Abstract
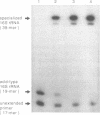
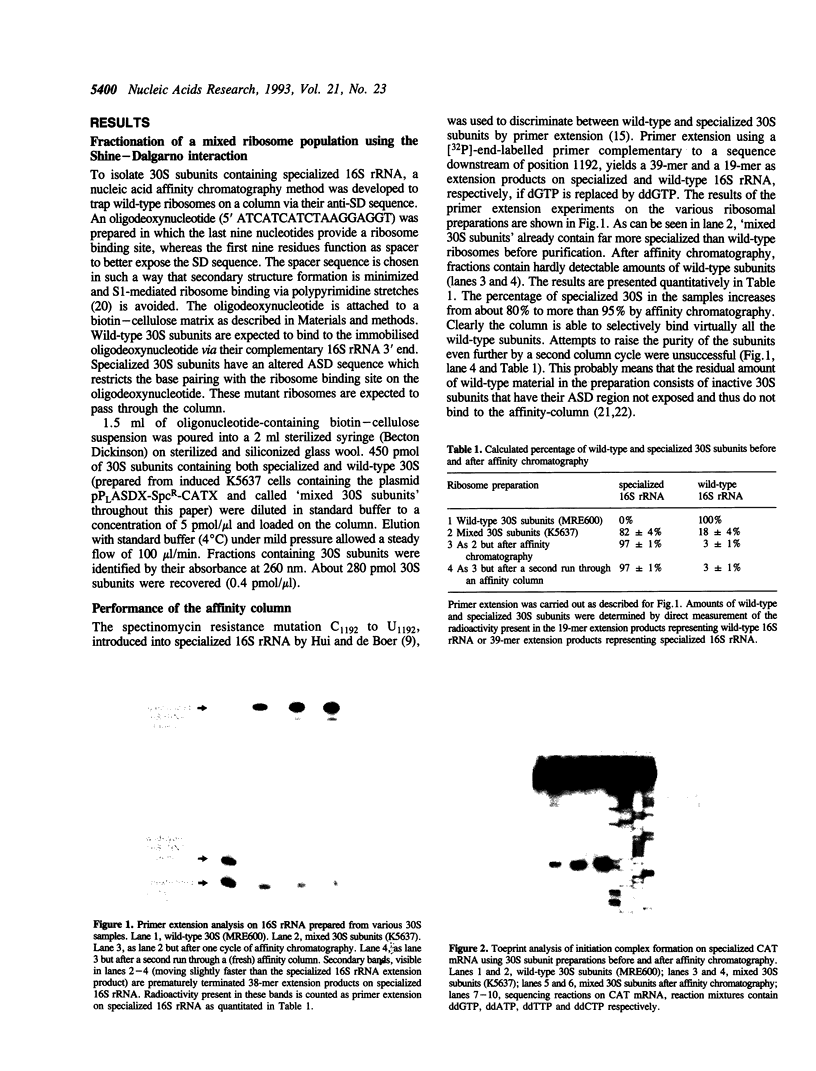
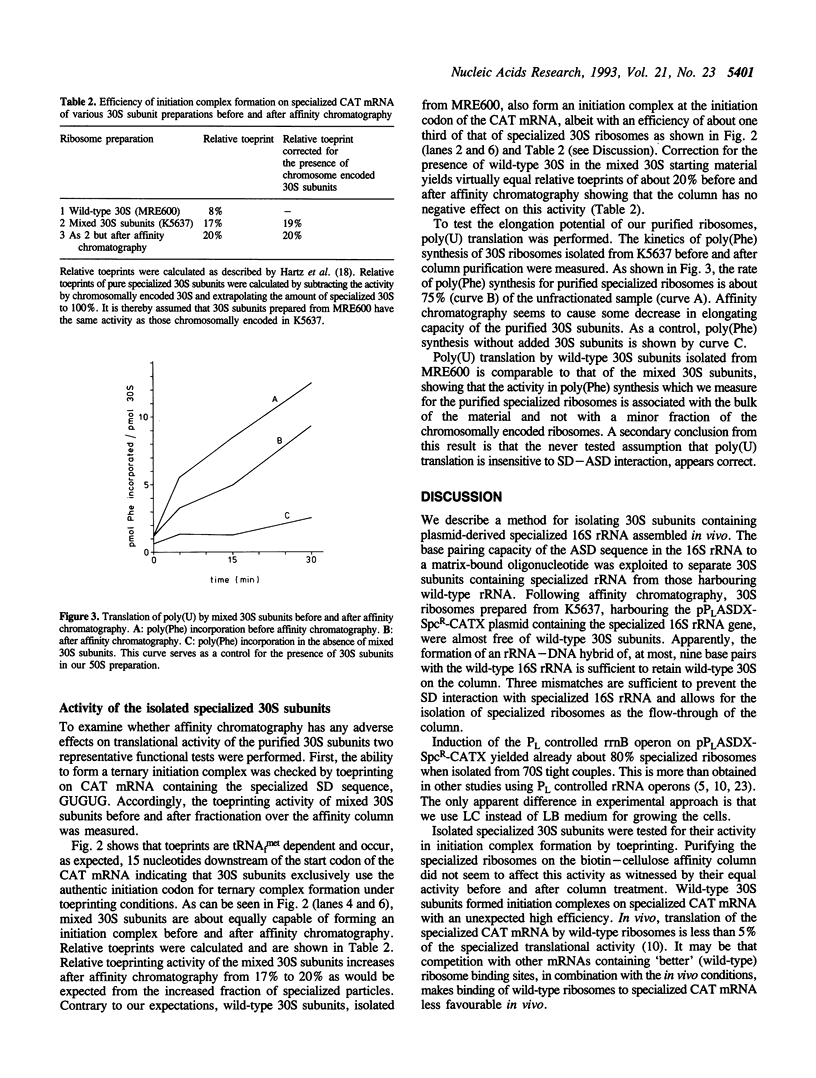
We describe a system to isolate 30S ribosomal subunits which contain targeted mutations in their 16S rRNA. The mutations of interest should be present in so-called specialized 30S subunits which have an anti-Shine-Dalgarno sequence that is altered from 5' ACCUCC to 5' ACACAC. These plasmid-encoded specialized 30S subunits are separated from their chromosomally encoded wild-type counterparts by affinity chromatography that exploits the different Shine-Dalgarno complementarity. An oligonucleotide complementary to the 3' end of wild-type 16S rRNA and attached to a solid phase matrix retains the wild-type 30S subunits. The flow-through of the column contains close to 100% mutant 30S subunits. Toeprinting assays demonstrate that affinity column treatment does not cause significant loss of activity of the specialized particles in initiation complex formation, whereas elongation capacity as determined by poly(Phe) synthesis is only slightly decreased. The method described offers an advantage over total reconstitution from in vitro transcribed mutant 16S rRNA since our 30S subunits contain the naturally occurring base modifications in their 16S rRNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Christodoulou C., Gait M. J. Efficient methods for attaching non-radioactive labels to the 5' ends of synthetic oligodeoxyribonucleotides. Nucleic Acids Res. 1986 Aug 11;14(15):6227–6245. doi: 10.1093/nar/14.15.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backendorf C., Ravensbergen C. J., Van der Plas J., van Boom J. H., Veeneman G., Van Duin J. Basepairing potential of the 3' terminus of 16S RNA: dependence on the functional state of the 30S subunit and the presence of protein S21. Nucleic Acids Res. 1981 Mar 25;9(6):1425–1444. doi: 10.1093/nar/9.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Brink M. F., Verbeet M. P., de Boer H. A. Formation of the central pseudoknot in 16S rRNA is essential for initiation of translation. EMBO J. 1993 Oct;12(10):3987–3996. doi: 10.1002/j.1460-2075.1993.tb06076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael G. G. Isolation of bacterial and phage proteins by homopolymer RNA-cellulose chromatography. J Biol Chem. 1975 Aug 10;250(15):6160–6167. [PubMed] [Google Scholar]
- Cunningham P. R., Nurse K., Weitzmann C. J., Nègre D., Ofengand J. G1401: a keystone nucleotide at the decoding site of Escherichia coli 30S ribosomes. Biochemistry. 1992 Aug 25;31(33):7629–7637. doi: 10.1021/bi00148a026. [DOI] [PubMed] [Google Scholar]
- Hartz D., McPheeters D. S., Gold L. Influence of mRNA determinants on translation initiation in Escherichia coli. J Mol Biol. 1991 Mar 5;218(1):83–97. doi: 10.1016/0022-2836(91)90875-7. [DOI] [PubMed] [Google Scholar]
- Hartz D., McPheeters D. S., Traut R., Gold L. Extension inhibition analysis of translation initiation complexes. Methods Enzymol. 1988;164:419–425. doi: 10.1016/s0076-6879(88)64058-4. [DOI] [PubMed] [Google Scholar]
- Hui A. S., Eaton D. H., de Boer H. A. Mutagenesis at the mRNA decoding site in the 16S ribosomal RNA using the specialized ribosome system in Escherichia coli. EMBO J. 1988 Dec 20;7(13):4383–4388. doi: 10.1002/j.1460-2075.1988.tb03337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui A., de Boer H. A. Specialized ribosome system: preferential translation of a single mRNA species by a subpopulation of mutated ribosomes in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4762–4766. doi: 10.1073/pnas.84.14.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemiolo D. K., Zwieb C., Dahlberg A. E. Point mutations in the 3' minor domain of 16S rRNA of E.coli. Nucleic Acids Res. 1985 Dec 9;13(23):8631–8643. doi: 10.1093/nar/13.23.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Krzyzosiak W., Denman R., Nurse K., Hellmann W., Boublik M., Gehrke C. W., Agris P. F., Ofengand J. In vitro synthesis of 16S ribosomal RNA containing single base changes and assembly into a functional 30S ribosome. Biochemistry. 1987 Apr 21;26(8):2353–2364. doi: 10.1021/bi00382a042. [DOI] [PubMed] [Google Scholar]
- Leclerc D., Melançon P., Brakier-Gingras L. Mutations in the 915 region of Escherichia coli 16S ribosomal RNA reduce the binding of streptomycin to the ribosome. Nucleic Acids Res. 1991 Jul 25;19(14):3973–3977. doi: 10.1093/nar/19.14.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Osswald M., Brimacombe R. Identification of intermolecular RNA cross-links at the subunit interface of the Escherichia coli ribosome. Biochemistry. 1992 Mar 24;31(11):3004–3011. doi: 10.1021/bi00126a023. [DOI] [PubMed] [Google Scholar]
- Noller H. F., Hoffarth V., Zimniak L. Unusual resistance of peptidyl transferase to protein extraction procedures. Science. 1992 Jun 5;256(5062):1416–1419. doi: 10.1126/science.1604315. [DOI] [PubMed] [Google Scholar]
- O'Connor M., Göringer H. U., Dahlberg A. E. A ribosomal ambiguity mutation in the 530 loop of E. coli 16S rRNA. Nucleic Acids Res. 1992 Aug 25;20(16):4221–4227. doi: 10.1093/nar/20.16.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers T., Noller H. F. A functional pseudoknot in 16S ribosomal RNA. EMBO J. 1991 Aug;10(8):2203–2214. doi: 10.1002/j.1460-2075.1991.tb07756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers T., Noller H. F. Dominant lethal mutations in a conserved loop in 16S rRNA. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1042–1046. doi: 10.1073/pnas.87.3.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Borden A., Morgan E. A. Antibiotic resistance mutations in ribosomal RNA genes of Escherichia coli. Methods Enzymol. 1988;164:673–690. doi: 10.1016/s0076-6879(88)64077-8. [DOI] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Morgan E. A. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1984 Jun 11;12(11):4653–4663. doi: 10.1093/nar/12.11.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. E., Cooperman B. S., Mitchell P. Methylation sites in Escherichia coli ribosomal RNA: localization and identification of four new sites of methylation in 23S rRNA. Biochemistry. 1992 Nov 10;31(44):10825–10834. doi: 10.1021/bi00159a025. [DOI] [PubMed] [Google Scholar]
- Thompson J., Cundliffe E., Dahlberg A. E. Site-directed mutagenesis of Escherichia coli 23 S ribosomal RNA at position 1067 within the GTP hydrolysis centre. J Mol Biol. 1988 Sep 20;203(2):457–465. doi: 10.1016/0022-2836(88)90012-5. [DOI] [PubMed] [Google Scholar]
- Triman K., Becker E., Dammel C., Katz J., Mori H., Douthwaite S., Yapijakis C., Yoast S., Noller H. F. Isolation of temperature-sensitive mutants of 16 S rRNA in Escherichia coli. J Mol Biol. 1989 Oct 20;209(4):645–653. doi: 10.1016/0022-2836(89)92000-7. [DOI] [PubMed] [Google Scholar]
- Van Dieijen G., Van Der Laken C. J., Van Knippenberg P. H., Van Duin J. Function of Escherichia coli ribosomal protein S1 in translation of natural and synthetic messenger RNA. J Mol Biol. 1975 Apr 15;93(3):351–366. doi: 10.1016/0022-2836(75)90282-x. [DOI] [PubMed] [Google Scholar]
- Weller J. W., Hill W. E. Probing dynamic changes in rRNA conformation in the 30S subunit of the Escherichia coli ribosome. Biochemistry. 1992 Mar 17;31(10):2748–2757. doi: 10.1021/bi00125a015. [DOI] [PubMed] [Google Scholar]
- Zipori P., Bosch L., van Duin J. Translation of MS2 RNA in vitro in the absence of initiation factor IF-3. Eur J Biochem. 1978 Dec 1;92(1):235–241. doi: 10.1111/j.1432-1033.1978.tb12741.x. [DOI] [PubMed] [Google Scholar]