Abstract
Cyclic guanosine monophosphate (cGMP) and its primary target kinase –protein kinase G (PKG) are well-recognized modulators of cardiac function and chronic stress response. Their enhancement appears to serve as a myocardial brake, reducing maladaptive hypertrophy, enhancing cell survival signaling and mitochondrial function, protecting against ischemia/reperfusion injury, and blunting the stimulatory effects from catecholamines. Translation of these effects into a chronic treatment in patients with heart failure based on enhancing cGMP generation has be difficult however, with tolerance and hypotension effects from nitrates, and neutral responses to natriuretic peptides (at least B-type). Inhibition of cGMP-targeted phosphodiesterases such as PDE5A is an alternative approach, and one that appears to have more potent effects. Recent studies in experimental models and patients are revealing benefits in heart failure syndromes, and ongoing multicenter trials are specifically testing efficacy of PDE5A inhibition. Here we discuss recent research findings and controversies regarding the PDE/cGMP/PKG signaling pathway, and suggest directions for further research.
Introduction
By the end of each day, the average human heart beats >100, 000 times to fulfill its job of delivering nutrients and oxygen to the peripheral tissues. This requires the ability to both rapidly respond to changes in demand and adapt to chronic stresses that ensue from physiologic or pathological stimuli. Central to this regulation are the cyclic nucleotide 3’, 5’-monophosphates cAMP and cGMP, and their respective effecter enzymes, protein kinase A and G (PKA and PKG). cAMP-PKA is a primary regulator of excitation-contraction associated with PKA-phosphorylation of the voltage-gated calcium channel, ryanodine receptor, phospholamban, and sarcomeric proteins to enhance and accelerate Ca2+ cycling through the sarcoplasmic reticulum and stimulate contraction and relaxation. cGMP/PKG by contrast is considered a sort of myocardial brake, countering cAMP stimulation as well as independently signaling alternative pathways to blunt contraction and growth, while still enhancing relaxation1.
The centrality of cAMP and cGMP regulation has made them attractive targets for therapeutic intervention in heart failure. To date, activating cAMP has proven a difficult tactic. Although it acutely enhances cardiac function, chronic stimulation of cAMP can worsen outcome and increase mortality. New approaches based on targeting distal G-coupled protein receptors such as receptor kinase2 or sarcoplasmic reticulum calcium ATPase(SERCA)3, 4 could provide alternatives that are beneficial, though clinical testing is at very early stages or not yet started.
Cyclic GMP has also been targeted, principally by enhancing synthesis by nitric oxide (NO) or natriuretic peptide stimulation. NO-activates soluble guanylate cyclase, thereby inducing vasodilation, with cardiac effects primarily being modestly enhanced diastolic function; however, tachyphylaxis has limited its sustained use. B-type natriuretic peptide was introduced into clinical practice in 2001 for the treatment of acute decompensated heart failure based on its capacity to lower central vascular pressures rather than its renal or cardiac modifications5, 6. Subsequent studies raised nephrotoxicity concerns7, though a recent trial found neutral results; no toxicity but also no benefits8. An alternative approach is to block specific phosphodiesterases (PDEs) that control cGMP hydrolysis. Yet as of early 2000, remarkably little was known about what enzymes accomplished this in the cardiac myocyte. This changed with evidence that PDE5A, the first discovered and still best understood cGMP-selective PDE, might be involved. Over the past decade, experimental and clinical studies have reported on the efficacy of PDE5A inhibition to treat various forms of heart disease. The work helped spawn the ongoing NIH-multicenter trial (RELAX: Evaluating the Effectiveness of Sildenafil at Improving Health Outcomes and Exercise Ability in People With Diastolic Heart Failure; NCT00763867) in patients with heart failure and a preserved ejection fraction (i.e. EF>50%).
Despite the recent work, plenty of questions and controversies persist. Are the drugs really targeting PDE5A, or are they inhibiting alternative PDEs, such as PDE1, which is more highly expressed in heart and also hydrolyzes cGMP? Is myocyte PDE5A important, or are cardiac effects seen from PDE5 inhibitors origniated from other cell types? Do PDEs selectively partner with specific intracellular cGMP pools, and if so, is this compartment altered by heart disease? How important is myocyte PKG activation to this process? And lastly, are effects of PDE5A inhibition similar in left ventricle (LV) and right ventricle (RV)? We discuss these issues and highlight areas for further research.
Identifying the PDEs involved in cardiac funtion
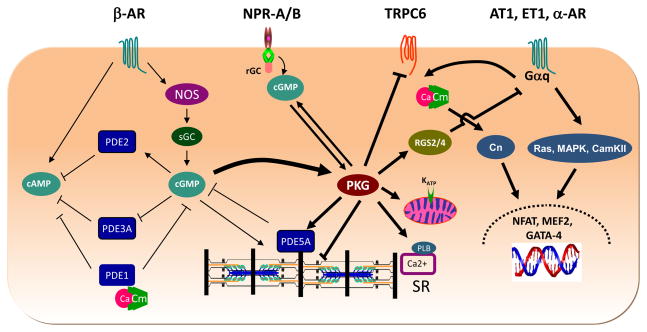
Five PDEs are capable of hydrolyzing cGMP (PDE1, PDE2, PDE3, PDE5 and PDE9), and genes for each are expressed in heart9. However, debate exists as to which are most important. Most analysis of PDE activity is based on the use of pharmacologic inhibitors in vitro, but this might not duplicate the complex regulation of the PDEs in vivo, particularly for dual-esterases and/or those are positively or negatively regulated by the cyclic nucleotides themselves. Recent evidence suggests more than one is involved, each likely targeting a selective pool of cGMP to regulate signal transduction. Furthermore, the precise role played by each PDE may vary among mamallian species. The various cGMP-regulating PDEs and their proposed interactions with cGMP, cAMP, and PKG myocyte targets are summarized in Figure 1.
Figure 1. Schematic diagram showing PDE/PKG regulation of cardiac myocytes.
Generation of cGMP is shown from both a nitric oxide synthase (NOS) –soluble guanylate cyclase (sGC) pathway, and natriuretic peptide receptor-receptor GC pathway. These are two very different pools, and appear to regulate different aspects of cardiac myocyte function and stress adapatation. Their stimulated PKG is also likely compartmentalized though this remains to be clarified and is depicted here for simplicity as a common pool. Once generated, cGMP can stimulate its effecter kinase PKG, and also regulates and is regulated by phosphodiesterases. Four PDEs (PDE1a, c; PDE2, PDE3a, and PDE5a) have been identified as contributing to hydrolytic regulation and/or signaling in myocytes. PDE1 is calcium-calmodulin (CaM) dependent and is a dual esterase, PDE2 is a cGMP-stimulated cAMP esterase, while PDE3A is principally a cAMP esterase that can be competitively inhibited by cGMP. PDE5a is the only selective cGMP esterase of this group. It has several post-translational activation mechanisms; cGMP binding to N-terminus GAF domains, and PKG phosphorylation at serine 92.
On the right of the figure are the targets of PKG. PKG can activate PDE5a – to lower cGMP, but may also target the rGC/NPR complex to stimulate cGMP generation, PKG also phosphorylates phospholamban (PLB) to enhance calcium uptake by the sarcoplasmic reticulum (SR), and mitochondrial proteins to regulate KATP channel opening and provide cardioprotection. Recent studies have also shown revealed it phosphoryates and binds to regulator of G-coupled protein signaling RGS2 and RGS4, both acting to suppress Gαq-coupled signaling cascades (e.g. Ras, MAP kinase, CamKII, and associated transcription factors, NFAT, MEF2 and GATA-4) Studies have also shown PKG directly phosphorylates and suppresses transient receptor potential channel 6, reducing the trigger calcium that has been linked to calcineurin (Cn) activation and subsequent dephosphorylation of NFAT, leading to the latter’s nuclear translocation.
PDE1 is a Ca2+/calmodulin stimulated dual-substrate esterase encoded by 3 genes with multiple splice variants. Humans express PDE1A and -1C, with PDE1C appearing to be the major isotype10, 11, whereas in rodents, PDE1A seems the major isoform12. Levels can rise with chronic heart disease, as shown in mice exposed to isoproterenol or pressure-overload12. Understanding of PDE1 in the intact heart remains very limited, with only one study testing a selective inhibitor IC86340 (no longer available) showing reduced ISO-induced hypertrophy in mice12. A cGMP/PKG dependent anti-hypertrophic effect was observed in cell culture from RNA silencing of PDE1A, and inhibiting both PDE1A and PDE5A yielded additive effects in these studies, supporting their regulation of different cGMP pools12.
PDE2 is also a dual-substrate esterase that can hydrolyze cGMP in vitro, yet it also is stimulated by cGMP to increase its hydrolyze of cAMP, behavior observed in the presence of adrenergic stimulation13. Which action occurs in vivo and under what conditions is unknown, because selective PDE2 inhibitors active in vivo have not been developed, and genetic deletion studies not yet reported. PDE3 principally targets cAMP, and its inhibition was widely tested as a potential positive inotropic therapy, (e.g. milrinone). Although it can hydroylze cGMP as well, this occurs at a much lower Vmax, so cAMP is favored.
PDE5A was the first cGMP-selective PDE discovered, and it is also activated by cGMP which binds to its GAF regulatory domain14. It is expressed in vascular smooth muscle, endothelium, and fibroblasts. Many laboratories have also reported expression in cardiac myocytes, albeit at much lower levels as compared to lung. Immunoblots and confocal imaging employing different commercial15–17 and custom generated/purified antibodies18 have generally found myocyte PDE5a expression and its localization to Z-discs16, 18, 19. However, myocyte expression had historically been considered negligible, and there remains some controversy as a recent study again reported no protein20. Differences in the antibody reagents may underlie the discrepancy. Importantly, the specificity of immune detection has been tested and confirmed in rat neonatal and adult mouse cardiomyocytes in which PDE5a was also genetically silenced21. The expression and activity of PDE5 rises in human and mouse cardiac hypertrophy and heart failure18, 22, 23, and could contribute to the pathophysiology by reducing cGMP and PKG activity. Such upregulation maybe coupled to myocardial oxidative stress24.
PDE5a is regulated by a negative feedback loop where by increased levels of cGMP and PKG activity (via phosphorylation at S92) stimulate hydrolytic activity to reduce cGMP. Through this mechanism, PKG activation reduces cGMP generated by nitric oxide synthase-sGC pathways. However, a recent study found the opposite held for cGMP generated by natriuretic peptide coupled receptor GC, where PKG further stimulated cGMP generation25. The PKG target in the latter case remains unknown and the subject of ongoing research.
PDE9 (9A, 9B) is the newest and highest affinity cGMP-selective PDE discovered, and is expressed in testes, brain, skeletal muscle, and heart. Currently most studies are focused on the effect of PDE9 in neural syndromes such as affective disorders and memory loss26–28. Research into its cardiac role is at an early stage.
Evidence and Controversy regarding myocardial PDE5A regulation
The introduction of sildenafil’ in 1998 for treating erectile dysfunction represented a major fork in the road for a drug being tested against hypertension and angina. A decade later, it was approved for pulmonary hypertension, but cardiac indications were still considered unlikely given its modest impact on arterial tone and low expression and activity in resting myocytes. However, in hearts under various forms of stress, the situation appeared quite different. Initial studies showed in intact dog, mouse, and human, that PDE5 inhibition blunted acute beta-adrenergic stimulated contractility coupled to protein kinase G activation. Kukreja and colleagues reported sildenafil acts in hearts and myocytes to suppress reperfusion injury and apoptosis15, 29, 30, and in 2005, Takimoto et al16 reported sildenafil had anti-hypertrophic effects in mice with pressure-overload in the absence of vascular unloading. The latter could be achieved well after pre-existing pathology was established31. These effects coexist with PKG activation, and targets such as RGS232, calcineurin-NFAT-TRPC623, 33, 34, ERK and GSK3β35 and mitochondrial KATP channels36–38 have been identified to be contributors. New work in the mdx mouse model of Duchenne muscular dystrophy found sildenafil improves cardiac function, sarcolemmal integrity, and suppresses abnormal fetal gene expression35, 39, effects coupled to reduced Ca2+ loading and mitochondrial Ca2+ uptake, preventing excessive mitochondrial permeability transition pore opening40. This work gave rise to a recently initiated clinical trial of sildenafil (REVERSE-DMD, NCT01168908) to treat Duchenne’s patients with cardiac disease, which is currently recruiting patients at the Johns Hopkins Medical Institutions.
These studies have mostly employed sildenafil, which is relatively but not fully selective for PDE5A, and some have argued the doses used might also target PDE141. Moreover, as the drug affects all cell types, myocytes might not be the primary target. However, PDE5 gene silencing blocks hypertrophy in neonatal myocytes21, and myocyte PDE5 overexpression worsens LV remodeling after infarction18. We recently developed a bi-directional PDE5 overexpression model, showing that myocyte PDE5 gene up- or down-regulation potently modulates cardiac responses to pressure-overload (worsens or ameliorates, respectively) while orchestrating extracellular matrix remodeling as well42. Reducing myocyte PDE5 gene expression/activity after establishing cardiac disease improved heart function and fibrosis, a response similar to that from sildenafil therapy. Other evidence for selectivity comes from studies with another PDE5 inhibitor-tadalafil, which is much specific for PDE5A over PDE1, yet also protects against myocardial ischemia/reperfusion injury43 and improves LV function and prevents apoptosis in doxorubicin-induced acute cardiomyopathy44. Admittedly, the role of PDE5 versus PDE1 could differ in humans. However, recent clinical studies in heart failure patients using sildenafil at doses considered fairly specific to PDE5A support benefits not only on exercise performance and symptoms45–47, but recently on both cardiac systolic and diastolic function and remodeling47.
Microdomains couple PDEs to specific guanylate cyclases
Compartmentation of cAMP is well established and thought regulated by anchoring proteins (AKAPs) that coordinate the cyclase with relevant PDE and PKA within a given microdonain. Growing evidence supports the existence of cGMP compartmentation as well, and although GKAP equivalents have not been found, localized PDE regulation appears important. In cardiac myocytes, PDE1C1 localizes to Z-discs and M-lines in a striated pattern10, and has been suggested to provide the majority of cAMP and cGMP hydrolytic activity in soluble fractions from human myocardium, and majority of cGMP hydrolytic activity in microsomal fractions10. This is less the case in rodent hearts12; but again these conclusions are based on in vitro assays. PDE2 localizes to the plasma membrane (particularly at cell-cell junctions) and Z-disc13. At the membrane, it resides along with beta-adrenergic receptors (β-AR), adenylate cyclase (AC), and nitric oxide synthase (NOS), all located in caveolin-rich lipid rafts. This localization could underlie its reported coupling to β3AR-dependent NOS/cGMP-activation of the PDE to hydrolyze cAMP and blunt adrenergic tone13. In isolated myocytes, PDE2 was found to selectively target the NP-receptor-coupled cGMP pool48. Yet even if PDE2 is inhibited, the cGMP generated from NP stimulation does not appear to replicate myocyte contractile regulation effects (e.g. blunt β-AR stimulation) as compared to that observed with NO-stimulation or PDE5 inhibition49. Something yet unknown appears to be constraining the NP signal.
PDE5 normally localizes to z-disc16, 19, 50, 51 where it modulates soluble but not particulate cGMP48, and this localization is also important for its contractile modulation. Cyclic GMP enhanced by PDE5 inhibition (but not NP-cGMP) blocks β-AR stimulation, an effect requiring β3-AR stimulated NOS3-sGC activation16, 49, 50, 52, 53. Intriguingly, PDE5 can move away from z-discs in myocytes from the failing or eNOS deficient heart16, 50, 51. The chaperone(s) involved and pathophysiological significance of this translocation, for example whether it alters that targeting to another cGMP pool, is under active investigation. Whether this applies to other cGMP-PDEs has not yet been reported.
Localized regulation could also be a function of post-translational modifications of a given cyclase or PDE by PKG. For example, Castro et al.54 recently showed that PKG activation in adult cardiomyocytes limits cGMP accumulation from NO stimuli by phosphorylating and activating PDE5, but increases cGMP generation via pGC-NP in a positive feed back loop. The protein and residue target for the latter modulation remains unknown. PDE localization and regulation within microdomains means that analyzing their activity from homogenized cell/tissues using labeled cyclic nucleotides may not provide accurate insight as to what their behavior would be in vivo. Fluorescent-based sensors and mutant membrane bound cGMP-stimulated channels (CNG) have been used48, 54, 55, though these remain challenging particularly for identifying non-membrane pools.
Is myocyte PKG important?
PKG is thought to be the primary downstream effecter of cGMP signaling pathway, and has long been thought to play a role in myocardial remodeling. The strongest evidence for this has been in cell culture studies in which selective PKG inhibitors, expression of dominant negative kinase (or gene silencing), or overexpression is used56, 57. In vivo evidence has been more “guilt by association”, that is a given maneuver coupled to cGMP generation improves heart disease and PKG activation also rises58–60. Global PKG gene silencing induces early lethality (largely from gut immotility), and conditional cardiac targeted knock-down models have yet to be successfully generated. The relevance of PKG activation as an anti-hypertrophic modulator also appears to depend upon the activation of particular target enzymes induced by the stress that can be in turn modulated by PKG. If these are not activated, PKG might have only minimal influence61.
A recent study has challenged the role of myocyte PKG by subjecting mice with global PKG-1 knockout and concomitant smooth muscle-targeted PKGIβ overexpression to both hormonal and mechanical stress20. Mice lacking myocyte PKG exposed to 7-days of intravenous isoproterenol infusion or transaortic constriction developed similar levels of ventricular hypertrophy as did controls. Fetal gene recapitulation including a fall in myosin heavy chain α/β ratio and rise in natriuretic peptide were also similar in both groups. However, the level of stress induced in both models was modest and the existence of maladaptive signaling known to be targeted by PKG was not shown. This could be important, as a prior study had shown that enhancing PKG activity during moderate pressure-overload stress had little impact, whereas similar activation with more severe stress was effective61. This correlated with the presence of PKG-targeted cascades with the latter but not former condition.
An alternative approach to selectively manipulating PKG activity was recently reported using a doxycycline-controllable (Tet-off) myocyte-targeted PDE5 overexpressor mouse42. When myocyte PDE5 was upregulated, PKG activity became depressed, and when combined with pressure-overload, resulted in marked worsening of cardiac hypertrophy, dysfunction, and fibrosis. Importantly, after inducing this pathophysiology, targeted suppression purely of myocyte PDE5 led to a rise in PKG activity, and subsequent improvement all aspects of the pathophysiology. Neither approach attacks the problem directly, and a further resolution awaits the development of selective and inducible PKG-knock down models, which are hopefully underway.
What about the right ventricle?
One might have presumed that, given the initial focus on PDE5 inhibition for pulmonary disease, studies regarding its impact on the RV would have come early. However, the concomitant decline in vascular afterload made this difficult to assess. Several experimental studies have since appeared, and the results seem quite different to those for the LV. In two models in which pulmonary arterial banding was used, and contrasted to a model of pulmonary vascular hypertension, sildenafil benefited the RV only in the latter setting62, 63. In a third study, investigators found that PDE5 inhibitors augmented contractility in RV hypertrophied rat hearts (unlike controls), accompanied by a rise in cAMP level similar to that from a direct β-agonist22. This was ascribed to cGMP-inhibition of PDE3 to elevated cAMP. This has not been reported for the LV, nor to our knowledge, in other studies on the RV. Still, more and more data supports fundamental differences in developmental biology, molecular and cellular physiology of RV versus LV, and their response to stress, so differences in cGMP/PDE/PKG signaling may well exist. This area is ripe for more detailed mechanistic analysis, and is important given the role cGMP-PDE inhibitors play in clinical pulmonary vascular disease.
Concluding remarks
Nearly two decades after the first clinical trials of a novel PDE5 inhibitor, and 12 years after it was turned into an drug for erectile dysfunction, therapeutic efforts to treat heart disease by this approach have been reignited. By the end of 2011, we should have important data on its efficacy in HFpEF patients, there is movement to develop a large scale trial of sildenafil in dilated cardiomyopathy, and other indications such as for muscular dystrophy syndromes may be forthcoming. In the meantime, much remains to be learned about the molecular targets, role of PKG, and importance of other cGMP PDEs and capacity of their selective inhibitors to ameliorate cardiac disease. New conditional and targeted mouse models are needed to selectively modify PDEs and confirm the specificity for observed changes with small molecule inhibitors. Limitations due to the inability to often target specific splice variants and thus compartmentalized PDE regulation need to be better understood or surmounted. One key lesson we have learned, and one that applies broadly to the field, is that expression levels of a given PDE do not directly translated to physiologic importance. Some are expressed at very low levels yet can exert a great effect by regulating a defined microdomain. In vitro activity removed from all of the localized signaling and regulation may not identify what is happening in tissues. The past decade has seen explosive interest in how PDE modulation can be used to impact cGMP/PKG signaling in the heart. The next one will undoubtedly reveal new actors and story lines that will hopefully further point to useful therapies for heart disease patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsai EJ, Kass DA. Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol Ther. 2009;122:216–238. doi: 10.1016/j.pharmthera.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rengo G, et al. Future g protein-coupled receptor targets for treatment of heart failure. Curr Treat Options Cardiovasc Med. 2009;11:328–338. doi: 10.1007/s11936-009-0033-5. [DOI] [PubMed] [Google Scholar]
- 3.Kawase Y, Hajjar RJ. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nat Clin Pract Cardiovasc Med. 2008;5:554–565. doi: 10.1038/ncpcardio1301. [DOI] [PubMed] [Google Scholar]
- 4.Micheletti R, et al. Istaroxime, a stimulator of sarcoplasmic reticulum calcium adenosine triphosphatase isoform 2a activity, as a novel therapeutic approach to heart failure. Am J Cardiol. 2007;99:24A–32A. doi: 10.1016/j.amjcard.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Colucci WS, et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000;343:246–253. doi: 10.1056/NEJM200007273430403. [DOI] [PubMed] [Google Scholar]
- 6.Colucci WS. Nesiritide for the treatment of decompensated heart failure. J Card Fail. 2001;7:92–100. doi: 10.1054/jcaf.2001.22999. [DOI] [PubMed] [Google Scholar]
- 7.Hiestand B, Abraham WT. Safety and efficacy of nesiritide for acute decompensated heart failure: recent literature and upcoming trials. Curr Cardiol Rep. 2007;9:182–186. doi: 10.1007/BF02938348. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez AF. Acute study of clinical effectiveness of Nesiratide in Decompensated Heart Failure. 2010. [Google Scholar]
- 9.Miller CL, Yan C. Targeting cyclic nucleotide phosphodiesterase in the heart: therapeutic implications. J Cardiovasc Transl Res. 2010;3:507–515. doi: 10.1007/s12265-010-9203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandeput F, et al. Cyclic nucleotide phosphodiesterase PDE1C1 in human cardiac myocytes. J Biol Chem. 2007;282:32749–32757. doi: 10.1074/jbc.M703173200. [DOI] [PubMed] [Google Scholar]
- 11.Lakics V, et al. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology. 2010;59:367–374. doi: 10.1016/j.neuropharm.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Miller CL, et al. Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res. 2009;105:956–964. doi: 10.1161/CIRCRESAHA.109.198515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mongillo M, et al. Compartmentalized phosphodiesterase-2 activity blunts beta-adrenergic cardiac inotropy via an NO/cGMP-dependent pathway. Circ Res. 2006;98:226–234. doi: 10.1161/01.RES.0000200178.34179.93. [DOI] [PubMed] [Google Scholar]
- 14.Rybalkin SD, et al. PDE5 is converted to an activated state upon cGMP binding to the GAF A domain. EMBO J. 2003;22:469–478. doi: 10.1093/emboj/cdg051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das A, et al. Phosphodiesterase-5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem. 2005;280:12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 16.Takimoto E, et al. cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res. 2005;96:100–109. doi: 10.1161/01.RES.0000152262.22968.72. [DOI] [PubMed] [Google Scholar]
- 17.Nagendran J, et al. Phosphodiesteras type 5 (PDE5) is highly expressed in the hypertrophied human right ventricle and acute inhibition of PDE5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 18.Pokreisz P, et al. Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation. 2009;119:408–416. doi: 10.1161/CIRCULATIONAHA.108.822072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Senzaki H, et al. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2001;15:1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- 20.Lukowski R, et al. Cardiac hypertrophy is not amplified by deletion of cGMP-dependent protein kinase I in cardiomyocytes. Proc Natl Acad Sci U S A. 2010;107:5646–5651. doi: 10.1073/pnas.1001360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, et al. Expression, activity, and pro-hypertrophic effects of PDE5A in cardiac myocytes. Cell Signal. 2008;20:2231–2236. doi: 10.1016/j.cellsig.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagendran J, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 23.Takimoto E, et al. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 24.Lu Z, et al. Oxidative stress regulates left ventricular PDE5 expression in the failing heart. Circulation. 2010;121:1474–1483. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castro LR, et al. Feedback control through cGMP-dependent protein kinase contributes to differential regulation and compartmentation of cGMP in rat cardiac myocytes. Circ Res. 2010;107:1232–1240. doi: 10.1161/CIRCRESAHA.110.226712. [DOI] [PubMed] [Google Scholar]
- 26.Menniti FS, et al. Phosphodiesterases in the CNS: targets for drug development. Nat Rev Drug Discov. 2006;5:660–670. doi: 10.1038/nrd2058. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt CJ. Phosphodiesterase inhibitors as potential cognition enhancing agents. Curr Top Med Chem. 2010;10:222–230. doi: 10.2174/156802610790411009. [DOI] [PubMed] [Google Scholar]
- 28.van der Staay FJ, et al. The novel selective PDE9 inhibitor BAY 73-6691 improves learning and memory in rodents. Neuropharmacology. 2008;55:908–918. doi: 10.1016/j.neuropharm.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Kukreja RC, et al. Cardioprotection with phosphodiesterase-5 inhibition--a novel preconditioning strategy. J Mol Cell Cardiol. 2004;36:165–173. doi: 10.1016/j.yjmcc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Kukreja RC, et al. Pharmacological preconditioning with sildenafil: Basic mechanisms and clinical implications. Vascul Pharmacol. 2005;42:219–232. doi: 10.1016/j.vph.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Nagayama T, et al. Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol. 2009;53:207–215. doi: 10.1016/j.jacc.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takimoto E, et al. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest. 2009;119:408–420. doi: 10.1172/JCI35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiedler B, et al. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci U S A. 2002;99:11363–11368. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koitabashi N, et al. Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation Novel mechanism of cardiac stress modulation by PDE5 inhibition. J Mol Cell Cardiol. 2010;48:713–724. doi: 10.1016/j.yjmcc.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamo CM, et al. Sildenafil reverses cardiac dysfunction in the mdx mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1013077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salloum FN, et al. Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K(ATP) channels when administered at reperfusion following ischemia in rabbits. J Mol Cell Cardiol. 2007;42:453–458. doi: 10.1016/j.yjmcc.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salloum FN, et al. Vardenafil: a novel type 5 phosphodiesterase inhibitor reduces myocardial infarct size following ischemia/reperfusion injury via opening of mitochondrial K(ATP) channels in rabbits. J Mol Cell Cardiol. 2006;40:405–411. doi: 10.1016/j.yjmcc.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Costa AD, et al. cGMP signalling in pre- and post-conditioning: the role of mitochondria. Cardiovasc Res. 2008;77:344–352. doi: 10.1093/cvr/cvm050. [DOI] [PubMed] [Google Scholar]
- 39.Khairallah M, et al. Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci U S A. 2008;105:7028–7033. doi: 10.1073/pnas.0710595105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ascah A, et al. Stress-induced opening of the permeability trnsition pore in the dystrophin-deficient heart is attenuated by acute treatment with sildenafil. Am J Physiol Heart Circ Physiol. 2010 doi: 10.1152/ajpheart.00522.2010. [DOI] [PubMed] [Google Scholar]
- 41.Vandeput F, et al. cGMP-hydrolytic activity and its inhibition by sildenafil in normal and failing human and mouse myocardium. J Pharmacol Exp Ther. 2009;330:884–891. doi: 10.1124/jpet.109.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, et al. Myocardial Remodeling Is Controlled by Myocyte-Targeted Gene Regulation of Phosphodiesterase Type-5. J Am Coll Cardiol. 2010 doi: 10.1016/j.jacc.2010.08.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salloum FN, et al. Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation. 2009;120:S31–S36. doi: 10.1161/CIRCULATIONAHA.108.843979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koka S, et al. Long-acting phosphodiesterase-5 inhibitor tadalafil attenuates doxorubicin-induced cardiomyopathy without interfering with chemotherapeutic effect. J Pharmacol Exp Ther. 2010;334:1023–1030. doi: 10.1124/jpet.110.170191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guazzi M, et al. Long-term use of sildenafil in the therapeutic management of heart failure. J Am Coll Cardiol. 2007;50:2136–2144. doi: 10.1016/j.jacc.2007.07.078. [DOI] [PubMed] [Google Scholar]
- 46.Lewis GD, et al. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 47.Guazzi M, et al. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: results of a 1-year, prospective, randomized, placebo-controlled study. Circ Heart Fail. 2011;4:8–17. doi: 10.1161/CIRCHEARTFAILURE.110.944694. [DOI] [PubMed] [Google Scholar]
- 48.Castro LR, et al. Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation. 2006;113:2221–2228. doi: 10.1161/CIRCULATIONAHA.105.599241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee DI, et al. PDE5A suppression of acute beta-adrenergic activation requires modulation of myocyte beta-3 signaling coupled to PKG-mediated troponin I phosphorylation. Basic Res Cardiol. 2010;105:337–347. doi: 10.1007/s00395-010-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kass DA, et al. Phosphodiesterase regulation of nitric oxide signaling. Cardiovasc Res. 2007;75:303–314. doi: 10.1016/j.cardiores.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 51.Nagayama T, et al. Sustained soluble guanylate cyclase stimulation offsets nitric-oxide synthase inhibition to restore acute cardiac modulation by sildenafil. J Pharmacol Exp Ther. 2008;326:380–387. doi: 10.1124/jpet.108.137422. [DOI] [PubMed] [Google Scholar]
- 52.Borlaug BA, et al. Sildenafil inhibits beta-adrenergic-stimulated cardiac contractility in humans. Circulation. 2005;112:2642–2649. doi: 10.1161/CIRCULATIONAHA.105.540500. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, et al. Phosphodiesterase 5 restricts NOS3/Soluble guanylate cyclase signaling to L-type Ca2+ current in cardiac myocytes. J Mol Cell Cardiol. 2009;47:304–314. doi: 10.1016/j.yjmcc.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Castro LR, et al. Feedback Control Through cGMP-Dependent Protein Kinase Contributes to Differential Regulation and Compartmentation of cGMP in Rat Cardiac Myocytes. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.110.226712. [DOI] [PubMed] [Google Scholar]
- 55.Russwurm M, et al. Design of fluorescence resonance energy transfer (FRET)-based cGMP indicators: a systematic approach. Biochem J. 2007;407:69–77. doi: 10.1042/BJ20070348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wegener JW, et al. cGMP-dependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ Res. 2002;90:18–20. doi: 10.1161/hh0102.103222. [DOI] [PubMed] [Google Scholar]
- 57.Fiedler B, et al. Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci U S A. 2002;99:11363–11368. doi: 10.1073/pnas.162100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holtwick R, et al. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003;111:1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kishimoto I, et al. A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc Natl Acad Sci U S A. 2001;98:2703–2706. doi: 10.1073/pnas.051625598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knowles JW, et al. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J Clin Invest. 2001;107:975–984. doi: 10.1172/JCI11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagayama T, et al. Pressure-overload magnitude-dependence of the anti-hypertrophic efficacy of PDE5A inhibition. J Mol Cell Cardiol. 2009;46:560–567. doi: 10.1016/j.yjmcc.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andersen A, et al. Effects of phosphodiesterase-5 inhibition by sildenafil in the pressure overloaded right heart. Eur J Heart Fail. 2008;10:1158–1165. doi: 10.1016/j.ejheart.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 63.Schafer S, et al. Chronic inhibition of phosphodiesterase 5 does not prevent pressure-overload-induced right-ventricular remodelling. Cardiovasc Res. 2009;82:30–39. doi: 10.1093/cvr/cvp002. [DOI] [PubMed] [Google Scholar]