Abstract
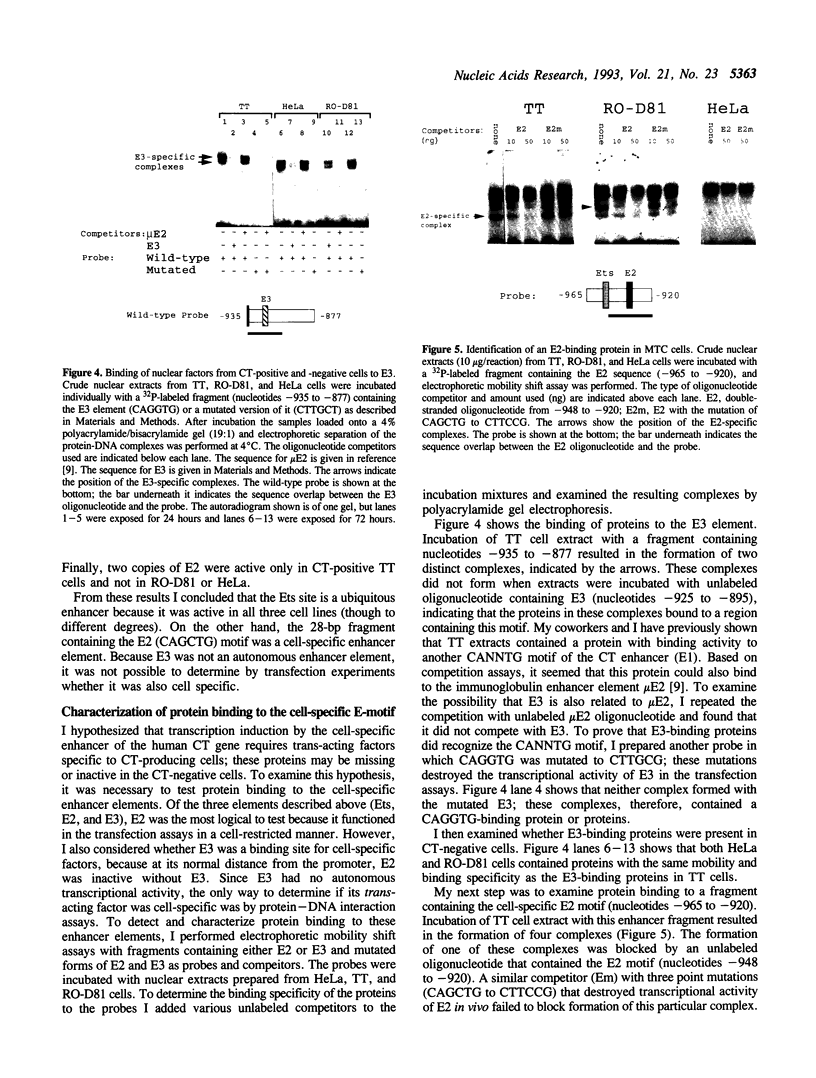
Transcription of the calcitonin (CT) gene in the medullary thyroid carcinoma (MTC) cell line TT is modulated by a neuroendocrine-specific enhancer fragment (nucleotides -965 to -905) containing two CANNTG motifs (E2 and E3) and an ETs-like response element. To determine the cell-specific component of this fragment, oligonucleotides containing the individual elements were inserted in front of a minimal CT promoter and tested for reporter protein production in CT-positive (TT) and -negative (RO-D81 and HeLa) cells. In TT cells, using two copies of E2 or four copies of Ets increased minimal promoter activity a 20-40 fold. Using two copies of E3 had no effect on minimal promoter activity. In CT-negative MTC cells (RO-D81), the Ets response element was active but the two copies of E2 were not. Similar results were obtained with the non-neuroendocrine cell-line HeLa. I therefore concluded that E2 was the cell-type-specific component of the enhancer. An E2-specific binding protein was detected in both MTC cell lines but not in HeLa. This protein had different mobility and DNA-binding specificity in CT-positive TT cells and CT-negative RO-D81 cells. In conclusion, the CAGCTG motif of E2 modulated the cell-specific transcription of the CT gene, and its inactivation in CT-negative MTC cells correlated with modifications in its binding proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abemayor E., Sidell N., Juillard G. Human medullary thyroid carcinoma. Initial characterization and in vitro differentiation of two new cell lines. Arch Otolaryngol Head Neck Surg. 1989 Apr;115(4):478–483. doi: 10.1001/archotol.1989.01860280076020. [DOI] [PubMed] [Google Scholar]
- Barad M., Jack T., Chadwick R., McGinnis W. A novel, tissue-specific, Drosophila homeobox gene. EMBO J. 1988 Jul;7(7):2151–2161. doi: 10.1002/j.1460-2075.1988.tb03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-David Y., Giddens E. B., Letwin K., Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991 Jun;5(6):908–918. doi: 10.1101/gad.5.6.908. [DOI] [PubMed] [Google Scholar]
- Blochlinger K., Bodmer R., Jack J., Jan L. Y., Jan Y. N. Primary structure and expression of a product from cut, a locus involved in specifying sensory organ identity in Drosophila. Nature. 1988 Jun 16;333(6174):629–635. doi: 10.1038/333629a0. [DOI] [PubMed] [Google Scholar]
- Cabrera C. V., Martinez-Arias A., Bate M. The expression of three members of the achaete-scute gene complex correlates with neuroblast segregation in Drosophila. Cell. 1987 Jul 31;50(3):425–433. doi: 10.1016/0092-8674(87)90496-x. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- German M. S., Blanar M. A., Nelson C., Moss L. G., Rutter W. J. Two related helix-loop-helix proteins participate in separate cell-specific complexes that bind the insulin enhancer. Mol Endocrinol. 1991 Feb;5(2):292–299. doi: 10.1210/mend-5-2-292. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Yaniv M. Nuclear oncogenes. Curr Opin Cell Biol. 1991 Jun;3(3):484–491. doi: 10.1016/0955-0674(91)90077-c. [DOI] [PubMed] [Google Scholar]
- Gutman A., Wasylyk B. Nuclear targets for transcription regulation by oncogenes. Trends Genet. 1991 Feb;7(2):49–54. doi: 10.1016/0168-9525(91)90231-E. [DOI] [PubMed] [Google Scholar]
- Lippman S. M., Mendelsohn G., Trump D. L., Wells S. A., Jr, Baylin S. B. The prognostic and biological significance of cellular heterogeneity in medullary thyroid carcinoma: a study of calcitonin, L-dopa decarboxylase, and histaminase. J Clin Endocrinol Metab. 1982 Feb;54(2):233–240. doi: 10.1210/jcem-54-2-233. [DOI] [PubMed] [Google Scholar]
- Moss L. G., Moss J. B., Rutter W. J. Systematic binding analysis of the insulin gene transcription control region: insulin and immunoglobulin enhancers utilize similar transactivators. Mol Cell Biol. 1988 Jun;8(6):2620–2627. doi: 10.1128/mcb.8.6.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Mabry M., de Bustros A., Ihle J. N., Nelkin B. D., Baylin S. B. Introduction of v-Ha-ras oncogene induces differentiation of cultured human medullary thyroid carcinoma cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5923–5927. doi: 10.1073/pnas.84.16.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C., Shen L. P., Meister A., Fodor E., Rutter W. J. Pan: a transcriptional regulator that binds chymotrypsin, insulin, and AP-4 enhancer motifs. Genes Dev. 1990 Jun;4(6):1035–1043. doi: 10.1101/gad.4.6.1035. [DOI] [PubMed] [Google Scholar]
- Peleg S., Abruzzese R. V., Cooper C. W., Gagel R. F. Down-regulation of calcitonin gene transcription by vitamin D requires two widely separated enhancer sequences. Mol Endocrinol. 1993 Aug;7(8):999–1008. doi: 10.1210/mend.7.8.8232320. [DOI] [PubMed] [Google Scholar]
- Peleg S., Abruzzese R. V., Cote G. J., Gagel R. F. Transcription of the human calcitonin gene is mediated by a C cell-specific enhancer containing E-box-like elements. Mol Endocrinol. 1990 Nov;4(11):1750–1757. doi: 10.1210/mend-4-11-1750. [DOI] [PubMed] [Google Scholar]
- Prendergast G. C., Ziff E. B. Methylation-sensitive sequence-specific DNA binding by the c-Myc basic region. Science. 1991 Jan 11;251(4990):186–189. doi: 10.1126/science.1987636. [DOI] [PubMed] [Google Scholar]
- Reddy M. A., Langer S. J., Colman M. S., Ostrowski M. C. An enhancer element responsive to ras and fms signaling pathways is composed of two distinct nuclear factor binding sites. Mol Endocrinol. 1992 Jul;6(7):1051–1060. doi: 10.1210/mend.6.7.1324418. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Amara S. G., Evans R. M. Alternative RNA processing: determining neuronal phenotype. Science. 1984 Sep 21;225(4668):1315–1320. doi: 10.1126/science.6089345. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Tverberg L. A., Russo A. F. Cell-specific glucocorticoid repression of calcitonin/calcitonin gene-related peptide transcription. Localization to an 18-base pair basal enhancer element. J Biol Chem. 1992 Sep 5;267(25):17567–17573. [PubMed] [Google Scholar]
- Wasylyk C., Gutman A., Nicholson R., Wasylyk B. The c-Ets oncoprotein activates the stromelysin promoter through the same elements as several non-nuclear oncoproteins. EMBO J. 1991 May;10(5):1127–1134. doi: 10.1002/j.1460-2075.1991.tb08053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Chalfie M. mec-3, a homeobox-containing gene that specifies differentiation of the touch receptor neurons in C. elegans. Cell. 1988 Jul 1;54(1):5–16. doi: 10.1016/0092-8674(88)90174-2. [DOI] [PubMed] [Google Scholar]
- de Bustros A., Lee R. Y., Compton D., Tsong T. Y., Baylin S. B., Nelkin B. D. Differential utilization of calcitonin gene regulatory DNA sequences in cultured lines of medullary thyroid carcinoma and small-cell lung carcinoma. Mol Cell Biol. 1990 Apr;10(4):1773–1778. doi: 10.1128/mcb.10.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]