Abstract
A stereochemically diverse array of monosaccharide analogues of the trisaccharide-based cardiac glycoside natural product digitoxin has been synthesized using a de novo asymmetric approach. The analogues were tested for cytotoxicity against the NCI panel of 60 human cancer cell lines and in more detail against nonsmall cell human lung cancer cells (NCI-H460). The results were compared with digitoxin and its aglycone digitoxigenin. Three novel digitoxin monosaccharide analogues with β-d-digitoxose, α-l-rhamnose, and α-l-amicetose sugar moieties showed excellent selectivity and activity. Further investigation revealed that digitoxin α-l-rhamnose and α-l-amicetose analogues displayed similar antiproliferation effects but with at least 5-fold greater potency in apoptosis induction than digitoxin against NCI-H460. This study demonstrates the ability to improve the digitoxin anticancer activity by modification of the stereochemistry and substitution of the carbohydrate moiety of this known cardiac drug.
Keywords: Digitoxin, digitoxin monosaccharide, rhamnose, amicetose, cancer, apoptosis, NCI-H460
Digitoxin (Figure 1, 1), a cardiac glycoside found in Digitalis purpurea, has been well studied and used over centuries and is still used today for treating congestive heart failure by the inhibition of plasma membrane Na+/K+ ATPase.1 The accumulated intracellular Na+ concentration from this inhibition causes an increase in Ca2+ concentration, which enhances myocardial cell contractility. In addition, digitoxin is known to have antiproliferative effects on cancer cells. Stenkvist found that breast cancer patients on digitalis (digitoxin 1, digoxin 1a) had better outcomes than untreated patients.2 They also found a 9.6-fold lower rate of recurrences for digitalis-treated patients after mastectomy.3 Because of its faster elimination rate from the body, digoxin (1a) is more widely prescribed as a cardiac drug than digitoxin (1). Conversely, we believe that pharmacokinetic properties may make digitoxin a better candidate for cancer therapy.
Figure 1.
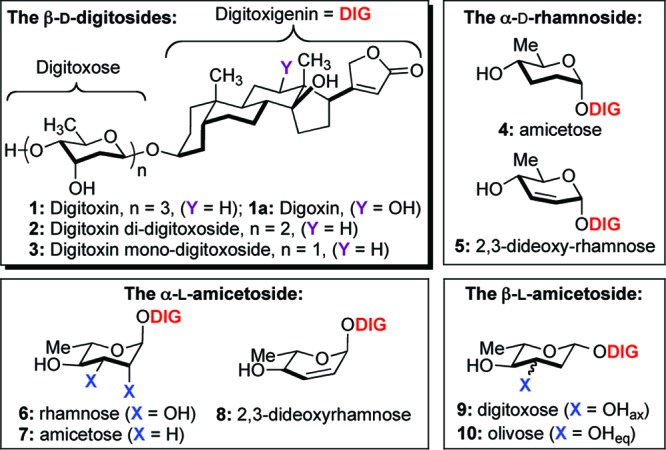
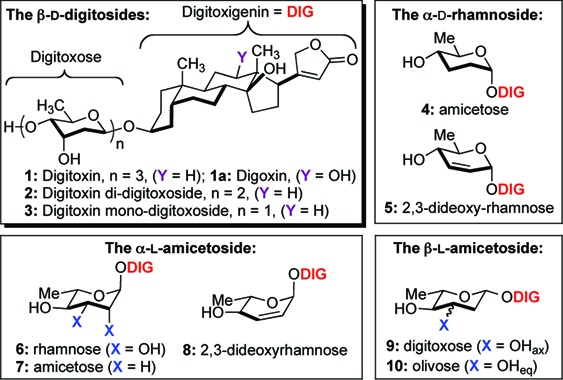
Digitoxin 1 and related carbohydrate analogues.
While no detailed mechanism of tumor cell death has been demonstrated, many apoptosis signal pathways have been found to be affected by digitoxin.4 For instance, digitoxin was shown in vitro to induce apoptosis and inhibit cancer cell growth at a subcardiotoxic concentration in plasma.5 It is known that the inhibition of Na+/K+ ATPase activates tyrosine kinase Src, phosphoinositide-3 kinase (PI3K), and phospholipase C signalosome complex, which can induce many antiproliferative downstream effects related to cell growth and apoptosis.6,7 Because the anticancer effects of digitoxin occur below its cardiotoxic concentration, many Na+/K+ ATPase independent pathways have also been suggested.8−10
Structurally, digitoxin (1) consists of digitoxigenin (aglycone/pharmacore) and the trisaccharide moiety, which is known to play a crucial role in cell growth inhibition and tumor cell death.11 Several research groups have studied the structure−activity relationship (SAR) of the carbohydrate moiety of digitoxin, although no systematic study of stereochemistry (α/β/d/l) has been conducted.12−14 To date, the most significant SAR study of carbohydrate portion of digitoxin on cancer cells is that of Thorson.15 Thorson demonstrated the potentiality to enhance the anticancer activity of digitoxin by screening a library of MeON-neoglycoside link monosaccharides to digitoxigenin.16 A limited SAR conclusion could be made to conclude that a C2′-axial alcohol in the d-sugar was essential for cytotoxicity in many human cancer cell lines. Importantly, they also noted that these substrates were inactive toward Na+/K+ ATPase inhibition in human embryonic kidney (HEK-293) and mammalian (CHO-K) cell lines. In a direct comparison of digitoxin MeON-neoglycoside to natural O-glycoside against a range of cancer cell lines, our recent study has found digitoxin O-glycoside to be more potent than MeON-neoglycoside in both apoptosis and caspases activity.17 This study showed the viability of digitoxin with C2′-deoxy d-sugar as a cytotoxic agent. In addition, we demonstrated that the cytotoxicity was dependent upon the sugar chain length with digitoxin monosaccharide 3 showing at least 10-fold stronger potency than disaccharide 2 and trisaccharide 1 (digitoxin) against a range of cancer cell lines.
Although Thorson's neoglycorandomization study was groundbreaking for digitoxin analogue drug discovery, it was limited in its search of stereochemistry (e.g., β-anomer predominately without C2′-deoxy sugar).16 Thus, we decided to test additional digitoxin monosaccharide analogues to further elucidate the role that carbohydrate chemistry plays in the anticancer effect. In this present study, we synthesized a series of digitoxin monosaccharide analogues (Figure 1, 4−10), as both α- and β-anomer, via a highly stereo/chemo-selective palladium-catalyzed glycosylation.18 This de novo sugar replacement strategy can be essentially used to systematically install a broad range of carbohydrates on natural products in a SAR-amenable fashion. Herein, we report the synthesis and the study of a range of digitoxin monosaccharides by the use of our de novo glycosylation strategy in a stereochemistry divergent manner. The analogues were screened against the National Cancer Institute's (NCI) panel of 60 human cell lines with further study of the apoptotic effects of the most potent analogue.
Recently, we have had success using our de novo asymmetric approach for the introduction of rare/unnatural sugars in biologically important natural products.19−21 This approach has great potential for the systematic synthesis of stereochemically diverse sets of carbohydrates (α/β-d/l). The route begins with the use of a palladium-catalyzed glycosylation to install any of the four corresponding digitoxigenin-pyranones (e.g., α-l-14a to α-l-16a, β-l-14b to β-l-16b, α-d-15a to α-d-17a, and β-d-15b to β-d-17b) from a given aglycone (Scheme 1).
Scheme 1. De Novo Synthesis of Digitoxin Pyranone Monosaccharides 16a, 16b, 17a, and 17b.
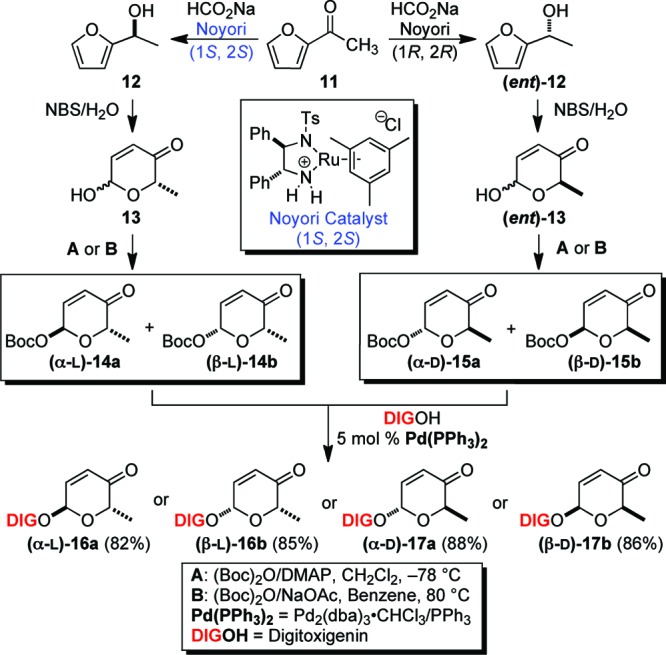
Reagents: NBS, N-bromosuccinimide; DMAP, 4-dimethylaminopyridine.
The desired glycosyl donor pyranones of any given stereochemistry were easily prepared by a three-step sequence from acylfuran 11 (Scheme 1). The absolute stereocenters were securely installed via a highly enantioselective Noyori asymmetric reduction to obtain the corresponding optically pure furanyl alcohols 12 and (ent)-12 with high enantioselectivity (ee > 98%).22,23 An Achmatowicz oxidative rearrangement and diastereoselective Boc protection give the resulting α-/β-diastereomeric mixtures, which were separated by flash chromatography to give a moderate yield of the diastereomeric pure α-/β-d- and α-/β-l-Boc-pyranones (15a/b and 14a/b).24,25 With a complete set of Boc-pyranones in hand, we employed our Pd-catalyzed glycosylation [2.5 mol % Pd2(dba)3CHCl3, 10 mol % PPh3] to convert each sugar building block 15a/b and 14a/b to the desired digitoxin monosaccharide precursors 16a/b and 17a/b with excellent yield and stereoretention at the anomeric center. The resulting enone functionality was then set for further postglycosylation transformation into various stereochemistries and functionalities without the reliance on protecting groups.
These efforts began with the synthesis of α-d-/l-rhamnose and α-d-/l-amicetose digitoxin monosaccharide analogues. The desired C4′-sugar stereochemistry was diastereoselectively installed via Luche reduction (NaBH4/CeCl3, 78 °C) to give α-d-/l-allylic alcohol 5 and 8 as a single isomer (Scheme 2). The resulting C2′-C3′ olefin was readily hydrolyzed to give exclusively rhamnose 18 and 6 through Upjohn dihydroxylation (OsO4/NMO) in excellent yield.26 In addition, this functional ene moiety was reduced under a facile diimide condition to generate α-d-/l-amicetose 4 and 7. The addition of NMM as a solvent seemed to improve the overall yield of this reaction.27,28
Scheme 2. Synthesis of α-d-/l-Rhamnose 18 and 6 and α-d-/l-Amicetose 4 and 7 Digitoxin Analogues.
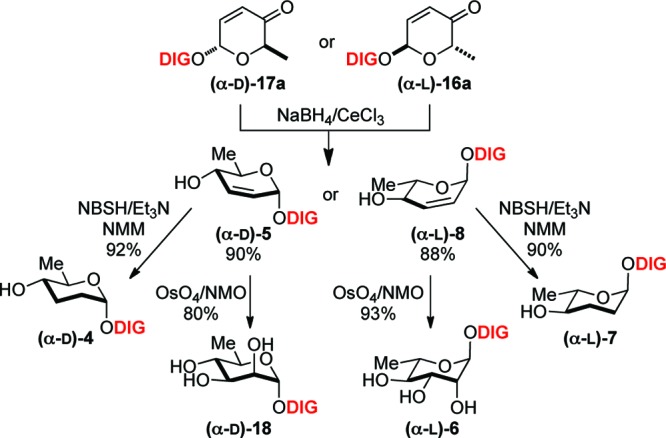
Reagents: NMO, N-methylmorpholine-N-oxide; NBSH, o-nitrobenzenesulfonylhydrazide; and NMM, N-methylmorpholine.
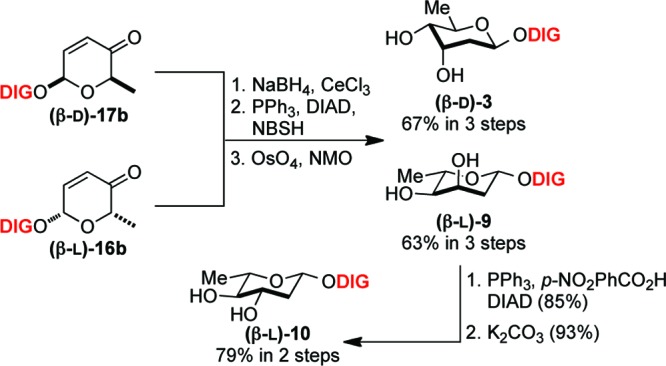
Our effort toward the β-d/l-sugars began from our previously reported synthesis of 3.29,30 Briefly, a reduction of digitoxin pyranones 17b and 16b under Luche reduction, followed by Myers reductive rearrangement (NBSH, PPh3/DIAD, NMM, −30 °C to room temperature) and Upjohn dihydroxylation gave exclusively diol 3 and 9 with excellent yield in three steps (Scheme 3).31 The resulting axial C3′-alcohol 9 was inverted via Mitsunobu reaction (DIAD/PPh3, p-NO2PhCO2H) and subsequently hydrolyzed with K2CO3 to generate β-l-olivose analogue 10. We then submitted our eight novel digitoxin monosaccharides (3−10), along with digitoxin disaccharide 2 and trisaccharide 1 to NCI for the evaluation of growth inhibitory effect toward panel of 60 human cancer cell lines.
Scheme 3. Synthesis of β-d-/l-Digitoxose 3 and 9 and β-l-Olivose 10 Digitoxin Analogues.
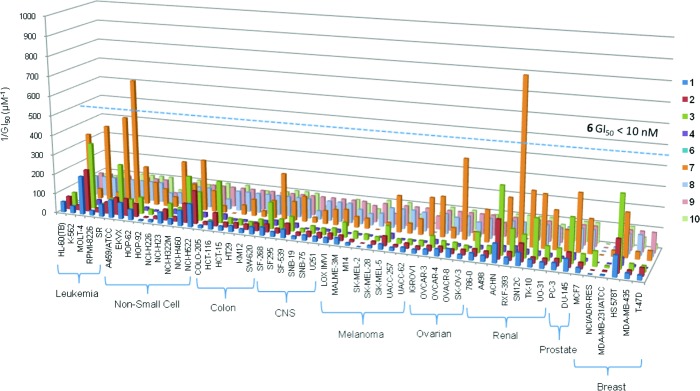
Previously, we have shown that 1, 2, and 3 possess potent antitumor activity against 60 members of NCI cancer cell lines.17 Expectedly, we found that nearly all of the cell lines significantly reduced growth ability when treated with digitoxin monosaccharide analogues (3−10, GI50 ∼ 1−10 nM), which showed at least 5-fold greater potency than digitoxin 1 and digitoxin disaccharide 2 (GI50 ∼ 50−100 nM, Figure 2). In particular, digitoxin monosaccharide 3 and α-l-amicetose 7 showed excellent selectivity and growth inhibition against several cancer cell lines, including leukemia (MOLT-4), human lung carcinoma (A459/ATCC and NCI-H460), human colon carcinoma (HCT-116), human ovary adenocarcinoma (OVACR-3/8), human prostate carcinoma (DU-145), and all six human renal adenocarcinoma cell lines. Interestingly, α-l-amicetose 7 (GI50 ∼ 12 nM across the 60 cell lines) appeared to be a better inhibitor of tumor cell growth by a factor of 10 than its d-sugar diastereomer α-d-amicetose 4 (GI50 ∼ 113 nM across the 60 cell lines). Similarly, we also found β-d-digitoxose 3 (GI50 ∼ 25 nM across the 60 cell lines) showing greater potency than its l-sugar diastereomer β-l-digitoxose 9 (GI50 > 50 nM across the 60 cell lines). Because of the instability of the glycosidic bond upon long-term storage, allylic alcohol 5 was not tested. We were, however, able to test the activity of its l-sugar diastereomer 8, although its decreased cytotoxicity could also be due to the same issue of instability. The GI50 value for α-l-rhamnose 6 was below the minimum concentration that could be accurately determined. On the basis of these findings, we decided to further test the most active digitoxin analogues β-d-digitoxose 3, α-l-amicetose 7 and α-l-rhamnose 6 against the nonsmall cell human lung cancer cell (NCI-H460) to investigate its mode of action. The choice of NCI-H460 was based on its intermediary activity (i.e., not the most or least sensitive cell line) to be the representative in vitro model for our SAR study.
Figure 2.
Carbohydrate survey of digitoxin monosaccharide analogues (1 to 5 and 7 to 10) against NCI panel cell lines, where the values for 6 are represented by the dotted line. Reciprocal GI50 value is displayed for clarity.
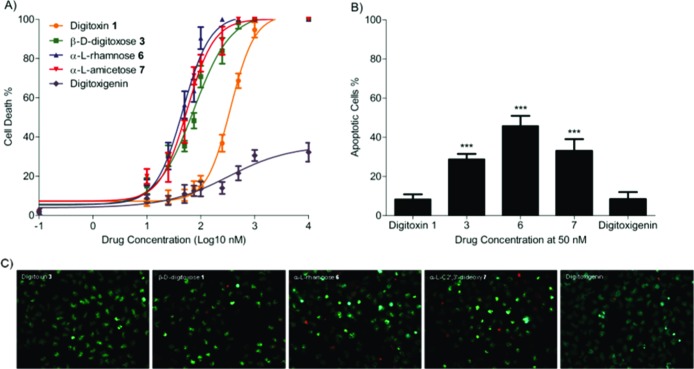
We have previously shown that β-d-digitoxose 3 induces apoptosis in SK-OV-3, NCI-H460, NCI/ADR-RES, and HT-29 cell lines, which is consistent with other findings for digitoxin.5,11,17 Digitoxin initiates apoptosis via both intrinsic and extrinsic pathways recognized to associate with different downstream effectors. To evaluate the pro-apoptotic properties of our novel digitoxin monosaccharide analogues 3, α-l-rhamnose 6, and α-l-amicetose 7, we conducted a cytotoxicity assay with both digitoxin 1 and the aglycone as a control. This assay was performed with a 12 h exposure of drugs in increasing concentration (10 nM to 10 μM). The apoptosis activity was differentiated by Hoechst 33342 nuclear stain and propidium iodide to score the cells having intensely condensed chromatin and/or fragmented nuclei from the cells that undergo necrosis.
Our result clearly showed that digitoxin monosaccharide analogues 3, 6, and 7 have significantly stronger potency than digitoxin 1 within a range of 10−500 nM (P < 0.001) in a dose-dependent manner (Figure 3A). A nonlinear regression analysis showed that α-l-rhamnose 6 was at least 7-fold more active (IC50 ∼ 46.7 nM) than digitoxin 1 (IC50 ∼ 357 nM). Interestingly, α-l-rhamnose 6 also showed better cytotoxicity than α-l-amicetose 7 (IC50 ∼ 55.7 nM) and β-d-digitoxose 3 (IC50 ∼ 74.8 nM) in a range of 50−250 nM (P < 0.001). In addition, α-l-amicetose 7 is comparatively more potent than β-d-digitoxose 3 at 50 nM (P < 0.01). The aglycone digitoxigenin was nearly ineffective (IC50 > 323 nM) against NCI-H460 cells in vitro, which was consistent to the results reported by Lopez-Lazaro.11
Figure 3.
(A) Concentration−response curve of the apoptosis-mediated total cell death by digitoxin analogues in 12 h treatment. (B) Apoptotic cell death percentage was compared for each compound at 50 nM concentration (one-way ANOVA; ***, P < 0.001). (C) Hoechst-stained apoptotic cells appear in blue, and propidium iodide-stained necrotic cells appear in red at 50 nM for compounds 1, 3, 6, 7, DIGOH.
To further differentiate the cytotoxicity between apoptosis and necrosis, the NCI-H460 cells were treated with Hoechst and propidium iodide. Cellular degradation with condensed and fragmented nuclei as shown by Hoechst stain in blue is indicative of apoptosis, while necrotic cells appear completely ruptured in red when stained with propidium iodide. We found for the most potent digitoxin analogue 6 that the ratio of apoptotic and necrotic cell death was 80:20 at 50 nM concentration (Figure 3C). It is worth noting that the ratio of apoptosis and necrosis becomes difficult to estimate at a high dose concentration (>500 nM) due to the high cell mortality rate. However, in the comparison of digitoxin and its analogues in apoptosis activity at 50 nM, we found that α-l-rhamnose 6 (45.8%) showed the highest apoptosis activity than the others (P < 0.001, Figure 3B). The β-d-digitoxose 3 (28.8%) and α-l-amicetose 7 (33%) displayed less activity, but both showed better apoptosis induction than digitoxin 1 (8.4%) and digitoxigenin (8.5%).
We next quantified NCI-H460 cell viability by measuring mitochondrial activity via MTT colorimetric assay to compare cell growth inhibition of our digitoxin analogues. Consistent with our apoptosis results, digitoxin monosaccharide analogues 3, 6, and 7 all showed significantly stronger cytotoxicity than digitoxin 1 in both time-and dose-dependent manner (P < 0.001, Figure 4A,B). In particular, we found that digitoxin monosaccharide analogues 3, 6, and 7 showed better growth inhibition than digitoxin 1 in a low concentration range (10−25 nM) over 48 h of treatment (P < 0.001, Figure 4B). Specifically, both α-l-rhamnose 6 (GI50 ∼ 2 nM) and α-l-amicetose 7 (GI50 ∼ 2.2 nM) showed a greater growth inhibitory effect than β-d-digitoxose 3 (GI50 ∼ 3.8 nM) as well as digitoxin 1 (GI50 ∼ 10.7 nM). These results also agree to the GI50 value from our NCI data, where α-l-rhamnose 6 (GI50 is undetermined), α-l-amicetose 7 (GI50 ∼ 3.8 nM), and β-d-digitoxose 3 (GI50 ∼ 4.5 nM) displayed at least 3-fold greater potency than digitoxin 1 (GI50 ∼ 12.8 nM) in NCI-H460 cells.
Figure 4.
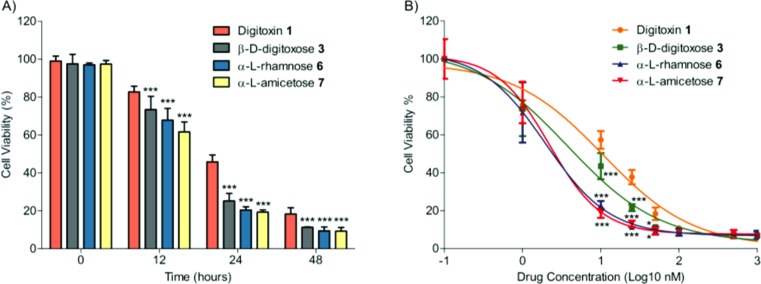
(A) Time-dependent experiment (50 nM). (B) Dose-dependent experiment (48 h). Both time- and dose-dependent data were analyzed by two-way ANOVA (N = 9, *, P < 0.05; ***, P < 0.001).
In summary, we investigated our newly synthesized digitoxin monosaccharide analogues, in which we note a distinct structural activity shift between the d- and the l-sugars in their NCI growth inhibition data. For instance, this data showed that α-l-amicetose 7 and β-d-digitoxose 3 with greater potency than their sugar enantiomers (i.e., diastereomer), α-d-amicetose 4 and β-l-digitoxose 9. We also found that the β-d-digitoxose 3, α-l-rhamnose 6, and α-l-amicetose 7 sugar moieties showed the most promising antineoplastic activity. Both of our apoptosis and cell viability assays showed enhanced activity for the digitoxin monosaccharide analogues 3, 6, and 7 as compared to natural digitoxin 1. Our results suggested an overall activity trend with α-l-rhamnose 6 ≥ α-l-amicetose 7 > β-d-digitoxose 3 > digitoxin 1 > digitoxigenin in both apoptosis induction and antiproliferation effects in NCI-H460 cells. It is interesting to note that the cancer cell cytotoxicity for structures 6, 7, and 3 is not inconsistent with the SAR conclusion by Fullerton for the cardioglycoside's cardiotonic activity.32
This study highlights our de novo asymmetric method for the unique introduction of different α/β-d/l stereoisomers, which can be easily established via palladium-catalyzed glycosylation coupled with a series of postglycosylation transformations. This approach not only shows an efficient preparation of novel digitoxin analogues but also demonstrates its use for systematic SAR studies. Moreover, this comparison of digitoxin monosaccharide stereoisomers provides better insight into the effect of the carbohydrate moiety toward anticancer activity for designing potential cardiac glycoside anticancer agents. Further investigations along this line are ongoing and will be reported in due course.
Acknowledgments
We thank Anand Krishnan Iyer (Hampton University) and Yongju Lu (West Virginia University) for advice on cell culturing and apoptosis assay.
Supporting Information Available
Assay protocols, statistical analysis data, synthetic procedures, characterization data, and NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
We thank the NIH (GM088839) and the NSF (CHE-0749451) for their support of our research program.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Greeff K.Cardiac Glycosides, Part 1: Experimental Pharmacology. Handbook of Experimental Pharmacology; Springer-Verlag: Berlin, NY, 1981; Vol. 56. [Google Scholar]
- Stenkvist B.; Bengtsson E.; Eriksson O.; Holmquist J.; Nordin B.; Westman-Naeser S. Cardiac glycosides and breast cancer. Lancet 1979, 1, 563. [DOI] [PubMed] [Google Scholar]
- Stenkvist B.; Bengtsson E.; Dahlqvist B.; Eriksson O.; Jarkrans T.; Nordin B. Cardiac glycosides and breast cancer, revisited. N. Engl. J. Med. 1982, 306, 484. [PubMed] [Google Scholar]
- Newman R. A.; Yang P.; Pawlus A. D.; Block K. I. Cardiac Glycosides as Novel Cancer Therapeutic Agents. Mol. Interventions 2008, 8, 36–49. [DOI] [PubMed] [Google Scholar]
- Haux J.; Solheim O.; Isaksen T.; Angelsen A. Digitoxin, in non-toxic concentrations, inhibits proliferation and induces cell death in prostate cancer cell lines. Z. Onkol. 2000, 32, 11–16. [Google Scholar]
- Haas M.; Askari A.; Xie Z. Involvement of Src and Epidermal Growth Factor Receptor in the Signal-transducing Function of Na+/K+-ATPase. J. Biol. Chem. 2000, 275, 27832–27837. [DOI] [PubMed] [Google Scholar]
- Xie Z.; Cai T. Na+-K+-ATPase-Mediated Signal Transduction: From Protein Interaction to Cellular Function. Mol. Interventions 2003, 3, 157–168. [DOI] [PubMed] [Google Scholar]
- Arispe N.; Diaz J. C.; Simakova O.; Pollard H. B. Heart failure drug digitoxin induces calcium uptake into cells by forming transmembrane calcium channels. Proc. Natl. Acad. Sci. U.S.A. 2008, 105, 2610–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Tian J.; Haas M.; Shapiro J. I.; Askari A.; Xie Z. Ouabain Interaction with Cardiac Na+/K+-ATPase Initiates Signal Cascades Independent of Changes in Intracellular Na+ and Ca2+ concentrations. J. Biol. Chem. 2000, 275, 27838–27844. [DOI] [PubMed] [Google Scholar]
- Saunders R.; Scheiner-Bobis G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. Eur. J. Biochem. 2004, 271, 1054–1062. [DOI] [PubMed] [Google Scholar]
- Lopez-Lazaro M.; Pastor N.; Azrak S. S.; Ayuso M. J.; Austin C. A.; Cortes F. Digitoxin Inhibits the Growth of Cancer Cell Lines at Concentrations Commonly Found in Cardiac Patients. J. Nat. Prod. 2005, 68, 1642–1645. [DOI] [PubMed] [Google Scholar]
- Yoda A. Structure-Activity Relationships of Cardiotonic Steroids for the inhibition of Sodium- and Potassium-Dependent Adenosine Triphosphatase. Mol. Pharmacol. 1973, 9, 51–60. [PubMed] [Google Scholar]
- Smith P.; Brown L.; Boutagy J.; Thomas R. Cardenolide Analogues. Synthesis and Biological Activity of Glucosides of C17b-Modified Derivatives of Digitoxigenin. J. Med. Chem. 1982, 25, 1222–1226. [DOI] [PubMed] [Google Scholar]
- Fullerton D. S.; Kihara M.; Deffo T.; Kitatsuji E.; Ahmed K.; Simat B.; From A. H. L.; Rohrer D. C. Cardiac Glycosides. A Systematic Study of Digitoxigenin D-Glycosides. J. Med. Chem. 1984, 27, 256–261. [DOI] [PubMed] [Google Scholar]
- Griffith B. R.; Langenhan J. M.; Thorson J. S. Sweetening natural products via glycorandomization. Curr. Opin. Biotechnol. 2005, 16, 622–630. [DOI] [PubMed] [Google Scholar]
- Langenhan L. M.; Peters N. R.; Guzei I. A.; Hoffmann M.; Thorson J. S. Enhancing the anticancer properties of cardiac glycosides by neoglycorandomization. Proc. Natl. Acad. Sci. U.S.A. 2005, 102, 12305–12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer A. K. V.; Zhou M.; Azad N.; Elbaz H.; Wang L.; Rogalsky D. K.; Rojanasakul Y.; O'Doherty G. A.; Langenhan J. M. A Direct Comparison of the Anticancer Activities of Digitoxin MeON-Neoglycosides and O-Glycosides: Oligosaccharide Chain Length-Dependent Induction of Caspase-9-Mediated Apoptosis. ACS Med. Chem. Lett. 2010, 1, 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu R. S.; O'Doherty G. A. A Palladium-Catalyzed Glycosylation Reaction: The De Novo Synthesis of Natural and Unnatural Glycosides. J. Am. Chem. Soc. 2003, 125, 12406–12407. [DOI] [PubMed] [Google Scholar]
- Shan M.; O'Doherty G. A. De Novo Asymmetric Syntheses of SL0101 and its Analogues via a Palladium-Catalyzed Glycosylation. Org. Lett. 2006, 8, 5149–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M.; O'Doherty G. A. De Novo Synthesis of the Trisaccharide Subunit of Landomycins A and E. Org. Lett. 2008, 10, 2283–2286. [DOI] [PubMed] [Google Scholar]
- Yu X.; O'Doherty G. A. De Novo Asymmetric Synthesis and Biological Evaluation of the Trisaccharide Portion of PI-080 and Vineomycin B2. Org. Lett. 2008, 10, 4529–4532. [DOI] [PubMed] [Google Scholar]
- Fujii A.; Hashiguchi S.; Uematsu N.; Ikariya T.; Noyori R. Ruthenium(II)-Catalyzed Asymmetric Transfer Hydrogenation of Ketones Using a Formic Acid-Triethylamine Mixture. J. Am. Chem. Soc. 1996, 118, 2521–2522. [Google Scholar]
- Li M.; O'Doherty G. A. An enantioselective synthesis of phomopsolide D. Tetrahedron Lett. 2004, 45, 6407–6411. [Google Scholar]
- Achmatowicz O.; Bukowski P.; Szechner B.; Zwierzchowska Z.; Zamojski A. Synthesis of methyl 2,3-dideoxy-DL-alk-2-enopyranosides from furan compounds: A general approach to the total synthesis of monosaccharides. Tetrahedron 1971, 27, 1973–1996. [Google Scholar]
- Guo H.; O'Doherty G. A. De Novo Asymmetric Synthesis of Daumone via a Palladium-Catalyzed Glycosylation. Org. Lett. 2005, 7, 3921–3924. [DOI] [PubMed] [Google Scholar]
- VanRheenen V.; Kelly R. C.; Cha D. Y. An improved catalytic OsO4 oxidation of olefins to cis-1,2-glycols using tertiary amine oxides as the oxidant. Tetrahedron Lett. 1976, 17, 1973–1976. [Google Scholar]
- Haukaas M. H.; O'Doherty G. A. Enantioselective Synthesis of 2-Deoxy- and 2,3-Dideoxyhexoses. Org. Lett. 2002, 4, 1771–1774. [DOI] [PubMed] [Google Scholar]
- Buszek K. R.; Brown N. Improved Method for the Diimide Reduction of Multiple Bonds on Solid-Supported Substrates. J. Org. Chem. 2007, 72, 3125–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M.; O'Doherty G. A. A Stereoselective Synthesis of Digitoxin and Digitoxigen Mono- and Bisdigitoxoside from Digitoxigenin via a Palladium-Catalyzed Glycosylation. Org. Lett. 2006, 8, 4339–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M.; O'Doherty G. A. De Novo Approach to 2-Deoxy-β-glycosides: Asymmetric Syntheses of Digoxose and Digitoxin. J. Org. Chem. 2007, 72, 2485–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A. G.; Zheng B. An efficient method for the reductive transposition of allylic alcohols. Tetrahedron Lett. 1996, 37, 4841–4844. [Google Scholar]
- Rathore H.; From A. H. L.; Ahmed K.; Fullerton D. S. Cardiac Glycosides. 7. Sugar Stereochemistry and Cardiac Glycoside Activity. J. Med. Chem. 1986, 29, 1945–1952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.