Abstract
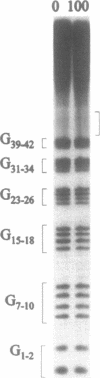
In Euplotes crassus millions of telomeres are synthesized during the sexual phase of the life cycle. Since these newly synthesized telomeres are longer than normal macronuclear telomeres, they must be trimmed to the mature size. We have examined the timing and mechanism of this trimming step. We have shown that a sudden decrease in telomere length takes place at a specific time during macronuclear development. The decrease in telomere length is not caused by incomplete replication of the most terminal DNA sequences; rather it is the result of an active processing event that occurs independently of DNA replication. The developmentally regulated telomere shortening that takes place in Euplotes is reminiscent of the sudden reductions in telomere length which have been observed in other eukaryotes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernards A., Michels P. A., Lincke C. R., Borst P. Growth of chromosome ends in multiplying trypanosomes. Nature. 1983 Jun 16;303(5918):592–597. doi: 10.1038/303592a0. [DOI] [PubMed] [Google Scholar]
- Biessmann H., Carter S. B., Mason J. M. Chromosome ends in Drosophila without telomeric DNA sequences. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1758–1761. doi: 10.1073/pnas.87.5.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessmann H., Mason J. M. Genetics and molecular biology of telomeres. Adv Genet. 1992;30:185–249. doi: 10.1016/s0065-2660(08)60321-1. [DOI] [PubMed] [Google Scholar]
- Biessmann H., Mason J. M. Progressive loss of DNA sequences from terminal chromosome deficiencies in Drosophila melanogaster. EMBO J. 1988 Apr;7(4):1081–1086. doi: 10.1002/j.1460-2075.1988.tb02916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E. H. Telomerases. Annu Rev Biochem. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomeres. Trends Biochem Sci. 1991 Oct;16(10):378–381. doi: 10.1016/0968-0004(91)90155-o. [DOI] [PubMed] [Google Scholar]
- Burr B., Burr F. A., Matz E. C., Romero-Severson J. Pinning down loose ends: mapping telomeres and factors affecting their length. Plant Cell. 1992 Aug;4(8):953–960. doi: 10.1105/tpc.4.8.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M. N., Wright J. H., Wolf A. J., Zakian V. A. RAP1 protein interacts with yeast telomeres in vivo: overproduction alters telomere structure and decreases chromosome stability. Cell. 1990 Nov 16;63(4):739–750. doi: 10.1016/0092-8674(90)90140-a. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darr S. C., Brown J. W., Pace N. R. The varieties of ribonuclease P. Trends Biochem Sci. 1992 May;17(5):178–182. doi: 10.1016/0968-0004(92)90262-8. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987 Dec 24;51(6):887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Klobutcher L. A., Swanton M. T., Donini P., Prescott D. M. All gene-sized DNA molecules in four species of hypotrichs have the same terminal sequence and an unusual 3' terminus. Proc Natl Acad Sci U S A. 1981 May;78(5):3015–3019. doi: 10.1073/pnas.78.5.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobutcher L. A., Turner L. R., Peralta M. E. Sequence of a Euplotes crassus macronuclear DNA molecule encoding a protein with homology to a rat form-I phosphoinositide-specific phospholipase C. J Protozool. 1991 Jul-Aug;38(4):425–427. doi: 10.1111/j.1550-7408.1991.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Kyrion G., Boakye K. A., Lustig A. J. C-terminal truncation of RAP1 results in the deregulation of telomere size, stability, and function in Saccharomyces cerevisiae. Mol Cell Biol. 1992 Nov;12(11):5159–5173. doi: 10.1128/mcb.12.11.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson D. D., Spangler E. A., Blackburn E. H. Dynamics of telomere length variation in Tetrahymena thermophila. Cell. 1987 Jul 31;50(3):477–483. doi: 10.1016/0092-8674(87)90501-0. [DOI] [PubMed] [Google Scholar]
- Levis R. W. Viable deletions of a telomere from a Drosophila chromosome. Cell. 1989 Aug 25;58(4):791–801. doi: 10.1016/0092-8674(89)90112-8. [DOI] [PubMed] [Google Scholar]
- Liu Z., Frantz J. D., Gilbert W., Tye B. K. Identification and characterization of a nuclease activity specific for G4 tetrastranded DNA. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3157–3161. doi: 10.1073/pnas.90.8.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pays E., Laurent M., Delinte K., Van Meirvenne N., Steinert M. Differential size variations between transcriptionally active and inactive telomeres of Trypanosoma brucei. Nucleic Acids Res. 1983 Dec 10;11(23):8137–8147. doi: 10.1093/nar/11.23.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz S. W., Jacobson A. mRNA stability: in trans-it. Curr Opin Cell Biol. 1992 Dec;4(6):979–983. doi: 10.1016/0955-0674(92)90129-z. [DOI] [PubMed] [Google Scholar]
- Price C. M., Skopp R., Krueger J., Williams D. DNA recognition and binding by the Euplotes telomere protein. Biochemistry. 1992 Nov 10;31(44):10835–10843. doi: 10.1021/bi00159a026. [DOI] [PubMed] [Google Scholar]
- Price C. M. Telomere structure in Euplotes crassus: characterization of DNA-protein interactions and isolation of a telomere-binding protein. Mol Cell Biol. 1990 Jul;10(7):3421–3431. doi: 10.1128/mcb.10.7.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman M. K., Dunn C. J., Hicke B. J., Cech T. R. Oxytricha telomeric nucleoprotein complexes reconstituted with synthetic DNA. Nucleic Acids Res. 1989 Jun 12;17(11):4235–4253. doi: 10.1093/nar/17.11.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. V., Ammermann D. Polytene chromosomes and nucleic acid metabolism during macronuclear development in Euplotes. Chromosoma. 1970;29(2):246–254. doi: 10.1007/BF00326082. [DOI] [PubMed] [Google Scholar]
- Rao M. V. Macronuclear development in Euplotes woodruffi following conjugation. Exp Cell Res. 1968 Feb;49(2):411–419. doi: 10.1016/0014-4827(68)90190-0. [DOI] [PubMed] [Google Scholar]
- Roth M., Lin M., Prescott D. M. Large scale synchronous mating and the study of macronuclear development in Euplotes crassus. J Cell Biol. 1985 Jul;101(1):79–84. doi: 10.1083/jcb.101.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shampay J., Blackburn E. H. Generation of telomere-length heterogeneity in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1988 Jan;85(2):534–538. doi: 10.1073/pnas.85.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaff R., Ilsley D., Kuchta R. Mechanism of DNA polymerase alpha inhibition by aphidicolin. Biochemistry. 1991 Sep 3;30(35):8590–8597. doi: 10.1021/bi00099a014. [DOI] [PubMed] [Google Scholar]
- Sheen J. Y., Seed B. Electrolyte gradient gels for DNA sequencing. Biotechniques. 1988 Nov-Dec;6(10):942–944. [PubMed] [Google Scholar]
- Walmsley R. M., Petes T. D. Genetic control of chromosome length in yeast. Proc Natl Acad Sci U S A. 1985 Jan;82(2):506–510. doi: 10.1073/pnas.82.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Skopp R., Scofield M., Price C. Euplotes crassus has genes encoding telomere-binding proteins and telomere-binding protein homologs. Nucleic Acids Res. 1992 Dec 25;20(24):6621–6629. doi: 10.1093/nar/20.24.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Raghuraman M. K., Cech T. R. Monovalent cation-induced structure of telomeric DNA: the G-quartet model. Cell. 1989 Dec 1;59(5):871–880. doi: 10.1016/0092-8674(89)90610-7. [DOI] [PubMed] [Google Scholar]
- Yu G. L., Bradley J. D., Attardi L. D., Blackburn E. H. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990 Mar 8;344(6262):126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]
- de Lange T., Shiue L., Myers R. M., Cox D. R., Naylor S. L., Killery A. M., Varmus H. E. Structure and variability of human chromosome ends. Mol Cell Biol. 1990 Feb;10(2):518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]