Abstract
We measured denitrification and nitrate removal rates in cold seep sediments from the Gulf of Mexico. Heterotrophic potential denitrification rates were assayed in time-series incubations. Surficial sediments inhabited by Beggiatoa exhibited higher heterotrophic potential denitrification rates (32 μ N reduced day−1) than did deeper sediments (11 μ N reduced day−1). Nitrate removal rates were high in both sediment horizons. These nitrate removal rates translate into rapid turnover times (<1 day) for the nitrate pool, resulting in a faster turnover for the nitrate pool than for the sulfate pool. Together, these data underscore the rigorous nature of internal nitrogen cycling at cold seeps and the requirement for novel mechanisms to provide nitrate to the sediment microbial community.
Keywords: nitrogen, denitrification, cold seep, Beggiatoa, sulfide oxidation, carbon remineralization
Introduction
Nitrate is abundant (∼30–40 μ) in the deep, oxygenated bottom waters that overlie cold seeps, but no data describing dissimilatory nitrate reduction (hereafter DNF) or total nitrate removal rates in such environments are available. Most studies of nitrate cycling at cold seeps have focused on qualitative descriptions of vacuolate sulfide-oxidizing bacteria (Teske and Nelson, 2006 and references therein), which concentrate nitrate in their vacuoles and couple nitrate reduction to sulfide oxidation (McHatton et al., 1996, Sayama et al., 2005).
Gulf of Mexico (GOM) cold seeps are characterized by abundant stocks of reduced organic carbon in the form of liquid and gaseous hydrocarbons (Arvidson et al., 2004, Joye et al., 2004). Denitrifying bacteria have a metabolic flexibility similar to that of sulfate-reducing bacteria and can degrade liquid hydrocarbons (Widdel and Rabus, 2001). Considering the abundant carbon sources and relatively high concentrations of nitrate in overlying waters (Joye et al., 2004, 2010), heterotrophic DNF is a likely metabolic pathway at cold seeps.
Heterotrophic, or non-sulfide-based, DNF has not been measured at cold seeps, which is surprising, as cold seeps support extremely high rates of heterotrophic metabolism, mainly sulfate reduction (Arvidson et al., 2004; Bowles et al., in press). Hexadecane oxidation coupled to DNF, for example, is a highly exergonic process generating 983 kJ per mole N2 formed (Widdel and Rabus, 2001):
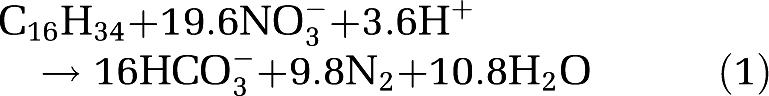 |
where activities were assumed to be 10−2, pH 7, and liquid n-hexadecane was considered. Here we present the first rate assays of heterotrophic potential denitrification from sediments from a northern GOM cold seep.
Materials and methods
Sediment covered by a Beggiatoa mat was collected from an active cold seep in the Northern GOM (lease block Mississippi Canyon 118; see Lapham et al., 2008 for details). Geochemistry (nitrate, nitrite, ammonium, sulfate and sulfide concentration determination) sampling and methods were as described in Joye et al. (2004). We measured heterotrophic potential denitrification rates in helium-purged, nitrate, nitrite, and ammonium-free, sulfide- and sulfate-free sediment slurries (2:1 artificial porewater (adapted from Weston and Joye, 2005) to sediment) from Beggiatoa-inhabited surface sediments (0–6 cm) and a deeper layer (6–12 cm) lacking visible filaments. Nitrate removal rates were determined from the linear decrease in nitrate concentration over time, while heterotrophic potential denitrification rates were estimated from the evolution of 29N2 and 30N2 over time (Kana et al., 1998). Briefly, ∼75 μ 15NO3− and 2 m C (equimolar C from lactate and acetate) were added to headspace-free hungate tubes and tubes were sampled for dissolved constituents at multiple time points (Porubsky et al., 2009).
Results and discussion
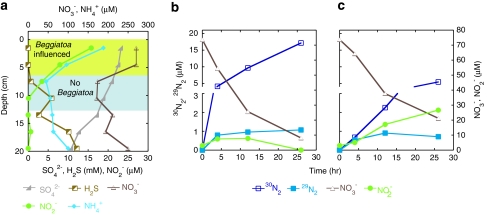
The concentration of total dissolved inorganic nitrogen species (ammonium, 260 μ; nitrate, 180 μ; and nitrite, 8 μ) was highest just below the sediment surface (1.5 cm, Figure 1a). Pore water sulfate concentration profiles illustrated significant removal at depth and a concomitant increase in sulfide (Figure 1b). The observation of low sulfide accumulation and significant concentrations of total dissolved inorganic nitrogen in upper sediments may reflect nitrate storage and sulfide oxidation by Beggiatoa (Joye et al., 2004).
Figure 1.
(a) Geochemical constituent NH4+ (μ), NO3− (μ), NO2− (μ), SO42− (m) and H2S (μ) versus depth (cm). (b) Average concentrations of reactant, NO3− (μ), and potential products, NO2−, 29N2 and 30N2 (μ) versus time (h) for slurried sediments from 0 to 6 cm, as well as (c) 6–12 cm horizons.
Both the upper and lower sediments supported significant rates of heterotrophic potential denitrification. In the surface sediments, nitrate was depleted rapidly and the production of 15N-labeled dinitrogen products (17 μ 30N2 and <1 μ 29N2) was easily detectable after 4 h (Figure 1b). Linear substrate removal and product accumulation over time yielded estimates of nitrate removal and heterotrophic potential denitrification rates of 96 and 32 μ N day−1, respectively. In the deeper sediments, nitrate was also rapidly consumed (Figure 1c) and at the same time 5 μ of 30N2 formed; 29N2 generation was low (<1 μ). Rates of nitrate removal and heterotrophic potential denitrification were lower, being 52 and 11 μ N reduced day−1, respectively.
At GOM cold seeps, sulfate reduction is the only terminal metabolic process that has been quantified (Arvidson et al., 2004; Joye et al., 2004). We show that denitrification and nitrate removal are also important processes at these seeps. Denitrification is well documented in numerous other environments (Seitzinger, 1988 and references therein), and integrated rates typically range from 1 to >1000 μmol N m−2 h−1 in freshwater and marine systems. The depth-integrated potential denitrification rates for GOM seeps are 80 and 27 μmol N m−2 h−1 for upper and lower sediment horizons, respectively. These rates are comparable with the rates from coastal marine sediments (Seitzinger, 1988), and from moderately eutrophic to eutrophic freshwater environments. Nitrate removal could also reflect additional processes such as assimilation, vacuole storage, reduction to ammonium (DNRA), or nitrite production and removal via anaerobic ammonium oxidation (ANAMMOX). Previously published data would suggest that DNRA may be more important in sulfidic and carbon-rich cold seep sediments than ANAMMOX (Burgin and Hamilton, 2007).
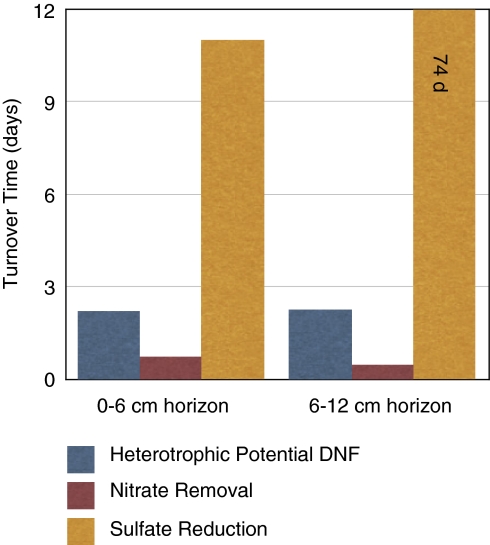
The integrated heterotrophic potential denitrification rates result in an extremely fast turnover time (on the order of 2 days) for the nitrate pool in these seep sediments (Figure 2). The sulfate pool turnover time was 11–72 days (Bowles et al., in press; Figure 2). When factoring in total nitrate removal, the turnover time of the nitrate pool is faster, being 0.7 and 0.4 days in the upper and lower sediment horizons, respectively. These results lead us to postulate that (1) heterotrophic denitrification is a relevant and important component of C and N cycling in cold seeps and (2) dissimilatory nitrate reduction, nitrate storage in vacuoles and/or assimilation into biomass require tight coupling of nitrogen-cycling processes and raises the potential for internal nitrate sources, such as anaerobic nitrification (Luther et al., 1997), and/or downward advection of bottom water nitrate within these habitats.
Figure 2.
Turnover times (day) of nitrate and sulfate from MC118 (sulfate turnover time calculated from the data in Bowles et al., in press) due to total nitrate removal and heterotrophic potential denitrification, and SR, respectively.
Acknowledgments
This research was supported by the NOAA National Institute for Undersea Science and Technology (award numbers 06-09-018, 07-10-028 and 08-10-031). The Gulf of Mexico Gas Hydrate Research Consortium provided ship time and at-sea logistical support. We thank W Porubsky for providing useful suggestions and assistance in the early stages of this research.
References
- Arvidson RS, Morse JW, Joye SB. The sulfur biogeochemistry of chemosynthetic cold seep communities, Gulf of Mexico, USA. Mar Chem. 2004;87:97–119. [Google Scholar]
- Bowles MW, Samarkin VA, Bowles KM, Joye SB. Weak coupling between sulfate reduction and the anaerobic oxidation of methane in methane-rich seafloor sediments. Geochim Cosmochim Acta. in press.
- Burgin AJ, Hamilton SK. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ. 2007;5:89–96. [Google Scholar]
- Joye SB, Boetius A, Orcutt BN, Montoya JP, Schulz HN, Erickson MJ, et al. The anaerobic oxidation of methane and sulfate reduction in sediments from Gulf of Mexico cold seeps. Chem Geol. 2004;205:219–238. [Google Scholar]
- Joye SB, Bowles MW, Samarkin VA, Hunter KS, Niemann H.2010Biogeochemical signatures and microbial activity of different cold seep habitats along the Gulf of Mexico lower slope Deep Sea Res,doi: 10.1016/j.dsr2.2010.06.001 [DOI]
- Kana TM, Sullivan MB, Cornwell JC, Groszkowski KM. Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnol Oceanogr. 1998;43:334–339. [Google Scholar]
- Lapham LL, Chanton JP, Martens CS, Higley PD, Jannasch HW, Woolsey JR. Measuring temporal variability in pore-fluid chemistry to assess gas hydrate stability: development of a continuous pore-fluid array. Environ Sci Technol. 2008;42:7368–7373. doi: 10.1021/es801195m. [DOI] [PubMed] [Google Scholar]
- Luther GW, Sunby B, Lewis BL, Brendel PJ, Silverberg N. Interactions of manganese with the nitrogen cycle: alternative pathways to dinitrogen. Geochim Cosmochim Acta. 1997;61:4043–4052. [Google Scholar]
- McHatton SC, Barry JP, Jannasch HW, Nelson DC. High nitrate concentrations in vacuolate, autotrophic marine Beggiatoa spp. Appl Environ Microbiol. 1996;62:954–958. doi: 10.1128/aem.62.3.954-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porubsky WP, Weston NB, Joye SB. Interactions between benthic primary production, denitrification and dissimilatory nitrate reduction to ammonium in intertidal sediments. Est Coast Shelf Sci. 2009;83:392–402. [Google Scholar]
- Sayama M, Risgaard-Petersen N, Nielsen LP, Fossing H, Christensen PB. Impact of bacterial NO3 transport on sediment biogeochemistry. Appl Environ Microbiol. 2005;71:7575–7577. doi: 10.1128/AEM.71.11.7575-7577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitzinger SP. Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol Oceanogr. 1988;33:702. [Google Scholar]
- Teske A, Nelson DC.2006The Genera Beggiatoa and Thioploca ProkaryotesVol. 3. Springer: New York; 784–810. [Google Scholar]
- Weston NB, Joye SB. Temperature driven accumulation of labile, dissolved organic matter in anoxic marine sediments. Proc Natl Acad Sci USA. 2005;102:17036–17040. doi: 10.1073/pnas.0508798102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdel F, Rabus R. Anaerobic biodegradation of saturated and aromatic hydrocarbons. Curr Opin Biotechnol. 2001;12:259–276. doi: 10.1016/s0958-1669(00)00209-3. [DOI] [PubMed] [Google Scholar]