Abstract
Oxidative-stress, in response to the activation of the superoxide-producing enzyme Nox2, has been implicated in the schizophrenia-like behavioral dysfunction that develops in animals that were subject to either neonatal NMDA receptor-antagonist treatment or social isolation. In both of these animal models of schizophrenia, an environmental insult occurring during the period of active maturation of the fast-spiking parvalbumin-positive (PV+) interneuronal circuit leads to a diminished expression of parvalbumin in GABA-inhibitory neurons when animals reach adulthood. The loss of PV+ interneurons in animal models had been tentatively attributed to the death of these neurons. However, present results show that for the perinatal NMDA-R antagonist model these interneurons are still alive when animals are 5–6 weeks of age even though they have lost their phenotype and no longer express parvalbumin. Alterations in parvalbumin expression and sensory-evoked gamma oscillatory activity, regulated by PV+ interneurons, are consistently observed in schizophrenia. We propose that cortical networks consisting of faulty PV+ interneurons interacting with pyramidal neurons may be responsible for the aberrant oscillatory activity observed in schizophrenia. Thus, oxidative stress during the maturation window for PV+ interneurons may, by alteration of normal brain development, lead to the emergence of schizophrenia-like behavioral dysfunctions when subjects reach early adulthood.
Keywords: redox, parvalbumin, fast-spiking, gamma oscillations, GABAergic, Interleukin-6, NADPH oxidase, schizophrenia
Introduction
There is increasing evidence that schizophrenia, which typically presents in adolescence or early adulthood, is a consequence of errors in early brain development (Rapoport et al., 2005; Fatemi and Folsom, 2009). Several animal models are being used to understand neurobiological processes relevant to the developmental hypothesis of schizophrenia (Lipska and Weinberger, 2000; Fatemi and Folsom, 2009; Meyer and Feldon, 2009; Powell, 2010). These models have provided insight into the vulnerability of the developing embryo and the importance of the early environment for normal maturation. Developmental models specific to schizophrenia have focused on epidemiological risk factors (e.g., prenatal viral insult, birth complications) or more heuristic models aimed at understanding the developmental neuropathology of the disease (e.g., neonatal NMDA receptor (NMDA-R) antagonist administration, neonatal ventral hippocampal lesions). Combined approach of behavioral and neuroanatomical evaluation of these models strengthens their utility in improving our understanding of the pathophysiology of schizophrenia and developing new treatment strategies.
Data from genetic and neurodevelopmental animal models show that alterations of brain development during specific periods of pre or postnatal life produce a decrease in the expression of the calcium-binding protein parvalbumin (PV) in frontal, limbic, and striatal brain regions and lead to behavioral and neurochemical alterations resembling those found in schizophrenia patients (Beasley et al., 2002; Reynolds et al., 2004; Lewis et al., 2005; Torrey et al., 2005). For example, both maternal immune activation, which produces many behavioral and neurochemical alterations relevant to schizophrenia, and early postnatal immune challenge show alterations in PV-expressing (PV+) interneurons. Mice exposed to PolyI:C in utero have decreased PV immunoreactivity in hippocampus and prefrontal cortex in adulthood (Meyer et al., 2008), and administration of lipopolysaccharide (LPS) on postnatal day 7 and 9 to rat pups also produced decreased PV immunoreactivity in the hippocampus (Jenkins et al., 2009). In response to neonatal ventral hippocampal lesion, the inhibitory GABAergic interneuron system is also dysregulated, and several studies have shown decreased expression of GAD67 and PV in the PFC (Lipska et al., 2003; Francois et al., 2009). Other studies, however, did not report changes in GAD67 or PV mRNA but did report abnormal responses to D2 stimulation in these interneurons (Tseng et al., 2008). The behavioral alterations produced by gestational methylazoxymethanol (MAM) exposure are also associated with decreased PV+ interneuron number in the PFC and hippocampus (Penschuck et al., 2006; Lodge et al., 2009). MAM treatment during the period of migration of PV+ interneurons, from the medial ganglionic eminence into cortex, produces offspring that show several schizophrenia-like behaviors, alteration in dopaminergic systems, and selective reductions in PV+ interneurons in the PFC and ventral subiculum (Lodge et al., 2009). Importantly, these animals show clear alterations in lateral inhibition and brain oscillatory activity resembling those found in schizophrenia patients (see Lodge and Grace, 2009 for a recent review). Moreover, reverse translational models using schizophrenia-risk genes such as DISC1, NRG1/ErbB4 and Reelin show selective alterations of PV expression and PV+ interneuron physiology (Hikida et al., 2007; Shen et al., 2008; Ammassari-Teule et al., 2009; Fisahn et al., 2009; Ayhan et al., 2010; Fazzari et al., 2010; Neddens and Buonanno, 2010; Wen et al., 2010). Finally, non-genetic or pharmacological models, such as social isolation rearing also produce a decrease in PV expression when animals reach adulthood (Harte et al., 2007; Schiavone et al., 2009). In summary, several neurodevelopmental models of schizophrenia converge on a sustained dysfunction of the fast-spiking PV+ interneuronal system (summarized in Table 1), which may start early during postnatal development.
Table 1.
PV+ Interneuron Alteration and Oxidative Stress mechanisms in Neurodevelopmental Models of Schizophrenia
| Rodent Model | PV expression (protein or mRNA) |
Oxidative Stress | |
|---|---|---|---|
| Risk Factor / Pathology |
|||
| Social isolation | Post-weaning social isolation rearing |
Decrease PV-IR in hippocampus (Harte et al. 2007) and PFC (Schiavone et al., 2009) |
Blockade of NOX-2 abolishes effect (Schiavone et al., 2009) Increase in other oxidative stress pathways (e.g., decreased oxidized:reduced GSH ratios, increased SOD) (Moller et al., 2010) |
| Neurodevelopmental NMDA-R hypofunction |
Neonatal NMDA antagonist |
Decreased PV-IR in frontal cortex and hippocampus (mice, (Nakatani-Pawlak et al., 2009); rat, (Wang et al., 2008)) exposed to perinatal PCP (PND 7,9,11) Decreased PV-IR in mice exposed to ketamine on PND 7,9,11) (Figure 1) |
Perinatal PCP (PND 2,6,9,12) reduced GSH and altered antioxidants ((Radonjic et al. 2010) Oxidative stress mechanisms of PCP – decreased Bcl- (X)L/Bax ratio (Wang et al., 2003). Absence of perinatal ketamine effects in Nox2- deficient animals (this paper). |
| Prenatal NMDA antagonist |
Decreased PV-IR in medial prefrontal cortex in rats exposed to MK801 prenatally (E15- E18; (Abekawa et al., 2007)) |
No data available | |
| Juvenile exposure to PCP |
Decreased PV mRNA in mPFC, orbital frontal cortex, nucleus accumbens shell in rats exposed to PCP (PND30–35; (Thomsen et al. 2010)) |
No data available | |
| Postnatal ablation of NMDA-Rs in GABAergic neurons |
Reduced expression of GAD67 and PV (Belforte et al., 2010) |
No data available | |
| Glutathione (GSH) deficit |
Transitory GSH deficit. GSH deficit produced by BSO (L-buthionine-(S,R)- sulfoximine) from PND 5–16 |
Reduced PV-IR in anterior cingulated (Cabungcal et al., 2007) |
GSH is a redox regulator and antioxidant |
| Prenatal/Neonatal Immune Activation |
Prenatal PolyI:C | Decreased PV-IR in PFC of mice (Meyer et al., 2008) and decreased PV mRNA in PFC of mice via microarray (Smith et al., 2007) |
No data available |
| Neonatal LPS | Decreased PV-IR interneurons in hippocampus of rats (Jenkins et al., 2009) |
No data available | |
| Developmental hippocampal pathology |
Neonatal ventral hippocampal lesion |
Decreased GAD67 and PV-IR in PFC adult rats with nVH lesion (Lipska et al., 2003; Francois et al., 2009) |
No data available |
| Disruption in neuronal migration | Prenatal MAM | Decreased PV-IR in PFC and hippocampus of rats (Penschuck et al., 2006; Lodge et al., 2009). | No data available |
| Genetic Susceptibility/ Candidate Genes | |||
| Reelin | Homozygous reeler mutants (rl/rl) |
Decreased PV-IR in striatum (Marrone et al., 2006) |
No data available |
| Heterozygous reeler mutants (rl/-) |
Decreased PV-IR in striatum (Ammassari-Teule et al., 2009) |
No data available | |
| Impaired glutathione synthesis |
Glutamate cysteine ligase modifier (GCLM) KO mice |
Decreased PV-IR in ventral hippocampus (VH) (dentate gyrus, CA3) but not dorsal hippocampus (DH) (Steullet et al., 2010) |
Increased oxidative stress as measured by 8-Oxo-dG in VH but not DH (Steullet et al., 2010) |
| Neuregulin-ErbB4 | ErbB4 KO mice | Decreased PV-IR in hippocampus ((Fisahn et al., 2009; Neddens and Buonanno 2010) |
No data available |
| DISC1 | DN-DISC1 (dominate negative DISC1) mice |
Decreased PV-IR in PFC (Hikida et al., 2007); additive effect on reduced PV-IR with PolyI:C on PND 2–6 (Ibi et al. 2010)& |
No data available |
| Truncated DISC1 | Decreased PV-IR in hippocampus and displaced PV-IR in PFC (Shen et al., 2008) |
No data available | |
| Inducible mutant human DISC1 (hDISC1) |
Decreased PV-IR in cortex of mice with hDISC1 expressed during prenatal, postnatal, or both prenatal and postnatal period (Ayhan et al., 2010) |
No data available | |
| Lisophosphatidic acid 1 receptor (LPA1) |
LPA1 KO mice | Decrease PV-IR interneurons in entorhinal cortex (Cunningham et al., 2006) |
No data available |
| 22q11 deletion (DiGeorge syndrome) |
LgDel mice (carrying hemizygous deletion from Idd to Hira on 22q11) |
Decreased PV-IR interneurons in upper and lower cortical layers (Meechan et al., 2009) |
No data available |
Note: similar loss of PV phenotype observed with adult administration of NMDA antagonists (Rujescu et al. 2006; Jenkins et al. 2010; Behrens et al. 2007,2008; Braun et al. 2007; Sorce et al. 2010). See text for discussion of important differences between developmental versus adult exposure.
No effect found with either PolyI:C or DN-DISC1 alone in this experiment.
Abbreviations: PV-IR parvalbumin immunoreactivity, DH dorsal hippocampus, DISC1 disrupted in schizophrenia, GABA gamma-aminobutyric acid, GAD glutamic acid decarboxylase, GSH glutathione, IR immunoreactivity, LPA lisophosphatidic acid 1, LPS lipopolysaccharide, MAM methylazoxymethanol acetate, NRG1 neuregulin 1, PND postnatal day, NMDAN-methyl-D-aspartate, Nox2 NADPH oxidase 2, NADPH Nicotinamide Adenine Dinucleotide Phosphate, PFC prefrontal cortex, pNM perinatal NMDA antagonist, PCP phencyclidine, PV parvalbumin, ROS reactive oxygen species, SI social isolation rearing, SOD superoxide dismutase, VH ventral hippocampus
This review explores the hypothesis that the dysfunction of this inhibitory interneuronal system in neurodevelopmental animal models results from oxidative-stress and highlights the early postnatal development of the PV+ interneuronal system as a sensitive period for such brain redox imbalance.
1.- Postnatal development of the PV+ interneuronal system
Inhibitory neurons and development of gamma oscillations
PV+ interneurons are involved in the generation of gamma oscillations, which regulate working memory and information transmission between cortical areas (Salinas and Sejnowski, 2001; Bartos et al., 2007; Gonzalez-Burgos and Lewis, 2008; Roopun et al., 2008; Cardin et al., 2009; Sohal et al., 2009; Uhlhaas et al., 2010). Alterations in brain oscillatory activity is a hallmark of schizophrenia pathophysiology, where derangements in both resting and evoked oscillatory activity are consistently found (Uhlhaas and Singer, 2010). Recent human data show that resting-state and task-related gamma-oscillatory activity emerges during early childhood and that temporal coordination by neural synchrony continues to mature until early adulthood (Uhlhaas et al., 2009; Uhlhaas et al., 2010). Additionally, adult levels of performance in delayed response tasks emerge relatively late in the postnatal development of primates (Alexander and Goldman, 1978) and rodents (Bachevalier and Beauregard, 1993; Cobb et al., 1995; de Lecea et al., 1995; Xu et al., 2010). The functional maturation of oscillatory activity and performance in delayed tasks appears to occur concomitantly with PV+ interneuron maturation (Wilson et al., 1994; Rao et al., 2000; Doischer et al., 2008). Thus, the protracted development of the PV+ interneuronal system may constitute a sensitive period where environmental derangements can lead to permanent alterations of inhibitory circuitry, as observed in schizophrenia (Behrens and Sejnowski, 2009).
Although great progress has been made toward the understanding of both the process of postnatal maturation of excitatory networks and the mechanisms underlying the activity-dependent modification of excitatory synapses in principal neurons, understanding of the maturation of inhibitory (GABAergic) circuits has emerged only recently. Unlike principal (excitatory) neurons, which have a relatively conserved set of characteristics, inhibitory interneurons include multiple phenotypes that vary in morphology, physiology and neurochemistry, and represent only 20–30% of neurons in cortex. Due to diversity, low numbers, and the relatively late and activity-dependent maturation of inhibitory neurons, it has been difficult to delineate the transcriptional control of their postnatal maturation.
Role of inhibition in the development of cortical circuitry
GABAergic interneurons profoundly affect the postnatal development of cortical circuitry (Cobb et al., 1995; Pouille and Scanziani, 2001). These effects are exerted by several interneuron subtypes that have distinct electrophysiological and morphological features, and have different synaptic targets (Kawaguchi, 1993; Krimer and Goldman-Rakic, 2001). In cortex, the different subtypes of GABAergic interneurons were originally classified by their expression of the calcium binding proteins PV, calretinin, or calbindin (Conde et al., 1994; Cauli et al., 1997). Recently, a more accurate classification through expression of several peptides suggest that most inhibitory interneurons in cortex can also be classified by their expression of PV, somatostatin, and vasointestinal peptide (Xu et al., 2010).
Convergent evidence suggests that contributions from both genes and neural activity affect the development of brain function, and that a correct balance between excitation and inhibition throughout the period of postnatal development is fundamental for the correct development of functional networks leading to the mature brain. In the immature neocortex, inhibitory interneurons generate excitatory depolarizing potentials that are important for the early development of the neural networks (Owens et al., 1999). At the end of the first postnatal week in rodents, this depolarizing activity is switched to inhibition upon expression of the potassium/chloride transporter KCC2 in the postsynaptic neuron (Rivera et al., 1999).
During postnatal development, activity-dependent regulation of gene expression is a major means of remodeling inhibitory networks through experiences (reviewed in Sun, 2007). Starting at the end of the first postnatal week in rodents, inhibitory networks play a crucial role in experience-dependent refinement of neural networks that last through week 4 (and beyond, depending on the cortical region Lema Tome et al., 2008; Huang, 2009). During this period, cortical inhibition is fundamental to the formation of critical periods for sensory plasticity (Hensch et al., 1998).
Alterations in GAD67 (the main enzyme responsible for GABA synthesis in cortex) and GABA levels profoundly influence interneuron axon growth and synapse formation during the postnatal development of inhibitory circuits (reviewed in Huang, 2009). Among all inhibitory neurons in cortex, the last to mature is the subtype of PV+ inhibitory neurons and in human and non-human primates (Grateron et al., 2003; Lewis et al., 2004; Hensch, 2005). GABA in these neurons was shown to act beyond inhibitory transmission in the juvenile and adolescent brain, regulating the maturation of inhibitory synapses and innervation patterns (Huang, 2009).
Developmental trajectory of PV+ interneurons: fine tuning the network
In mice, the maturational process of PV+ interneurons does not start until the end of the first postnatal week (de Lecea et al., 1995; Doischer et al., 2008; Okaty et al., 2009), although migration is complete by around embryonic day 15–17 (Tanaka et al., 2006; Liodis et al., 2007; Wang et al., 2010). What prevents these neurons from maturing until postnatal day 5 (P5) is not known. At around P5, these cells start responding to GABA and glutamate transmission (Sauer and Bartos, 2010) and by P7 they start expressing PV (de Lecea et al., 1995). Throughout the next 3 weeks they slowly mature into fast-spiking inhibitory neurons (Doischer et al., 2008; Okaty et al., 2009; Goldberg et al. 2010). This period of maturation occurs concomitantly with a striking transcriptional change (Okaty et al., 2009; Goldberg et al. 2010) in which most of the genes characteristically expressed in mature PV+ interneurons are turned on in an orchestrated fashion between the second and fourth postnatal weeks, coinciding with their electrophysiological maturation (Doischer et al., 2008; Okaty et al., 2009).
Among all interneuron subtypes, PV+ interneurons receive the highest number of glutamatergic synapses from thalamic afferents in the adult rodent brain (Gulyas et al., 1999). These afferents, by contacting both pyramidal and inhibitory neurons, ensure the correct timing of cortical excitation by local feedforward inhibition (Hull and Scanziani, 2007). Glutamatergic neurotransmission preferentially activates Ca2+ permeable AMPA-type glutamate receptors on mature PV+ interneurons (Goldberg et al., 2003; Wang and Gao, 2010). However, Ca2+ AMPA-mediated currents develop slowly during postnatal maturation, with most immature PV+ interneurons expressing Ca2+ impermeable AMPA receptors (Wang and Gao, 2010). On the other hand, the expression and function of NMDA-Rs in PV+ interneurons changes during postnatal development, with high levels being expressed early during postnatal development and profound functional changes occurring during adolescence (Boctor and Ferguson, 2009; Wang and Gao, 2009). Furthermore, confirming our results on developing PV+ interneurons in vitro (Kinney et al., 2006), recent results show that GluN2 (NR2) subunits undergo a developmental switch early during postnatal development in PV+ interneurons, and that activity of NMDA receptors in these neurons is fundamental for their maturational process in vivo (Zhang and Sun, 2010). This high NMDA/AMPA ratio may make PV+ interneurons extremely sensitive to alterations in NMDA-mediated transmission during their maturational phase
Thus, it is possible to postulate that environmental inputs that alter the orchestrated process of PV+ interneuron maturation could have profound consequences at the level of experience-dependent plasticity during brain postnatal development, leading to dysfunctional networks when the system reaches adulthood.
Behavioral and neuroanatomical consequences of environmental manipulations during the maturation of PV+ interneurons
Perinatal NMDA receptor antagonist exposure
Alteration of glutamatergic transmission, specifically blockade of NMDA receptors during the postnatal period leads to a range of behavioral abnormalities that are relevant to schizophrenia, from enhancement of exploration and increased phencyclidine-induced hyperactivity, mimicking psychomotor agitation, to impaired working memory in the delayed alternation task (reviewed in Mouri et al., 2007). Perinatal NMDA receptor antagonist exposure also leads to impairments in sensorimotor gating (i.e. prepulse inhibition of acoustic startle response), spatial memory, social interaction behavior, and cognitive flexibility in adulthood (Wang et al., 2003; Mouri et al., 2007; Broberg et al., 2008; Boctor and Ferguson, 2009; Broberg et al., 2009). In addition to cognitive deficits typical of schizophrenia, rodents treated postnatally with NMDA receptor antagonists showed higher level of fear exhibited in the elevated plus maze (Wedzony et al., 2008) and impairments in conditioned fear (Hunt, 2006). A decrease in the number of PV+ interneurons and spine density in the frontal cortex, nucleus accumbens and hippocampus was also shown in both rats (Wang et al., 2008; Boctor and Ferguson, 2009) and mice (Nakatani-Pawlak et al., 2009) exposed to early postnatal NMDA-R blockade when analyzed in adulthood.
Furthermore, direct confirmation of the role of NMDA-R function during postnatal development in the expression of schizophrenia-like behaviors comes from results showing that genetic ablation of these receptors from GABAergic neurons from birth decreases the expression of parvalbumin in PV+ interneurons, produces disinhibition of pyramidal neurons, and leads to schizophrenia-related behaviors when mice reach adulthood (Belforte et al. 2010).
Post-weaning social isolation
Social isolation rearing of young rodents provides a non-pharmacologic method of inducing long-term alterations reminiscent of several symptoms seen in schizophrenia patients (Geyer et al., 1993; Powell et al., 2002). Rearing animals in social isolation is particularly consequential for species that rely on social contact after being weaned from the mother. Specifically, isolation rearing deprives rodents of social interactions during a developmental period in which play behavior emerges (Einon and Morgan, 1977). Thus, as a consequence of social isolation, animals are deprived of stimuli critical to behavioral and neurobiological development (reviewed in Hall et al., 1998)). The lack of early social contact provides a model of the social isolation and social withdrawal which occurs early in the course of schizophrenia and predicts conversion to psychosis in patients at a high risk of developing psychosis (Cannon et al., 2008). Rodents reared in social isolation exhibit profound abnormalities in behavior, drug responses, and neurochemistry compared to animals reared in social groups (Hall et al., 1998; Powell and Geyer, 2002; Powell et al., 2002; Fone and Porkess, 2008). In addition to alterations in striatal dopamine function, isolation-reared animals display abnormalities in the hippocampus and frontal cortex, (Del-Bel et al., 2002; Silva-Gomez et al., 2003; Preece et al., 2004; Scaccianoce et al., 2006). Specifically, isolation-reared rodents show abnormal firing of pyramidal cells in the PFC upon dopamine stimulation from VTA neurons (Peters and O'Donnell, 2005), decreased volume of PFC (Silva-Gomez et al., 2003; Day-Wilson et al., 2006), and decreased dendritic arborization in the PFC (Silva-Gomez et al., 2003; Pascual et al., 2006). Particularly relevant is the observation that the decreased dendritic arborization in the PFC occurs as early as 2 weeks post-weaning (Pascual et al., 2006), and that social isolation also induces deficits in PV+ interneurons in the brain (Harte et al., 2007; Schiavone et al., 2009).
Rats reared in social isolation show deficits in prepulse inhibition and slow rates of startle habituation (Weiss and Feldon, 2001; Powell and Geyer, 2002). More recent studies have also shown that several different strains of mice exhibit deficits in PPI when reared in social isolation from weaning (Sakaue et al., 2003; Dai et al., 2004; Varty et al., 2006) but see (Pietropaolo et al., 2008). Additionally, isolation-reared rodents also display alterations in anxiety-like behavior (Wright et al., 1991; Da Silva et al., 1996), deficits in fear learning (Weiss et al., 2004; Voikar et al., 2005) and deficits visual learning and memory (e.g.,novel object recognition test) (Voikar et al., 2005; Bianchi et al., 2006), and cognitive inflexibility as demonstrated by deficits in reversal learning (Krech et al., 1962; Schrijver and Wurbel, 2001) and extradimensional set-shifting tasks (Schrijver and Wurbel, 2001). Thus, isolation rearing is associated with impaired sensorimotor gating, cognitive inflexibility, reductions in PFC volume and hippocampal synaptic plasticity, loss of PV+ interneurons, hyperfunction of mesolimbic dopaminergic systems, and hypofunction of mesocortical dopamine. The isolation rearing model shows strikingly similar behavioral and neuroanatomical abnormalities as those observed in the perinatal NMDA-R antagonist model and in schizophrenia.
Redox dysregulation during postnatal development leads to enduring loss of parvalbumin expression in PV+ interneurons
Redox dysregulation in schizophrenia
Several studies have reported an altered oxidative state in schizophrenia patients (reviewed in Do et al., 2009). Glutathione (GSH), responsible for detoxification of reactive oxygen and other radical species, is consistently decreased in cerebrospinal fluid of drug-naïve schizophrenia patients (Browne et al., 2000; Do et al., 2000; Lipska and Weinberger, 2000; Rao et al., 2000), as well as in postmortem tissue (Yao et al., 2006). Polymorphisms in genes coding for enzymes that participate in GSH synthesis have been linked to schizophrenia risk (Tosic et al., 2006; Gysin et al., 2007), and recent results show that genetically compromised GSH synthesis affects the morphological and functional integrity of hippocampal PV+ interneurons (Steullet et al., 2010). Furthermore, results showing that treatment with N-acetyl-cysteine, a precursor of GSH, improves negative symptoms, and corrects mismatch negativity in schizophrenia patients, support the idea of a redox imbalance in schizophrenia (Berk et al., 2008; Lavoie et al., 2008; Steullet et al., 2008).
Redox dysregulation in response to NMDA antagonists
In addition to direct manipulations of the redox pathway, other developmental perturbations have been shown to exert their effects through oxidative stress mechanisms. Using adult repetitive exposures of mice to NMDA-R antagonists, as a model of schizophrenia pathophysiology, we showed that injections of ketamine on two consecutive days induced an increased oxidative-state in brain that was sufficient to produce the loss of phenotype (i.e. loss of GAD67 and parvalbumin expression) of PV+ interneurons and an enduring inhibitory dysfunction in the rodent prelimbic region (Behrens et al., 2007; Zhang et al., 2008). The altered oxidative state produced by ketamine was due to a sustained increase in the proinflammatory cytokine interleukin-6 (IL-6) and activation of the superoxide producing enzyme NADPH oxidase-2 (Nox2). Ketamine effects were absent in Nox2-deficient and IL-6-deficient animals, confirming the role of the IL-6/Nox2 pathway as mediator of ketamine effects (Behrens et al., 2008). Exposure to other non-subunit selective NMDA-R antagonists such as PCP and MK801 were previously shown to produce a rapid increase in brain reactive oxygen- and nitrogen-species (Zuo et al., 2007; Fejgin et al., 2008). To confirm the specific role of NMDA-R blockade in the induction of oxidative stress, we used an NR2A-preferring antagonist (NVP-AAM007), at concentrations known to preferentially affect NR2A-containing receptors (3 mg/kg) (Fantin et al., 2007), and observed a similar increase in superoxide production as that produced by ketamine (values are means ± SEM. Ketamine: 68 ± 10 % increase over saline; NVP-AAM007: 62 ± 9 % increase over saline. F(2,9) = 12.636. P < 0.05). These results strongly support a role of increased oxidative-stress in the effects of antagonism of NMDA-Rs in the adult brain. Furthermore, a specific role of Nox2 in the acute effects of ketamine was recently shown: using Nox2-deficient mice, Sorce and collaborators (Sorce et al. 2010) demonstrated the requirement of this superoxide-producing enzyme in the effects of ketamine, suggesting that even the acute propsychotic effects of NMDA-R antagonists are due to their ability to activate Nox2 and thus produce a redox imbalance in brain.
Redox dysregulation during development
Increased oxidative-stress mechanisms during development are hypothesized to be involved in the origin of schizophrenia pathophysiology. Acute decrease in antioxidant capacity during early postnatal periods in rodents, as well as genetic deficiency in GSH produces a loss in PV+ interneurons and induces cognitive derangements relevant to the disease (Cabungcal et al., 2007; Steullet et al., 2010. Summarized in Table 1). Furthermore, genetic dysregulation of glutathione synthesis predicts the alteration in thiol redox status in schizophrenia patients (Gysin et al., 2010). Patients showing high-risk glutamate cysteine ligase, catalytic subunit (GCLC) genotypes had reduced fibroblast production of GSH, decreased total cysteine plasma levels, and increased levels of Cystine. Increased levels of plasma free serine, glutamine, citrulline, and arginine were also observed in the high-risk genotype.
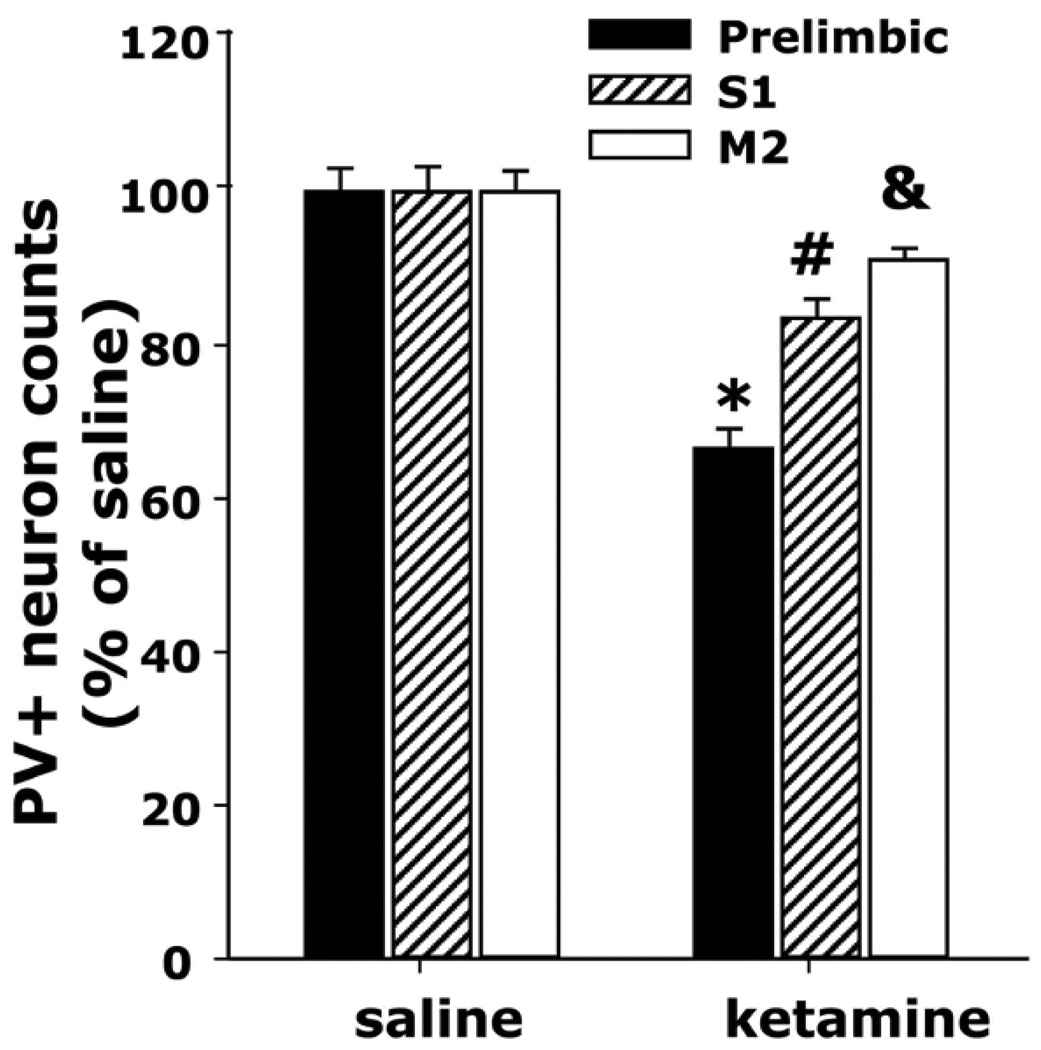
Accumulating evidence shows that embryonic and perinatal NMDA-R antagonist exposures, contrary to the reversible effects observed in adults (Behrens et al., 2008), can produce the loss of PV+ interneurons in several regions when animals reach adulthood (Fig 1) and persistent behavioral and neurochemical deficits (Sircar and Rudy, 1998; Wiley et al., 2003; Andersen and Pouzet, 2004; Stefani and Moghaddam, 2005; Wang et al., 2008; Boctor and Ferguson, 2009; du Bois et al., 2009; Nakatani-Pawlak et al., 2009). Oxidative mechanisms in this model were previously suggested by results showing that antioxidants can prevent the appearance of behavioral disruptions in adult animals that were treated with phencyclidine during the perinatal period (Wang et al., 2003).
Figure 1. Perinatal exposure to ketamine reduces the number of PV-expressing interneurons in the adult frontal cortex.
Litters from two C57BL/6 females per treatment condition were injected subcutaneously with either saline or ketamine (30 mg/kg) on postnatal days 7, 9, and 11. Males, 5 for each treatment condition, were perfused with 4 % paraformaldehyde when they had reached 8 weeks of age, and their brains sliced for floating section immunohistochemistry as described (Behrens et al., 2007). Six consecutive slices encompassing the frontal region were immunostained with Vectastain for detection of parvalbumin using a polyclonal antibody (Swant, Switzerland). Cells in each region were counted as described (Dugan et al., 2009). All cells in the region were counted and total numbers per slice were corrected using Abercrombie’s correction algorithm (Abercrombie, 1946) and normalized to the saline samples. Bar graphs represent means ± SEM. *,#,& indicates statistical significance with respect to saline on each brain region as assessed by ANOVA followed by Tukey’s test (* F(1,8) = 105.351, p < 0.05; # F(1,8) = 25.460, p < 0.05; & F(1,8) = 9.366, p = 0.05
Nox2 mediates effects of perinatal ketamine and social isolation on PV+ interneurons
To test the role of Nox2 in the loss of PV+ interneurons in this model, we exposed Nox2-deficient animals and their wild type littermates to ketamine (30 mg/kg, SC) on postnatal days 7, 9, and 11, and counted the PV+ interneuronal population in the prelimbic region when animals had reached adulthood (8 weeks). Nox2-deficiency completely prevented the loss of PV+ interneurons in the prelimbic region. PV-expressing neurons were counted as described in (Dugan et al., 2009) and expressed as percent of wild type saline conditions (results are means ± SEM. Nox2+/+ –saline = 100 ± 5%, Nox2+/+ -ketamine = 63 ± 1,7%; Nox2−/−-saline = 99 ± 2%, Nox2−/−-ketamine = 97± 3%). A two-way ANOVA revealed a significant effect of treatment (F(1,15) = 52.10, p < 0.001), genotype (F(1,15) = 36.62, P < 0.001) and a significant genotype x treatment interaction (F(1,15) = 58.71, p < 0.001). Tukey’s post hoc analysis revealed a statistically significant difference between Nox2+/+ -saline and Nox2+/+ -ketamine (p < 0.001), and between Nox2+/+ -ketamine and Nox2−/−-saline (p < 0.001) and between Nox2+/+ -ketamine and Nox2−/−-ketamine (p < 0.001).
Recent studies have implicated Nox2-dependent oxidative mechanisms in the loss of PV+ interneurons and development of schizophrenia-like behavior in the isolation rearing model (Schiavone et al., 2009). Corroborating earlier work (Harte et al., 2007), Schiavone and collaborators (Schiavone et al., 2009) found decreased PV immunoreactivity in the brains of rats reared in social isolation. This loss of PV+ interneurons was associated with elevations in Nox2, and the decrease in PV-staining and deficits in novel object recognition were blocked by treatment with the Nox2 inhibitor apocynin (Schiavone et al., 2009). Studies should be done to determine whether other behavioral abnormalities (e.g. disruptions in PPI) can be prevented by treatment with apocynin in this model. Recent studies have shown that isolation rearing is associated with oxidative stress as measured by increased superoxide dismutase, decrease oxidized/reduced glutathione ratio, and increased concentrations of malondialdehyde (Moller et al. 2010). Interestingly, these markers of oxidative stress were all reversed with chronic clozapine administration (Moller et al. 2010).
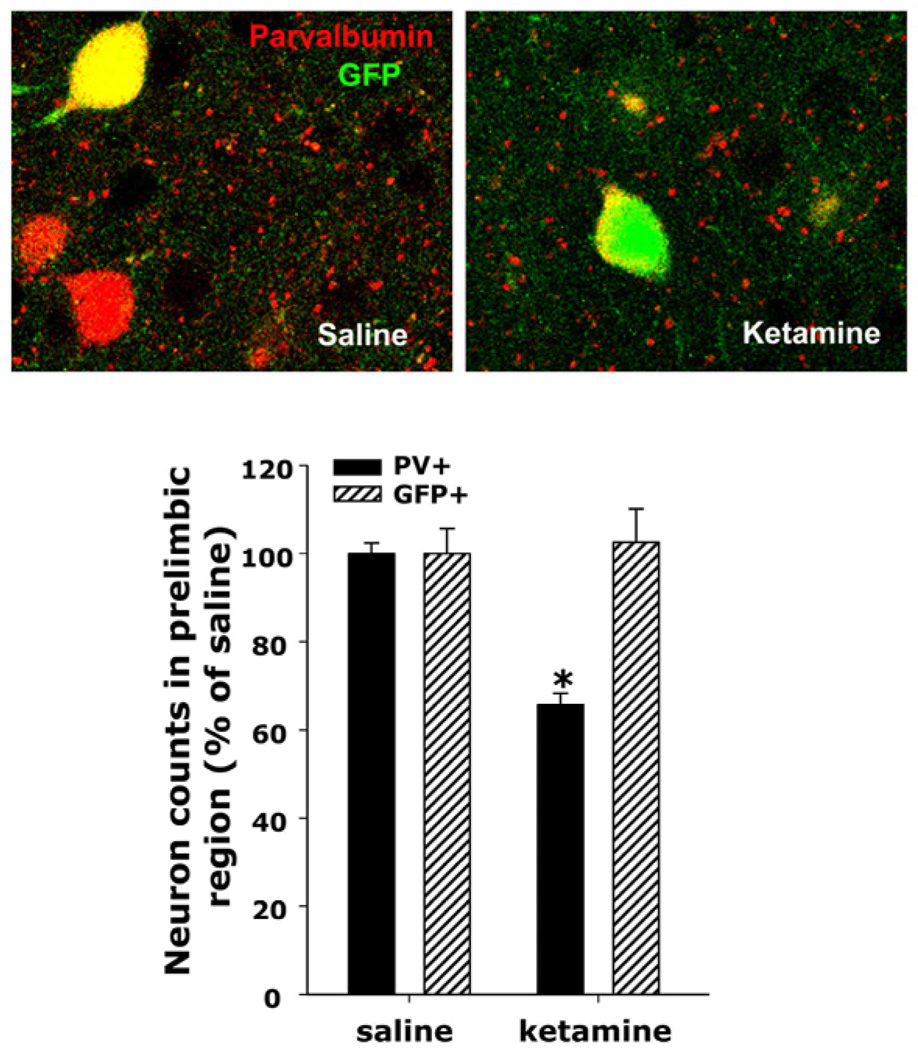
The wide-spread apoptosis in retrosplenial cortex caused by high doses of NMDA antagonists during the perinatal period had led to the conclusion that the reduced number of PV+ interneurons observed (see Fig 1) was due to their death (Olney et al., 1999; Wang et al., 2003; Wang et al., 2008). To test whether this was the case, we took advantage of a mouse line expressing green fluorescent protein exclusively in PV+ interneurons (G42 line, Chattopadhyaya et al., 2004; Di Cristo et al., 2004) and exposed these animals during the second postnatal week (postnatal days 7, 9, and 11) to ketamine (30 mg/kg. s.c). Analysis of parvalbumin and GFP co-expression when animals were in their 5th-6th week of age showed a similar decrease in PV+ neurons as we had observed in wild type adult animals (Fig 1). However, there was no decrease in GFP-expressing neurons (Fig 2), suggesting that while the cells are still alive their developmental maturation may be affected. As commented above, PV+ interneurons reach maturity at around week 5 (deLecea et al., 1995; Doischer et al., 2009; Lema Tome et al., 2008; Huang, 2009). The similarity in the results depicted in Figures 1 and 2 strongly suggest that perinatal ketamine exposures/oxidative-stress mechanisms produce an alteration in the normal development of PV+ interneurons which is already evident during adolescence. Similar results were recently obtained using a mouse line expressing GFP in all GABAergic neurons (Zhang and Sun, 2010). In this study, and using an NR2A-preferring antagonist, the authors show that prolonged blockade of NR2A-containing receptors in vivo during the critical period of plasticity in the barrel cortex produced a decrease in PV expression and an alteration of fast-spiking-mediated IPSCs onto principal neurons.
Figure 2. Loss of PV-expression without death of the interneurons.
The heterozygous progeny of G42 X C57BL/6 crossings was treated with saline or ketamine during the second postnatal week as described in figure 1 and the animals were perfused between the 5th and 6th week of age. The G42 line expresses GFP in approximately 50% of PV+ interneurons (Chattopadhyaya et al., 2004; Di Cristo et al., 2004). (A) Colocalization of GFP and PV was determined by confocal microscopy using a Zeiss Pascal system and AlexaFluor 568 as secondary antibody for detection of PV. (B) The prelimbic region on each slice was imaged across six consecutive slices, and across 16 mm on the Z axis. Images were then collapsed and the number of cells expressing PV or GFP were counted as described in figure 1 and normalized by the mean of the saline controls. As described in figure 1, there was a statistically significant decrease in the number of PV-expressing neurons, but there was no decrease in the GFP-expressing population. Since all GFP expressing neurons were also positive for PV in saline controls, as previously described (Chattopadhyaya et al., 2004; Di Cristo et al., 2004), these results suggest that the PV-interneuronal population is still present in these animals, but the neurons do not express their characteristic marker, parvalbumin.
Bar graphs represent means ± SEM. * indicates statistical significance (p < 0.005) with respect to saline as determined by ANOVA (F(3,12) = 12.278, p < 0.001) followed by Tukey’s pos hoc test.
Functional alteration in cortical networks
Networks of PV+ interneurons tightly interact with excitatory-neuron firing and affect dynamics of cortical activity (Borgers et al., 2008; Tiesinga and Sejnowski, 2009). Each PV-interneuron makes contacts with many principal neurons, and dysfunction of a single interneuron may desynchronize a large portion of the local network and disrupt cortical information processing. Thus, when the PV+ interneuronal system contains a proportion of cells with altered electrophysiological characteristics, a functional consequence may be altered sensory processing and cognitive dysfunction as observed in schizophrenia (Javitt, 2009; Uhlhaas and Singer, 2010). Support for this hypothesis comes from recent data showing that decreased expression of parvalbumin in synaptic terminals leads to increased asynchronous GABA release from PV+ interneurons (Manseau et al. 2010). This asynchronous GABA release in turn reduces the ability of principal neurons to integrate incoming stimuli into precise firing.
Discussion
In summary, there is converging genetic, pharmacological and non-pharmacological evidence that oxidative stress in the early postnatal period affects the normal neurodevelopment of PV+ interneurons and increases the risk of schizophrenia in adulthood. The mechanism by which oxidative stress leads to the enduring dysfunction of PV+ interneurons is unknown. As we previously suggested, acute redox imbalances in brain may affect several neurotransmitter systems, with the glutamatergic synapse being particularly sensitive to oxidative stress (Behrens and Sejnowski, 2009). However glutamatergic synapses are found on many types of cortical neurons, but only the PV+ interneurons show redox sensitivity during postnatal maturation. This increased sensitivity could be due to the fundamental role that glutamatergic transmission has on the maturation of these interneurons. Alternatively, redox-mediated changes in transcription activity, per se, may affect the orchestrated maturational process of PV+ interneurons. Under physiological conditions, nuclear antioxidants (specifically GSH) are critical for maintaining the reducing environment needed to ensure proper gene transcription (Green et al., 2006). A number of transcription factors contain redox-sensitive cysteine residues at their DNA-binding sites (Haddad, 2002), and in most cases, oxidation of these proteins inhibits their DNA-binding activities (Turpaev, 2002). The transcriptional activation responsible for PV+ interneuronal maturation occurring during the early postnatal period may be in fact what makes this interneuronal system so highly redox sensitive. Increased oxidative stress during this postnatal period may halt or alter the maturational process, and in this way lead to a dysfunctional PV+ interneuronal network in adulthood. Detailed understanding of the mechanisms controlling the maturation program of PV+ interneurons and how they are affected by redox dysregulation will shed light into the mechanisms underlying experience-dependent maturation of inhibition, and may unravel the origins of schizophrenia. Moreover, detailed understanding of the electrophysiological consequences that alteration of PV+ interneuron maturation has at the network level, will give insights into the derangements in oscillatory activity observed at the systems level in schizophrenia patients.
PV-expressing interneurons first appear in the human cortex postnatally, between the 3rd and the 5th month of age (Reynolds and Beasley, 2001; Grateron et al., 2003). However, the time course of their maturation is not known. In other species, the period of maturation of PV+ interneuronal circuits in cortex ends at different times during postnatal development, with somatosensory and visual cortex maturing first and prefrontal regions maturing last (Hensch, 2005). In rodent cortex, this maturation coincides with the appearance of α1 GABA(A) containing receptors (Fritschy et al., 1994), which are characteristic of mature PV-synaptic contacts (Klausberger et al., 2002). The development of synchronized oscillatory activity also follows the same time course as the maturation of PV+ interneurons in each cortical region (Doischer et al., 2008). Based on postnatal changes in synchronization of oscillatory activity (Uhlhaas et al., 2009; Uhlhaas et al., 2010) and the switch in GABA(A) receptor subunit expression in primate frontal cortex (Hashimoto et al., 2009), the period of maturation of the cortical PV+ interneuronal system in this brain region could range from childhood to early adulthood in humans. Thus, a better understanding of the sensitive periods to oxidative stress mechanisms that alter the development of the PV+ interneuronal system may shed light on efforts at early intervention for psychosis.
The prognosis of schizophrenia is much improved when the psychotic symptoms are treated early in the course of the illness (Browne et al., 2000; Wyatt and Henter, 2001; Rosen et al., 2002; Melle et al., 2008). Indeed, prolonged periods of untreated psychosis are associated with an unfavorable treatment outcome and reduced quality of life (Wyatt and Henter, 2001), (Browne et al., 2000). If the emergence of schizophrenia is associated with a prolonged oxidative state in the brain, treatment of oxidative stress may be an effective early intervention. Indeed, several studies have shown that blockade of redox pathways prevents the behavioral and neurochemical effects of neonatal NMDA-R antagonists (Wang et al., 2003) and isolation rearing (Schiavone et al., 2009; Moller et al. 2010). The GABAergic inhibitory system, specifically the PV+ interneurons, may be uniquely sensitive to various environmental perturbations during early development and thus may be uniquely responsive to early interventions.
Conclusions
Pathophysiological studies of schizophrenia are beginning to converge on a specific set of inhibitory neurons, the PV-immunoreactive fast-spiking interneurons, which are critically positioned to modulate higher order cognition, and which are clearly implicated in schizophrenia. However, when and how the selective dysfunction of these neurons occurs has not yet been established. Our results using adult and perinatal exposures to NMDA-R antagonist suggest that activation of the IL-6/Nox2 pathway and subsequent increase in oxidative stress may be responsible for the dysfunction and loss of PV+ interneurons. Support for this hypothesis comes from recent data showing that activation of this same oxidative pathway is responsible for the neurochemical and behavioral outcomes in the isolation rearing model. Together, these results point to the period between the second and fourth postnatal week in rodents as a sensitive period where environmental perturbations, by producing increased oxidative stress in brain, may indelibly affect the maturation of the PV-inhibitory circuitry and increase the risk for the development of mental diseases in adulthood.
This interneuron-development hypothesis suggests novel therapeutic interventions for treating schizophrenia, such as brain-targeted anti-IL-6/Nox2 strategies during postnatal manipulations, which could be tested in model systems to determine whether psychosis and cognitive deficits in early adulthood can be prevented.
Acknowledgements
We were supported by the National Institute of Mental Health grant MH091407-01 (SBP and MMB), the Howard Hughes Medical Institute (TJS), and NARSAD (MMB). We would like to thank Dr. Mark Geyer for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abekawa T, Ito K, Nakagawa S, Koyama T. Prenatal exposure to an NMDA receptor antagonist, MK-801 reduces density of parvalbumin-immunoreactive GABAergic neurons in the medial prefrontal cortex and enhances phencyclidine-induced hyperlocomotion but not behavioral sensitization to methamphetamine in postpubertal rats. Psychopharmacology (Berl) 2007;192:303–316. doi: 10.1007/s00213-007-0729-8. [DOI] [PubMed] [Google Scholar]
- Abercrombie M. Estimation of nuclear population from microtome sections. The Anatomical Record. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Goldman PS. Functional development of the dorsolateral prefrontal cortex: an analysis utlizing reversible cryogenic depression. Brain Res. 1978;143:233–249. doi: 10.1016/0006-8993(78)90566-8. [DOI] [PubMed] [Google Scholar]
- Ammassari-Teule M, Sgobio C, Biamonte F, Marrone C, Mercuri NB, Keller F. Reelin haploinsufficiency reduces the density of PV+ neurons in circumscribed regions of the striatum and selectively alters striatal-based behaviors. Psychopharmacology (Berl) 2009;204:511–521. doi: 10.1007/s00213-009-1483-x. [DOI] [PubMed] [Google Scholar]
- Andersen JD, Pouzet B. Spatial memory deficits induced by perinatal treatment of rats with PCP and reversal effect of D-serine. Neuropsychopharmacology. 2004;29:1080–1090. doi: 10.1038/sj.npp.1300394. [DOI] [PubMed] [Google Scholar]
- Ayhan Y, Abazyan B, Nomura J, Kim R, Ladenheim B, Krasnova IN, Sawa A, Margolis RL, Cadet JL, Mori S, Vogel MW, Ross CA, Pletnikov MV. Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders. Mol Psychiatry. 2010 doi: 10.1038/mp.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Beauregard M. Maturation of medial temporal lobe memory functions in rodents, monkeys, and humans. Hippocampus. 1993;3 Spec No: 191–201. [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52:708–715. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Sejnowski TJ. Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology. 2009;57:193–200. doi: 10.1016/j.neuropharm.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dugan LL. Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of schizophrenia. J Neurosci. 2008;28:13957–13966. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI. N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Fone KF, Azmi N, Heidbreder CA, Hagan JJ, Marsden CA. Isolation rearing induces recognition memory deficits accompanied by cytoskeletal alterations in rat hippocampus. Eur J Neurosci. 2006;24:2894–2902. doi: 10.1111/j.1460-9568.2006.05170.x. [DOI] [PubMed] [Google Scholar]
- Boctor SY, Ferguson SA. Neonatal NMDA receptor antagonist treatments have no effects on prepulse inhibition of postnatal day 25 Sprague-Dawley rats. Neurotoxicology. 2009;30:151–154. doi: 10.1016/j.neuro.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Borgers C, Epstein S, Kopell NJ. Gamma oscillations mediate stimulus competition and attentional selection in a cortical network model. Proc Natl Acad Sci U S A. 2008;105:18023–18028. doi: 10.1073/pnas.0809511105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun I, Genius J, Grunze H, Bender A, Möller HJ, Rujescu D. Alterations of hippocampal and prefrontal GABAergic interneurons in an animal model of psychosis induced by NMDA receptor antagonism. Schizophr Res. 2007;97(1–3):254–263. doi: 10.1016/j.schres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Broberg BV, Dias R, Glenthoj BY, Olsen CK. Evaluation of a neurodevelopmental model of schizophrenia--early postnatal PCP treatment in attentional set-shifting. Behav Brain Res. 2008;190:160–163. doi: 10.1016/j.bbr.2008.02.020. [DOI] [PubMed] [Google Scholar]
- Broberg BV, Glenthoj BY, Dias R, Larsen DB, Olsen CK. Reversal of cognitive deficits by an ampakine (CX516) and sertindole in two animal models of schizophrenia--sub-chronic and early postnatal PCP treatment in attentional set-shifting. Psychopharmacology (Berl) 2009;206:631–640. doi: 10.1007/s00213-009-1540-5. [DOI] [PubMed] [Google Scholar]
- Browne S, Clarke M, Gervin M, Waddington JL, Larkin C, O'Callaghan E. Determinants of quality of life at first presentation with schizophrenia. Br J Psychiatry. 2000;176:173–176. doi: 10.1192/bjp.176.2.173. [DOI] [PubMed] [Google Scholar]
- Cabungcal JH, Preissmann D, Delseth C, Cuenod M, Do KQ, Schenk F. Transitory glutathione deficit during brain development induces cognitive impairment in juvenile and adult rats: relevance to schizophrenia. Neurobiol Dis. 2007;26:634–645. doi: 10.1016/j.nbd.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, Seidman LJ, Perkins D, Tsuang M, McGlashan T, Heinssen R. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B, Angulo MC, Ropert N, Tsuzuki K, Hestrin S, Rossier J. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- Cunningham MO, Hunt J, Middleton S, LeBeau FE, Gillies MJ, Davies CH, Maycox PR, Whittington MA, Racca C. Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J Neurosci. 2006;26:2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva NL, Ferreira VM, Carobrez Ade P, Morato GS. Individual housing from rearing modifies the performance of young rats on the elevated plus-maze apparatus. Physiol Behav. 1996;60:1391–1396. doi: 10.1016/s0031-9384(96)00254-5. [DOI] [PubMed] [Google Scholar]
- Dai H, Okuda H, Iwabuchi K, Sakurai E, Chen Z, Kato M, Iinuma K, Yanai K. Social isolation stress significantly enhanced the disruption of prepulse inhibition in mice repeatedly treated with methamphetamine. Ann N Y Acad Sci. 2004;1025:257–266. doi: 10.1196/annals.1316.032. [DOI] [PubMed] [Google Scholar]
- Day-Wilson KM, Jones DN, Southam E, Cilia J, Totterdell S. Medial prefrontal cortex volume loss in rats with isolation rearing-induced deficits in prepulse inhibition of acoustic startle. Neuroscience. 2006;141:1113–1121. doi: 10.1016/j.neuroscience.2006.04.048. [DOI] [PubMed] [Google Scholar]
- de Lecea L, del Rio JA, Soriano E. Developmental expression of parvalbumin mRNA in the cerebral cortex and hippocampus of the rat. Brain Res Mol Brain Res. 1995;32:1–13. doi: 10.1016/0169-328x(95)00056-x. [DOI] [PubMed] [Google Scholar]
- Del-Bel EA, Joca SR, Padovan CM, Guimaraes FS. Effects of isolation-rearing on serotonin-1A and M1-muscarinic receptor messenger RNA expression in the hipocampal formation of rats. Neurosci Lett. 2002;332:123–126. doi: 10.1016/s0304-3940(02)00933-3. [DOI] [PubMed] [Google Scholar]
- Di Cristo G, Wu C, Chattopadhyaya B, Ango F, Knott G, Welker E, Svoboda K, Huang ZJ. Subcellular domain-restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat Neurosci. 2004;7:1184–1186. doi: 10.1038/nn1334. [DOI] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, Cuenod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I, Bartos M. Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci. 2008;28:12956–12968. doi: 10.1523/JNEUROSCI.2890-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Bois TM, Newell KA, Han M, Deng C, Huang XF. Perinatal PCP treatment alters the developmental expression of prefrontal and hippocampal muscarinic receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:37–40. doi: 10.1016/j.pnpbp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Ali SS, Shekhtman G, Roberts AJ, Lucero J, Quick KL, Behrens MM. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One. 2009;4:e5518. doi: 10.1371/journal.pone.0005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einon DF, Morgan MJ. A critical period for social isolation in the rat. Dev Psychobiol. 1977;10:123–132. doi: 10.1002/dev.420100205. [DOI] [PubMed] [Google Scholar]
- Fantin M, Marti M, Auberson YP, Morari M. NR2A and NR2B subunit containing NMDA receptors differentially regulate striatal output pathways. J Neurochem. 2007;103:2200–2211. doi: 10.1111/j.1471-4159.2007.04966.x. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Lujan R, Lloyd K, Lerma J, Marin O, Rico B. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- Fejgin K, Palsson E, Wass C, Svensson L, Klamer D. Nitric oxide signaling in the medial prefrontal cortex is involved in the biochemical and behavioral effects of phencyclidine. Neuropsychopharmacology. 2008;33:1874–1883. doi: 10.1038/sj.npp.1301587. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–1102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Francois J, Ferrandon A, Koning E, Angst MJ, Sandner G, Nehlig A. Selective reorganization of GABAergic transmission in neonatal ventral hippocampal-lesioned rats. Int J Neuropsychopharmacol. 2009;12:1097–1110. doi: 10.1017/S1461145709009985. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Wilkinson LS, Humby T, Robbins TW. Isolation rearing of rats produces a deficit in prepulse inhibition of acoustic startle similar to that in schizophrenia. Biol Psychiatry. 1993;34:361–372. doi: 10.1016/0006-3223(93)90180-l. [DOI] [PubMed] [Google Scholar]
- Goldberg EM, Jeong HY, Kruglikov I, Tremblay R, Lazarenko RM, Rudy B. Rapid Developmental Maturation of Neocortical FS Cell Intrinsic Excitability. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Tamas G, Aronov D, Yuste R. Calcium microdomains in aspiny dendrites. Neuron. 2003;40:807–821. doi: 10.1016/s0896-6273(03)00714-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grateron L, Cebada-Sanchez S, Marcos P, Mohedano-Moriano A, Insausti AM, Munoz M, Arroyo-Jimenez MM, Martinez-Marcos A, Artacho-Perula E, Blaizot X, Insausti R. Postnatal development of calcium-binding proteins immunoreactivity (parvalbumin, calbindin, calretinin) in the human entorhinal cortex. J Chem Neuroanat. 2003;26:311–316. doi: 10.1016/j.jchemneu.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Green RM, Graham M, O'Donovan MR, Chipman JK, Hodges NJ. Subcellular compartmentalization of glutathione: correlations with parameters of oxidative stress related to genotoxicity. Mutagenesis. 2006;21:383–390. doi: 10.1093/mutage/gel043. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Sandell J, Bovet P, Chappuis C, Conus P, Deppen P, Preisig M, Ruiz V, Steullet P, Tosic M, Werge T, Cuenod M, Do KQ. Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A. 2007;104:16621–16626. doi: 10.1073/pnas.0706778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gysin R, Kraftsik R, Boulat O, Bovet P, Conus P, Emily CK, Polari A, Steullet P, Preisig M, Teichmann T, Cuenod M, Do KQ. Genetic dysregulation of glutathione synthesis predicts alteration of plasma thiol redox status in schizophrenia. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3463. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Haddad JJ. Antioxidant and prooxidant mechanisms in the regulation of redox(y)- sensitive transcription factors. Cell Signal. 2002;14:879–897. doi: 10.1016/s0898-6568(02)00053-0. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GF, Pert A. The effects of social isolation on the forced swim test in Fawn hooded and Wistar rats. J Neurosci Methods. 1998;79:47–51. doi: 10.1016/s0165-0270(97)00155-6. [DOI] [PubMed] [Google Scholar]
- Harte MK, Powell SB, Swerdlow NR, Geyer MA, Reynolds GP. Deficits in parvalbumin and calbindin immunoreactive cells in the hippocampus of isolation reared rats. J Neural Transm. 2007;114:893–898. doi: 10.1007/s00702-007-0627-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M, Gonzalez-Burgos G, Lewis DA. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 2009;65:1015–1023. doi: 10.1016/j.biopsych.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Jaaro-Peled H, Seshadri S, Oishi K, Hookway C, Kong S, Wu D, Xue R, Andrade M, Tankou S, Mori S, Gallagher M, Ishizuka K, Pletnikov M, Kida S, Sawa A. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ. Activity-dependent development of inhibitory synapses and innervation pattern: role of GABA signalling and beyond. J Physiol. 2009;587:1881–1888. doi: 10.1113/jphysiol.2008.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Scanziani M. It's about time for thalamocortical circuits. Nat Neurosci. 2007;10:400–402. doi: 10.1038/nn0407-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PS. Neonatal treatment with a competitive NMDA antagonist results in response-specific disruption of conditioned fear in preweanling rats. Psychopharmacology (Berl) 2006;185:179–187. doi: 10.1007/s00213-005-0291-1. [DOI] [PubMed] [Google Scholar]
- Ibi D, Nagai T, Koike H, Kitahara Y, Mizoguchi H, Niwa M, Jaaro-Peled H, Nitta A, Yoneda Y, Nabeshima T, Sawa A, Yamada K. Combined effect of neonatal immune activation and mutant DISC1 on phenotypic changes in adulthood. Behav Brain Res. 2010;206:32–37. doi: 10.1016/j.bbr.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Sensory processing in schizophrenia: neither simple nor intact. Schizophr Bull. 2009;35:1059–1064. doi: 10.1093/schbul/sbp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins TA, Harte MK, Reynolds GP. Effect of subchronic phencyclidine administration on sucrose preference and hippocampal parvalbumin immunoreactivity in the rat. Neurosci Lett. 2010;471(3):144–147. doi: 10.1016/j.neulet.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Jenkins TA, Harte MK, Stenson G, Reynolds GP. Neonatal lipopolysaccharide induces pathological changes in parvalbumin immunoreactivity in the hippocampus of the rat. Behav Brain Res. 2009;205:355–359. doi: 10.1016/j.bbr.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krech D, Rosenzweig MR, Bennett EL. Relations between chemistry and problem-solving among rats raised in enriched and impoverished environments. J Comp Physiol Psychol. 1962;55:801–807. doi: 10.1037/h0044220. [DOI] [PubMed] [Google Scholar]
- Krimer LS, Goldman-Rakic PS. Prefrontal microcircuits: membrane properties and excitatory input of local, medium, and wide arbor interneurons. J Neurosci. 2001;21:3788–3796. doi: 10.1523/JNEUROSCI.21-11-03788.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, Bovet P, Bush AI, Conus P, Copolov D, Fornari E, Meuli R, Solida A, Vianin P, Cuenod M, Buclin T, Do KQ. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- Lema Tome CM, Miller R, Bauer C, Smith C, Blackstone K, Leigh A, Busch J, Turner CP. Decline in age-dependent, MK801-induced injury coincides with developmental switch in parvalbumin expression: somatosensory and motor cortex. Dev Psychobiol. 2008;50:665–679. doi: 10.1002/dev.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Cruz D, Eggan S, Erickson S. Postnatal development of prefrontal inhibitory circuits and the pathophysiology of cognitive dysfunction in schizophrenia. Ann N Y Acad Sci. 2004;1021:64–76. doi: 10.1196/annals.1308.008. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Lerman DN, Khaing ZZ, Weinberger DR. The neonatal ventral hippocampal lesion model of schizophrenia: effects on dopamine and GABA mRNA markers in the rat midbrain. Eur J Neurosci. 2003;18:3097–3104. doi: 10.1111/j.1460-9568.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav Brain Res. 2009;204:306–312. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manseau F, Marinelli S, Mendez P, Schwaller B, Prince DA, Huguenard JR, Bacci A. Desynchronization of neocortical networks by asynchronous release of GABA at autaptic and synaptic contacts from fast-spiking interneurons. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000492. pii: e1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrone MC, Marinelli S, Biamonte F, Keller F, Sgobio CA, Ammassari-Teule M, Bernardi G, Mercuri NB. Altered cortico-striatal synaptic plasticity and related behavioural impairments in reeler mice. Eur J Neurosci. 2006;24:2061–2070. doi: 10.1111/j.1460-9568.2006.05083.x. [DOI] [PubMed] [Google Scholar]
- Meechan DW, Tucker ES, Maynard TM, LaMantia AS. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc Natl Acad Sci U S A. 2009;106:16434–16445. doi: 10.1073/pnas.0905696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melle I, Larsen TK, Haahr U, Friis S, Johannesen JO, Opjordsmoen S, Rund BR, Simonsen E, Vaglum P, McGlashan T. Prevention of negative symptom psychopathologies in first-episode schizophrenia: two-year effects of reducing the duration of untreated psychosis. Arch Gen Psychiatry. 2008;65:634–640. doi: 10.1001/archpsyc.65.6.634. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav Brain Res. 2009;204:322–334. doi: 10.1016/j.bbr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Moller M, Du Preez JL, Emsley R, Harvey BH. Isolation rearing-induced deficits in sensorimotor gating and social interaction in rats are related to cortico-striatal oxidative stress, and reversed by sub-chronic clozapine administration. Eur Neuropsychopharmacol. 2010 doi: 10.1016/j.euroneuro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Enomoto T, Nabeshima T. Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem Int. 2007;51:173–184. doi: 10.1016/j.neuint.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Nakatani-Pawlak A, Yamaguchi K, Tatsumi Y, Mizoguchi H, Yoneda Y. Neonatal phencyclidine treatment in mice induces behavioral, histological and neurochemical abnormalities in adulthood. Biol Pharm Bull. 2009;32:1576–1583. doi: 10.1248/bpb.32.1576. [DOI] [PubMed] [Google Scholar]
- Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2010;20:724–744. doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB. Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci. 2009;29:7040–7052. doi: 10.1523/JNEUROSCI.0105-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Owens DF, Liu X, Kriegstein AR. Changing properties of GABA(A) receptor-mediated signaling during early neocortical development. J Neurophysiol. 1999;82:570–583. doi: 10.1152/jn.1999.82.2.570. [DOI] [PubMed] [Google Scholar]
- Pascual R, Zamora-Leon SP, Valero-Cabre A. Effects of postweaning social isolation and re-socialization on the expression of vasoactive intestinal peptide (VIP) and dendritic development in the medial prefrontal cortex of the rat. Acta Neurobiol Exp (Wars) 2006;66:7–14. doi: 10.55782/ane-2006-1582. [DOI] [PubMed] [Google Scholar]
- Penschuck S, Flagstad P, Didriksen M, Leist M, Michael-Titus AT. Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. Eur J Neurosci. 2006;23:279–284. doi: 10.1111/j.1460-9568.2005.04536.x. [DOI] [PubMed] [Google Scholar]
- Peters YM, O'Donnell P. Social isolation rearing affects prefrontal cortical response to ventral tegmental area stimulation. Biol Psychiatry. 2005;57:1205–1208. doi: 10.1016/j.biopsych.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S, Singer P, Feldon J, Yee BK. The postweaning social isolation in C57BL/6 mice: preferential vulnerability in the male sex. Psychopharmacology (Berl) 2008;197:613–628. doi: 10.1007/s00213-008-1081-3. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Powell SB. Models of neurodevelopmental abnormalities in schizophrenia. In: Swerdlow N, editor. Behavioral Neurobiology of Schizophrenia and Its Treatment. Curr Topics Behav Neurosci. Heidelberg: Springer; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Geyer MA. Developmental markers of psychiatric disorders as identified by sensorimotor gating. Neurotox Res. 2002;4:489–502. doi: 10.1080/10298420290030578. [DOI] [PubMed] [Google Scholar]
- Powell SB, Swerdlow NR, Pitcher LK, Geyer MA. Isolation rearing-induced deficits in prepulse inhibition and locomotor habituation are not potentiated by water deprivation. Physiol Behav. 2002;77:55–64. doi: 10.1016/s0031-9384(02)00817-x. [DOI] [PubMed] [Google Scholar]
- Preece MA, Dalley JW, Theobald DE, Robbins TW, Reynolds GP. Region specific changes in forebrain 5-hydroxytryptamine1A and 5-hydroxytryptamine2A receptors in isolation-reared rats: an in vitro autoradiography study. Neuroscience. 2004;123:725–732. doi: 10.1016/j.neuroscience.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Radonjic NV, Knezevic ID, Vilimanovich U, Kravic-Stevovic T, Marina LV, Nikolic T, Todorovic V, Bumbasirevic V, Petronijevic ND. Decreased glutathione levels and altered antioxidant defense in an animal model of schizophrenia: long-term effects of perinatal phencyclidine administration. Neuropharmacology. 2010;58:739–745. doi: 10.1016/j.neuropharm.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Beasley CL. GABAergic neuronal subtypes in the human frontal cortex-- development and deficits in schizophrenia. J Chem Neuroanat. 2001;22:95–100. doi: 10.1016/s0891-0618(01)00113-2. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Abdul-Monim Z, Neill JC, Zhang ZJ. Calcium binding protein markers of GABA deficits in schizophrenia--postmortem studies and animal models. Neurotox Res. 2004;6:57–61. doi: 10.1007/BF03033297. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Roopun AK, Cunningham MO, Racca C, Alter K, Traub RD, Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–973. doi: 10.1093/schbul/sbn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JL, Woods SW, Miller TJ, McGlashan TH. Prospective observations of emerging psychosis. J Nerv Ment Dis. 2002;190:133–141. doi: 10.1097/00005053-200203000-00001. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Bender A, Keck M, Hartmann AM, Ohl F, Raeder H, Giegling I, Genius J, McCarley RW, Möller HJ, Grunze H. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biol Psychiatry. 2006;59(8):721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Sakaue M, Ago Y, Baba A, Matsuda T. The 5-HT1A receptor agonist MKC-242 reverses isolation rearing-induced deficits of prepulse inhibition in mice. Psychopharmacology (Berl) 2003;170:73–79. doi: 10.1007/s00213-003-1515-x. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer JF, Bartos M. Recruitment of early postnatal parvalbumin-positive hippocampal interneurons by GABAergic excitation. J Neurosci. 2010;30:110–115. doi: 10.1523/JNEUROSCI.4125-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaccianoce S, Del Bianco P, Paolone G, Caprioli D, Modafferi AM, Nencini P, Badiani A. Social isolation selectively reduces hippocampal brain-derived neurotrophic factor without altering plasma corticosterone. Behav Brain Res. 2006;168:323–325. doi: 10.1016/j.bbr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Schiavone S, Sorce S, Dubois-Dauphin M, Jaquet V, Colaianna M, Zotti M, Cuomo V, Trabace L, Krause KH. Involvement of NOX2 in the development of behavioral and pathologic alterations in isolated rats. Biol Psychiatry. 2009;66:384–392. doi: 10.1016/j.biopsych.2009.04.033. [DOI] [PubMed] [Google Scholar]
- Schrijver NC, Wurbel H. Early social deprivation disrupts attentional, but not affective, shifts in rats. Behav Neurosci. 2001;115:437–442. [PubMed] [Google Scholar]
- Shen S, Lang B, Nakamoto C, Zhang F, Pu J, Kuan SL, Chatzi C, He S, Mackie I, Brandon NJ, Marquis KL, Day M, Hurko O, McCaig CD, Riedel G, St Clair D. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci. 2008;28:10893–10904. doi: 10.1523/JNEUROSCI.3299-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Sircar R, Rudy JW. Repeated neonatal phencyclidine treatment impairs performance of a spatial task in juvenile rats. Ann N Y Acad Sci. 1998;844:303–309. [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorce S, Schiavone S, Tucci P, Colaianna M, Jaquet V, Cuomo V, Dubois-Dauphin M, Trabace L, Krause KH. The NADPH oxidase NOX2 controls glutamate release: a novel mechanism involved in psychosis-like ketamine responses. J Neurosci. 2010;30:11317–11325. doi: 10.1523/JNEUROSCI.1491-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Transient N-methyl-D-aspartate receptor blockade in early development causes lasting cognitive deficits relevant to schizophrenia. Biol Psychiatry. 2005;57:433–436. doi: 10.1016/j.biopsych.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Steullet P, Lavoie S, Kraftsik R, Guidi R, Gysin R, Cuenod M, Do KQ. A glutathione deficit alters dopamine modulation of L-type calcium channels via D2 and ryanodine receptors in neurons. Free Radic Biol Med. 2008;44:1042–1054. doi: 10.1016/j.freeradbiomed.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Steullet P, Cabungcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, Cuenod M, Do KQ. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. J Neurosci. 2010;30:2547–2558. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QQ. The missing piece in the 'use it or lose it' puzzle: is inhibition regulated by activity or does it act on its own accord? Rev Neurosci. 2007;18:295–310. doi: 10.1515/revneuro.2007.18.3-4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka DH, Maekawa K, Yanagawa Y, Obata K, Murakami F. Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex. Development. 2006;133:2167–2176. doi: 10.1242/dev.02382. [DOI] [PubMed] [Google Scholar]
- Thomsen MS, Hansen HH, Mikkelsen JD. Opposite effect of phencyclidine on activity-regulated cytoskeleton-associated protein (Arc) in juvenile and adult limbic rat brain regions. Neurochem Int. 2010;56:270–275. doi: 10.1016/j.neuint.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Tiesinga P, Sejnowski TJ. Cortical enlightenment: are attentional gamma oscillations driven by ING or PING? Neuron. 2009;63:727–732. doi: 10.1016/j.neuron.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Tosic M, Ott J, Barral S, Bovet P, Deppen P, Gheorghita F, Matthey ML, Parnas J, Preisig M, Saraga M, Solida A, Timm S, Wang AG, Werge T, Cuenod M, Do KQ. Schizophrenia and oxidative stress: glutamate cysteine ligase modifier as a susceptibility gene. Am J Hum Genet. 2006;79:586–592. doi: 10.1086/507566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Hashimoto T, Sesack SR, Kloc M, Lewis DA, O’Donnell P. A neonatal ventral hippocampal lesion causes functional deficits in adult prefrontal cortical interneurons. J Neurosci. 2008;28:12691–12699. doi: 10.1523/JNEUROSCI.4166-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpaev KT. Reactive oxygen species and regulation of gene expression. Biochemistry (Mosc) 2002;67:281–292. doi: 10.1023/a:1014819832003. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn Sci. 2010;14:72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci U S A. 2009;106:9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varty GB, Powell SB, Lehmann-Masten V, Buell MR, Geyer MA. Isolation rearing of mice induces deficits in prepulse inhibition of the startle response. Behav Brain Res. 2006;169:162–167. doi: 10.1016/j.bbr.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Wang C, McInnis J, West JB, Bao J, Anastasio N, Guidry JA, Ye Y, Salvemini D, Johnson KM. Blockade of phencyclidine-induced cortical apoptosis and deficits in prepulse inhibition by M40403, a superoxide dismutase mimetic. J Pharmacol Exp Ther. 2003;304:266–271. doi: 10.1124/jpet.102.041798. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008;33:2442–2455. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology. 2009;34:2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Gao WJ. Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex. J Physiol. 2010;588:2823–2838. doi: 10.1113/jphysiol.2010.187591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dye CA, Sohal V, Long JE, Estrada RC, Roztocil T, Lufkin T, Deisseroth K, Baraban SC, Rubenstein JL. Dlx5 and Dlx6 regulate the development of parvalbumin-expressing cortical interneurons. J Neurosci. 2010;30:5334–5345. doi: 10.1523/JNEUROSCI.5963-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzony K, Fijal K, Mackowiak M, Chocyk A, Zajaczkowski W. Impact of postnatal blockade of N-methyl-D-aspartate receptors on rat behavior: a search for a new developmental model of schizophrenia. Neuroscience. 2008;153:1370–1379. doi: 10.1016/j.neuroscience.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Feldon J. Environmental animal models for sensorimotor gating deficiencies in schizophrenia: a review. Psychopharmacology (Berl) 2001;156:305–326. doi: 10.1007/s002130100800. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Wen L, Lu YS, Zhu XH, Li XM, Woo RS, Chen YJ, Yin DM, Lai C, Terry AV, Jr, Vazdarjanova A, Xiong WC, Mei L. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Buhler KG, Lavecchia KL, Johnson KM. Pharmacological challenge reveals long-term effects of perinatal phencyclidine on delayed spatial alternation in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:867–873. doi: 10.1016/S0278-5846(03)00146-5. [DOI] [PubMed] [Google Scholar]
- Wilson FA, O'Scalaidhe SP, Goldman-Rakic PS. Functional synergism between putative gamma-aminobutyrate-containing neurons and pyramidal neurons in prefrontal cortex. Proc Natl Acad Sci U S A. 1994;91:4009–4013. doi: 10.1073/pnas.91.9.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IK, Upton N, Marsden CA. Resocialisation of isolation-reared rats does not alter their anxiogenic profile on the elevated X-maze model of anxiety. Physiol Behav. 1991;50:1129–1132. doi: 10.1016/0031-9384(91)90572-6. [DOI] [PubMed] [Google Scholar]
- Wyatt RJ, Henter I. Rationale for the study of early intervention. Schizophr Res. 2001;51:69–76. doi: 10.1016/s0920-9964(01)00242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010;518:389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Behrens MM, Lisman JE. Prolonged exposure to NMDAR antagonist suppresses inhibitory synaptic transmission in prefrontal cortex. J Neurophysiol. 2008;100:959–965. doi: 10.1152/jn.00079.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Sun QQ. Development of NMDA NR2 subunits and their roles in critical period maturation of neocortical gabaergic interneurons. Dev Neurobiol. 2010 doi: 10.1002/dneu.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo DY, Wu YL, Yao WX, Cao Y, Wu CF, Tanaka M. Effect of MK-801 and ketamine on hydroxyl radical generation in the posterior cingulate and retrosplenial cortex of free-moving mice, as determined by in vivo microdialysis. Pharmacol Biochem Behav. 2007;86:1–7. doi: 10.1016/j.pbb.2006.05.010. [DOI] [PubMed] [Google Scholar]