Abstract
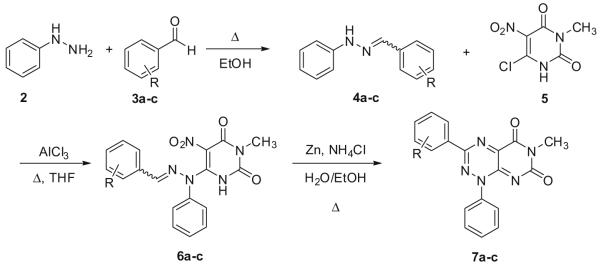
A new synthesis of N1-(substituted)-pyrimido[5,4-e]-1,2,4-triazine-5,7(1H,6H)-diones, which are analogues of the natural product toxoflavin, is reported. Condensation of preformed alkyl or aryl hydrazones with 6-chloro-3-methyl-5-nitrouracil efficiently provides pyrimidotriazinediones in a three-step process that broadens the scope of R1 substituents.
Keywords: Pyrimido[5,4-e]-1,2,4-triazine-5,7(1H,6H)-diones; Toxoflavin; Hydrazones; Cyclizations; Zinc
Toxoflavin (1; Fig. 1), which was originally isolated from Pseudomonas cocovenenans, was first synthesized in 1961.1 Since then, a number of analogues have been prepared in order to explore the potent antibiotic and pharmacological properties of the pyrimido[5,4-e]-1,2,4-triazine-5,7(1H,6H)-dione class represented by this natural product.2 Many of these analogues have the same methyl substituent found at the N1 position of toxoflavin, partially due to the ease with which a methyl group can be incorporated via the use of methylhydrazine early in the synthesis.3 Other analogues have either a proton at N1 or a variety of alkyl and benzyl groups. Conspicuously absent, however, are pyrimido[5,4-e]-1,2,4-triazine-5,7(1H,6H)-diones with an aryl substituent at the N1 position. This can be attributed to the classical route that has been utilized, which requires that nucleophilic attack of 6-chloro-3-methyluracil proceed via the secondary nitrogen of the mono-substituted hydrazine. However, for mono-aryl hydrazines, such attack typically proceeds via the primary nitrogen. Herein, we report a novel and efficient route to pyrimido[5,4-e]-1,2,4-triazine-5,7(1H,6H)-diones that allows for incorporation of aryl and alkyl substituents at N1 of this ring system, thus broadening the scope of accessible analogues.
Figure 1.
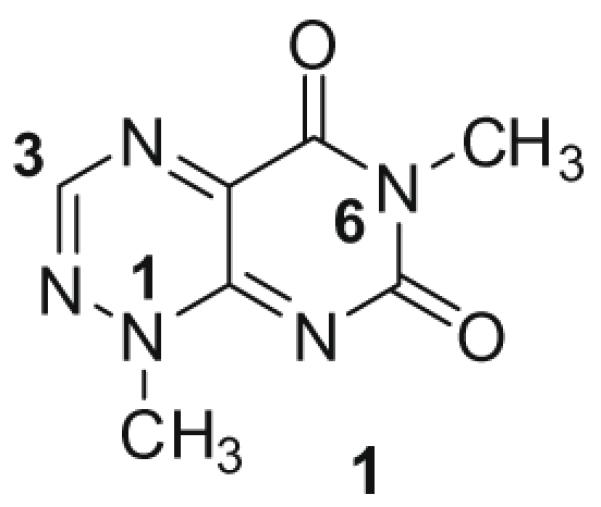
Structure and numbering of 1,6-dimethylpyrimido[5,4-e][1,2,4]triazine-5,7(1H,6H)-dione (toxoflavin).
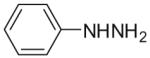
Our strategy is shown in Scheme 1 and is exemplified with phenylhydrazine. First, phenylhydrazine (2) was treated with p-anisaldehyde (3a) under reflux conditions to form hydrazone 4a, which readily precipitated out of ethanol upon cooling. The resulting hydrazone (4a) was then treated with 6-chloro-3-methyl-5-nitrouracil (5), derived from nitration of commercially-available 6-chloro-3-methyluracil by the published method.4 The resultant 6-(2-(4-methoxybenzylidene)-1-phenyl)hydrazinyl-3-methyl-5-nitrouracil (6a) was cyclized to 3-(4-methoxyphenyl)-6-methyl-1-phenylpyrimido[5,4-e][1,2,4]triazine-5,7(1H,6H)-dione (7a) upon treatment with zinc (4 equiv) and ammonium chloride (2 equiv) while exposed to air and with vigorous stirring in refluxing 50% aq ethanol. The N1-phenylpyrimidotriazinedione was isolated cleanly by treating the suspension with 1 N aq HCl to dissolve residual zinc and then washing the precipitate with 1 N aq HCl while filtering.5 The zinc-mediated cyclization was also tried in pure water or ethanol as the solvent, but the yields were considerably diminished. While hydrogenation over Pd/C has been shown to yield pyrimidotriazinediones previously from intermediates analogous to 6a, the reported yields are deplorable, usually only 2–5%.6 In stark contrast, the cyclization conditions reported here yield the desired pyrimidotriazinediones in 51–81% yield.
Scheme 1.
Synthesis of N1-phenylpyrimido[5,4-e]-1,2,4-triazine-5,7(1H,6H)-diones.
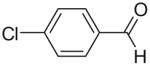
Moreover, as shown in Table 1, the method is applicable to substituted phenylhydrazines and alkyl hydrazines, although the yields in the ring closure are slightly lower with alkyl substituents at N1. Unlike aryl hydrazones derived from aryl hydrazines, those derived from alkyl hydrazines do not require the use of AlCl3 in the condensation with 6-chloro-3-methyl-5-nitrouracil, and the reaction occurs much faster, usually within minutes. Attempts were also made to condense the hydrazones with the less activated 6-chloro-3-methyluracil. These efforts were unsuccessful, however, as the presence of the nitro group at the 5-position of the uracil was found to be critical for making the uracil sufficiently electrophilic for Michael-type addition of the hydrazone, even in the presence of AlCl3.
Table 1.
Substrates and reaction yields for hydrazone (4) addition to 6-chloro-3-methyl-5-nitrouracil (5) and zinc-mediated ring closure to pyrimidotriazinediones (7)
| Aldehyde (3) | Hydrazine (2) | Reaction yield for addition of hydrazone to uracil (%) |
Reaction yield for ring closure (%) |
|---|---|---|---|

|

|
65 | 78 (7a) |
|
|
53 | 72 (7b) |
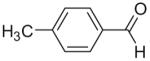
|
|
73 | 81 (7c) |
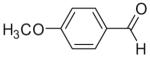
|
NH2NHCH2CH2CH(CH3)2 | 52 | 62 (7d) |

|
NH2NHCH2CH2OH | 67 | 51 (7e) |
In conclusion, we describe a novel and efficient route to pyrimido[5,4-e]-1,2,4-triazine-5,7(1H,6H)-diones. The methodology allows for the incorporation of both aryl and alkyl groups at the R1 position of this ring system, which broadens the scope of accessible substituents. Future reports will describe expanding the scope of R1 substituents reported herein as well the R3 and R6 positions of the pyrimido[5,4-e]-1,2,4-triazine-5,7(1H, 6H)-dione core toward uncovering agents with novel pharmacological properties.
Acknowledgments
A.J.T. gratefully acknowledges financial support of the NIGMS (grant number GM007767), the American Chemical Society Division of Medicinal Chemistry, Sheila B. Cresswell, and Fred and Dee Lyons Graduate Fellowships.
References and notes
- 1.Daves GD, Robins RK, Cheng CC. J. Am. Chem. Soc. 1961;83:3904–3905. [Google Scholar]
- 2.Levenberg B, Linton SN. J. Biol. Chem. 1966;241:846–852. [PubMed] [Google Scholar]
- 3.Nagamatsu T, Yamasaki H, Hirota T, Yamato M, Kido Y, Shibata M, Yoneda F. Chem. Pharm. Bull. 1993;41:362–368. doi: 10.1248/cpb.41.362. [DOI] [PubMed] [Google Scholar]
- 4.Black HT. J. Heterocyclic Chem. 1987;24:1373–1375. [Google Scholar]
- 5.Typical experimental procedures. 1-(4-Methoxybenzylidene)-2-phenylhydrazine (4a): A mixture of phenylhydrazine (2.0 mL, 20.3 mmol) and p-anisaldehyde (2.7 mL, 22.3 mmol) was dissolved in 30 mL abs EtOH. The solution was heated at reflux for 90 min and then cooled to 25 °C. The precipitated solid was collected by filtration and rinsed thoroughly with EtOH to afford 3.91 g (85%) of pale peach product; mp 226–228 °C: 1H NMR (DMSO-d6, 500 MHz) δ 3.78 (s, 3H), 6.72 (t, J = 7.2 Hz, 1H), 6.96 (d, J = 8.7 Hz, 2H), 7.06 (d, J = 7.75 Hz, 2H), 7.21 (t, J = 7.8 Hz, 2H), 7.59 (d, J = 8.7 Hz, 2H), 7.84 (s, 1H). 6-(2-(4-Methoxybenzylidene)-1-phenylhydrazinyl)-3-methyl-5-nitropyrimidine-2,4(1H,3H)-dione (6a): Hydrazone 4a (200 mg, 0.88 mmol) was suspended in anhydrous THF under nitrogen. AlCl3 (118 mg, 0.88 mmol) and 6-chloro-3-methyl-5-nitrouracil (5)4 (164 mg, 0.80 mmol) were added, and the mixture was heated at reflux for 4 h, during which time it became homogeneous and dark green. The mixture was cooled to 25 °C and then on ice, and the precipitated solid was collected by filtration and washed with THF and EtOH to leave 490 mg (65%) of product as a pale lime green powder; mp 225–229 °C: 1H NMR (DMSO-d6, 500 MHz) δ 3.16 (s, 3H), 3.80 (s, 3H), 6.97 (d, J = 8.7 Hz, 2H), 7.39 (d, J = 6.05 Hz, 2H), 7.43 (s, 1H), 7.60 (m, 5H), 11.50 (br s, 1H); 13C NMR (DMSO-d6, 125 MHz) δ 27.69, 55.80, 114.65, 122.21, 125.98, 128.78, 129.37, 130.57, 130.84, 134.62, 145.07, 147.27, 149.19, 157.44, 161.74. 3-(4-Methoxyphenyl)-6-methyl-1-phenylpyrimido[5,4-e][1,2,4]triazine-5,7(1H,6H)-dione (7a): To a suspension of 6a (1.25 mmol) in 6 mL of 50% aq EtOH were added zinc dust (327 mg, 5.0 mmol) and ammonium chloride (134 mg, 2.5 mmol). The suspension was stirred vigorously and heated at reflux overnight with exposure to the air. The mixture was cooled to 25 °C, diluted with 5 mL of 1 N aq HCl, and stirred further for 1 h at 25 °C. The precipitated solid was collected by filtration, washed with 1 N aq HCl and then EtOH, and dried to leave 84 mg (78%) of product as a red powder; mp 315–317 °C (dec): 1H NMR (DMSO-d6, 500 MHz) δ 3.34 (s, 3H), 3.88 (s, 3H), 7.14 (d, J = 8.4 Hz, 2H), 7.67 (m, 3H), 7.76 (m, 2H), 8.15 (d, J = 8.3 Hz, 2H); 13C NMR (TFA-d, 125 MHz) δ 24.68, 50.69, 117.96, 119.92, 126.77, 127.18, 130.03, 132.30, 137.09, 139.86, 143.29, 153.81, 160.79; MS m/z 362.2 (M+H).
- 6.Lacrampe JFA, Connors RW, Ho CY, Richardson A, Freyne EJE, Buijnsters PJJ, Bakker AC. WO 2004007498 A3. Janssen Pharmaceutica N.V.; Jan 22, 2004. 3-Phenyl Analogs of Toxoflavine as Kinase Inhibitors; p. 70. [Google Scholar]