 205–248. [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Stoeck T, Behnke A, Christen R, Amaral-Zettler L, Rodriguez-Mora MJ, Chistoserdov A, et al. Massively parallel tag sequencing reveals the complexity of anaerobic marine protistan communities. BMC Biol. 2009;7:72. doi: 10.1186/1741-7007-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeck T, Hayward B, Taylor GT, Varela R, Epstein SS. A multiple PCR-primer approach to access the microeukaryotic diversity in environmental samples. Protist. 2006;157:31–43. doi: 10.1016/j.protis.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Teske A, Sigalevich P, Cohen Y, Muyzer G. Molecular identification of bacteria from a coculture by denaturing gradient gel electrophoresis of 16S ribosomal DNA fragments as a tool for isolation in pure cultures. Appl Environ Microbiol. 1996;62:4210–4215. doi: 10.1128/aem.62.11.4210-4215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbach E, Vergin KL, Young L, Morse A. Unusual bacterioplankton community structure in ultra-oligotrophic Crater Lake. Limnol Oceanogr. 2001;46:557–572. [Google Scholar]
- Van Dreiessche E, Houf K, Vangroenweghe F, Nollet N, Zutter L, Vandamme P, et al. Occurrence and strain diversity of Arcobacter species isolated from healthy Belgian pigs. Res Microbiol. 2004;155:662–666. doi: 10.1016/j.resmic.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Vismann B. Sulfide tolerance and detoxification in Arenicola marina and Sipunculus nudus. Am Zool. 1991;35:145–153. [Google Scholar]
- Wallner G, Amman RI, Beisker W. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry. 1993;14:136–143. doi: 10.1002/cyto.990140205. [DOI] [PubMed] [Google Scholar]
- Wirsen CO, Sievert SM, Cavanaugh CM, Molyneaux SJ, Ahmad A, Taylor LT, et al. Characterization of an autotrophic sulfide-oxidizing marine Arcobacter sp. that produces filamentous sulfur. Appl Environ Microbiol. 2002;68:316–325. doi: 10.1128/AEM.68.1.316-325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolowski K. Dylakosoma pelophilum Skuja, a rare colourless euglenophyte found in Poland. Algol studies. 1995;76:75–78. [Google Scholar]
- Yeates C, Saunders AM, Crocetti GR, Blackall LL. Limitations of the widely used GAM42a and BET42a probes targeting bacteria in the Gammaproteobacteria radiation. Microbiology. 2003;149:1239–1247. doi: 10.1099/mic.0.26112-0. [DOI] [PubMed] [Google Scholar]
- Yubuki N, Edgcomb VP, Bernhard JM, Leander BS. Ultrastructure and molecular phylogeny of Calkinsia aureus: cellular identity of a novel clade of deep-sea euglenozoans with epibiotic bacteria. BMC Microbiol. 2009;9:16. doi: 10.1186/1471-2180-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]