Abstract
Objective
To study the longitudinal rate of (and sensitivity to) change of knee cartilage thickness across defined stages of radiographic osteoarthritis (ROA), specifically healthy knees and knees with end-stage ROA.
Methods
One knee of 831 Osteoarthritis Initiative (OAI) participants was examined: 112 healthy, without ROA or risk factors for knee OA, and 719 ROA knees: 310 calculated Kellgren Lawrence [cKLG] grade 2, 300 cKLG3, and 109 cKLG4. Subregional change in thickness was assessed after segmentation of weight-bearing femorotibial cartilage at baseline and at one year from coronal MRI. Regional and ordered values (OV) of change were compared by baseline ROA status.
Results
Healthy knees displayed small changes in plates and subregions (±0.7%; standardized response mean [SRM] ±0.15), with OVs being symmetrically distributed around zero. In cKLG2 knees, changes in cartilage thickness were small (≤1%; minimal SRM -0.22) and not significantly different from healthy knees. Knees with cKLG3 showed substantial loss of cartilage thickness (up to -2.5%; minimal SRM -0.35), with OV changes being significantly (p<0.05) greater than those in healthy knees. cKLG4 knees displayed the largest rate of loss across ROA grades (up to -3.9%; minimal SRM -0.51), with OV changes also significantly (p<0.05) greater than in healthy knees.
Conclusion
MRI-based cartilage thickness showed high rates of loss in knees with moderate and end-stage ROA, and small rates (indistinguishable from healthy knees) in mild ROA. From the perspective of sensitivity to change, end-stage ROA knees need not be excluded from longitudinal studies using MRI cartilage morphology as an endpoint.
Keywords: End-stage, Radiographic osteoarthritis, sensitivity to change, cartilage thickness, magnetic resonance imaging
Introduction
Joint space width (JSW) in weight-bearing radiographs has been commonly used as outcome measures of progression in osteoarthritis (OA) (1-3) and represents an endpoint for disease modification that is accepted by regulatory agencies. Radiograhic studies for identification of OA risk factors and treatment efficacy have generally excluded knees with late- or end-stage radiographic OA (ROA) at baseline (4-7), since no further reduction in JSW can be expected when its value approaches zero (bone to bone contact, Kellgren-Lawrence grade [KLG] 4). Hence, no information on the structural progression of end-stage ROA knees is currently available. However, KLG4 knees may be of particular interest, since they are likely to encounter total knee arthroplasty (TKA) in the near future, with TKA representing an important clinical endpoint (8).
The Osteoarthritis Initiative (OAI) is an ongoing multi-center study (http://www.oai.ucsf.edu) targeted at identifying sensitive (imaging) biomarkers for onset and progression of knee OA. 4674 participants with all grades of ROA and 122 healthy knees without symptoms, signs and risk factors of knee OA are studied using radiographs (3,9) and magnetic resonance imaging (MRI) (10). MRI can be used to obtain technically validated, quantitative, structural outcomes in OA, including cartilage area, (subregional) cartilage thickness, and volume, (11-15). Prior work showed that high rates of cartilage loss, measured quantitatively with MRI, were associated with an increased risk of having future TKA, whereas radiographic outcomes were not (16).
Although studies have shown that substantial amounts of cartilage are still present at end-stage ROA (17) or when a TKA is performed (12,18), longitudinal studies using MRI (19-30) have generally avoided the inclusion of such knees. No prior study has compared the rate of (and sensitivity to) change in cartilage thickness in end-stage ROA knees with that in knees with less severe ROA grades, or that in healthy reference OAI knees with that in ROA OAI knees.
The objective of this study was therefore to study the one year rate of (and sensitivity to) change in femorotibial cartilage thickness in a large subset of OAI knees across a wide range of radiographic disease stages (17), specifically healthy reference knees and knees with end-stage ROA. We tested the hypotheses that end-stage ROA knees display a rate of change similar to knees with less severe stages of ROA, and that all ROA knees display larger rates of change than healthy knees. The overarching question was whether, from a perspective of sensitivity to change, knees with end-stage ROA need to be excluded from longitudinal studies of OA progression. Answering the above questions can inform clinical trials with regard to sample size calculations per specific radiographic disease stages, including end-stage ROA.
Methods
OAI cohort and MRI sequences
OAI participants were 45-79 years old, with or at risk of symptomatic knee OA in at least one knee. General exclusion criteria were rheumatoid or inflammatory arthritis, bilateral end-stage knee OA, inability to walk without aids, and MRI contraindications. The OAI also includes “non-exposed” healthy reference participants (n=122) without clinical or radiographic signs of knee OA, and free of exposure to potential risk factors of knee OA. The following inclusion criteria applied to this cohort: a) no pain, aching or stiffness in either knee in the past year; b) no radiographic femorotibial OA (OARSI osteophyte grade 0 and joint space narrowing grade 0) in either knee using the clinical site readings of the baseline bilateral fixed flexion radiographs (3); c) no risk factors for OA, including obesity, knee injury, knee surgery, a family history of TKA in a biological parent or sibling, Heberden's nodes (defined as self-reported bony enlargements), or repetitive knee bending (defined as current daily activity).
Fixed flexion radiographs (3,9) and 3 Tesla MRIs (using Magnetom Trio magnets [Siemens Erlangen, Germany] and quadrature transmit-receive knee coils [USA Instruments, Aurora, OH]) were acquired according to a standardized protocol (http://www.oai.ucsf.edu/datarelease/) (10,31). The current longitudinal analysis was performed in a subsample of OAI knees analyzed by a consortium of industry partners (see acknowledgments), the OAI coordinating center at University of California San Francisco (UCSF), and an image analysis company (Chondrometrics GmbH)(17). The analysis only included the knees that were imaged using the double oblique coronal FLASH water excitation sequence (10).
Radiographic grading and sample selection
The baseline calculated KL grades (cKLG), derived from OARSI atlas osteophyte and JSN grades (32,33) (public-use data set 0.2.2) were used in this study, because central readings were only available for a small part of the cohort. The cKL grades were assigned by centrally trained and certified readers (one to three validated radiologists in each of four clinical sites. Readers assessed each knee for presence/absence of definite marginal osteophytes (OARSI atlas grade1-3, any medial and lateral, tibial and femoral osteophytes), and medial and lateral JSN grades1 (OARSI atlas grades1-2) or 2 (OARSI atlas grade3). The cKLG was defined as:
cKLG0 = grade0 for both osteophytes and JSN scores
cKLG1 = questionable osteophyte and grade0-1 JSN (or grade 0 osteophyte and grade1 JSN)
cKLG2 = definite osteophyte and grade0 JSN (or no / questionable osteophyte and grade2 JSN)
cKLG3 = definite osteophyte and grade1 JSN
cKLG4 = definite osteophyte and grade2 JSN.
Grade1 JSN in the OAI corresponds to “definite mild” and not to “possible or uncertain” JSN. Based on previous recommendations (34,35), the OAI therefore graded knees with definite osteophytes and OARSI JSN grade1 and 2 as cKLG3. Six knees without definite osteophytes but with OARSI JSN grade3 were also classified as cKLG2 by the OAI (see above), but as these represented a distinct (and very small) group they were eliminated from the analysis. OARSI JSN grade3 (OAI JSN grade2 = cKLG 4) ranged from a 67% to a 100% reduction in JSW (100%=bone-on-bone contact).
A sample of 831 knees (825 right, six left, one per participant) was studied: 112 from the non-exposed healthy reference sample, 310 with cKLG2, 300 with cKLG3, and 109 with cKLG4. Of the 122 participants in the non-exposed healthy reference sample (public use data 0.F.1 and 1.F.1), 112 had usable coronal FLASH acquisitions at baseline and year-1 follow-up. The selection criteria of the entire cohort studied here has been reported in detail in a recent (cross-sectional) report (17). In brief, the ROA subgroups were comprised of knees from three samples:
139 knees from an age- and gender-stratified sub sample from the progression subcohort (first release; public-use dataset 0.B.1 and 1.B.1) (26,27). This subsample included participants with frequent symptoms and ROA in at least one knee: 52 knees were cKLG2, 68 cKLG3, and 19 cKLG4. Note that these radiographic grades deviate from the “centrally read” KLG readings used in previous publications (26,27) for reasons explained above. Three cKLG0 and 15 cKLG1 knees (knees contra-lateral to those that qualified the participant to be in the progression subcohort), and two knees with unusable FLASH acquisitions were excluded from the current analysis.
490 knees with cKLG2 or 3 (clinical site readings), selected by ascending OAI IDs from the first half of the OAI cohort (public-use data set 0.C.1 and 1.C.1), but excluding those described under a). Only knees with definite ROA, e.g. KLG2 or higher (but not cKLG1), were selected, as these are of particular relevance for clinical trials. At the time of sample selection, the imaging data of the 2nd half of the cohort were not yet publicly available.
90 knees from participants with cKLG4. These represent all cKLG4 knees from the first half of the OAI cohort with usable baseline and year-1 follow-up coronal FLASH acquisitions.
MRI analysis
The baseline and year-1 follow up MR images (10,31) were shipped from the OAI coordinating center at UCSF to the image analysis center (Chondrometrics GmbH, Ainring, Germany). After quality control of appropriate coverage, orientation and absence of artefacts (M.H.), segmentation of paired baseline and year-1 follow-up images was performed by seven operators, each with more than three years of experience in cartilage segmentation. The operators were blinded to the order of the acquisition and to the baseline radiographic status. The total area of subchondral bone (tAB) and cartilage surface (AC) were segmented manually in the medial (MT) and lateral tibiae (LT), and in the weight-bearing (central) part of the medial (cMF) and lateral femoral condyles (cLF). Each of these regions of interest was addressed as a cartilage “plate” throughout this manuscript. From MT and cMF, aggregate values were obtained for the medial femorotibial compartment (MFTC), and from LT and cLF aggregate values for the lateral femorotibial compartment (LFTC). The posterior aspects of the femoral condyles were not included, because segmentation in these areas is not supported by the coronal imaging protocol and because the sensitivity to change in these regions was shown to be less than in the tibia and in the weight-bearing femoral regions (29). To minimize segmentation errors and deviations between readers, all segmentations were quality controlled by one expert (S.M.). tAB or AC entries were corrected by the operators, if found necessary by the expert. The mean cartilage thickness over the tAB (ThCtAB.Me), including denuded areas but excluding osteophytes, was determined in all cartilage plates. The subregional thickness was determined in the central, external, internal, anterior, and posterior aspect of MT and LT, and in the central, external, and internal aspects of cMF and cLF, as described previously (13). The test-retest precision of the methodology has also been previously reported (13,36-38).
Statistical analysis
The statistical analyses were performed using Version 17 of SPSS (SPSS Inc., Chicago, MI). The mean change (MC) and standard deviation of change in ThCtAB (μm) between baseline and year-1 follow-up were determined as a measure of progression. Percent changes were derived by relating the MC in a group to the mean ThCtAB at baseline for the same group. The SRM (defined as mean change divided by the standard deviation of change) was used as a measure of the sensitivity to change. An extended ordered values (OV) approach (39,40) was applied comprising all 16 subregions in the femorotibial joint (5 in MT and LT, and 3 in cMF and cLF, respectively). This approach sorts subregional changes (in ThCtAB) in each subject in ascending order, i.e. the subregion in each knee showing the largest decrease in ThCtAB is assigned to OV1, the subregion showing the second largest decrease to OV2, and so forth, with the subregion showing the smallest decrease (or largest increase) assigned to OV16.
OV1 was defined as the primary outcome, to evaluate whether the rate of subregional cartilage loss differed between KL grades, independent of where it was located in the femorotibial joint. Exploratory and comparative tests were also performed for cartilage compartments, plates, subregions, and OV2 through 16.
To test for potential differences in the rate of change (OV1) between healthy knees and knees with different cKLGs the Kruskal-Wallis test was applied. When this test revealed significant differences (p<0.05), the Mann-Whitney-U test, and the t-test for unequal variances (Welch's test) were used to compare rates of change of cKLG2, cKLG3 and cKLG4 knees with non-exposed healthy knees, respectively. To correct for multiplicity of these three parallel tests (cKLG2, cKLG3 and cKLG4 knees versus healthy), the required p-value was set to <0.0167 to maintain a global error level of 5%. For exploratory reasons, the same series of tests were used for the OVs 2-16 and region-based results, with no adjustment for multiple testing of several OVs, plates and subregions.
Results
The demographic data of the non-exposed control participants and those of the participants with different cKL grades are shown in Table 1.
Table 1. Demographic data (mean ± standard deviation) for the 831 study participants, including non-exposed controls and participants with knees with definite radiographic osteoarthritis.
| Non-exposed controls (n=112) | cKLG2 (n=310) | cKLG3 (n=300) | cKLG4 (n=109) | |
|---|---|---|---|---|
| Sex (M/W) | 43/69 | 113/197 | 113/187 | 55/54 |
| Age (years) | 55.0±7.7 | 60.5±9.0 | 64.2±9.4 | 63.7±9.0 |
| Height (cm) | 168±86 | 166±87 | 168±94 | 169±89 |
| Weight (kg) | 68.6±11.8 | 81.8±15.3 | 84.4±16.8 | 85.3±15.3 |
| BMI (kg/m2) | 24.3±3.0 | 29.5±4.6 | 29.8±4.7 | 29.7±4.9 |
cKLG = calculated Kellgren-Lawrence grade at baseline, obtained at the clinical sites from osteophyte and joint space narrowing (JSN) readings in fixed flexion radiographs based on the OARSI atlas; M = men, W = women, BMI = body mass index.
In the non-exposed control knees, the rate of changes across the OVs were symmetrically distributed around zero (OV1=-121μm; OV16=+124 μm; 8 OVs with negative and 8 with positive changes; Table 2). In anatomical regions (Table 3), the changes were small and within ±0.3% for compartments and plates, and within ±0.7% for the subregions.
Table 2. Ordered values (OV) of longitudinal subregional cartilage thickness changes over one year as measured with magnetic resonance imaging (MRI).
| Non-exposed Controls (n=112) | cKLG2 (n=310) | cKLG3 (n=300) | cKLG4 (n=109) | Kruskal-Wallis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MC | SD | MC% | MC | SD | MC% | MC | SD | MC% | MC | SD | MC% | ||
| OV 1 | -121 | 81 | -6.2 | -137 | 104 | -6.6 | -180* | 144 | -9.4 | -200* | 117 | -12.5 | 5.54E-014 |
| OV 2 | -80 | 45 | -4.3 | -98 | 80 | -5.3 | -126* | 95 | -7.3 | -139* | 86 | -8.7 | 6.03E-013 |
| OV 3 | -60 | 42 | -3.3 | -69 | 50 | -3.7 | -95* | 78 | -5.4 | -108* | 71 | -7.3 | 4.01E-013 |
| OV 4 | -46 | 38 | -2.6 | -53 | 43 | -2.9 | -73* | 61 | -4.2 | -85* | 60 | -6.3 | 8.43E-011 |
| OV 5 | -31 | 36 | -1.7 | -40 | 40 | -2.1 | -57* | 55 | -3.2 | -68* | 55 | -4.8 | 1.06E-009 |
| OV 6 | -20 | 36 | -1.2 | -29 | 39 | -1.6 | -43* | 50 | -2.5 | -53* | 49 | -3.8 | 6.21E-009 |
| OV 7 | -11 | 36 | -0.6 | -19 | 37 | -1.0 | -31* | 45 | -1.8 | -40* | 47 | -2.8 | 9.12E-008 |
| OV 8 | -1 | 35 | 0.0 | -9 | 36 | -0.5 | -19* | 43 | -1.1 | -27* | 45 | -1.8 | 2.95E-006 |
| OV 9 | 8 | 34 | 0.4 | 1 | 34 | 0.0 | -7* | 42 | -0.4 | -16* | 44 | -1.2 | 7.88E-006 |
| OV 10 | 18 | 34 | 1.0 | 10 | 32 | 0.5 | 4* | 43 | 0.2 | -6* | 47 | -0.3 | 4.12E-005 |
| OV 11 | 29 | 34 | 1.6 | 20* | 32 | 1.2 | 16 | 43 | 1.0 | 6* | 46 | 0.6 | 8.91E-005 |
| OV 12 | 37 | 36 | 2.1 | 31 | 31 | 1.7 | 28 | 43 | 1.7 | 19* | 46 | 1.2 | .001 |
| OV 13 | 48 | 37 | 2.8 | 44 | 33 | 2.5 | 42 | 44 | 2.5 | 33* | 47 | 2.3 | .007 |
| OV 14 | 65 | 42 | 3.8 | 59 | 37 | 3.3 | 61 | 48 | 3.5 | 51 | 54 | 3.6 | .021 |
| OV 15 | 85 | 48 | 4.4 | 79 | 43 | 4.3 | 81 | 55 | 4.7 | 78 | 63 | 5.3 | .352 |
| OV 16 | 124 | 83 | 6.1 | 112 | 55 | 5.8 | 116 | 71 | 6.5 | 118 | 79 | 7.7 | .453 |
cKLG = calculated Kellgren-Lawrence grade at baseline, obtained from osteophyte and joint space narrowing (JSN) readings in fixed flexion radiographs, based on the OARSI atlas; MC = mean change in μm, SD = standard deviation of the change, MC% = mean change in % (expressed as ratio of mean change in μm and average cartilage thickness at baseline in each group. The Kruskal-Wallis was used to test whether or not there were any significant differences in the rate of change between the different subcohorts;
rate of change significantly different from non-exposed healthy controls when applying a Mann-Whitney-U test at p<0.0167 (global error level = 5%, since 3 parallel tests are applied). Results obtained with the Welch's test were very similar to those reported above for the Mann-Whitney-U.
Table 3. Subregional cartilage thickness changes over one year as measured with magnetic resonance imaging (MRI).
| Non-exposed Controls(n=112) | cKLG2 (n=310) | cKLG3 (n=300) | cKLG4 (n=109) | Kruskal-Wallis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MC | SRM | MC% | MC | SRM | MC% | MC | SRM | MC% | MC | SRM | MC% | ||
| MFTC | 2 | 0.02 | 0.1 | -13 | -0.13 | -0.3 | -40* | -0.31 | -1.2 | -55* | -0.38 | -2.0 | 8.89E-005 |
| LFTC | 7 | 0.09 | 0.2 | -9 | -0.11 | -0.2 | -29* | -0.23 | -0.8 | -49* | -0.39 | -1.5 | 4.89E-004 |
| MT | 2 | 0.05 | 0.1 | -2 | -0.05 | -0.1 | -10 | -0.18 | -0.6 | -27* | -0.42 | -1.9 | 2.64E-004 |
| cMF | 0 | 0.00 | 0.0 | -11 | -0.14 | -0.6 | -30* | -0.32 | -1.8 | -28* | -0.28 | -2.1 | .001 |
| LT | 2 | 0.05 | 0.1 | -10 | -0.22 | -0.5 | -21* | -0.31 | -1.1 | -27* | -0.51 | -1.7 | .001 |
| cLF | 6 | 0.10 | 0.3 | 2 | 0.03 | 0.1 | -7 | -0.09 | -0.4 | -22* | -0.25 | -1.3 | .044 |
| cMT | 4 | 0.05 | 0.2 | -9 | -0.09 | -0.4 | -23 | -0.22 | -1.0 | -58* | -0.44 | -3.0 | 1.22E-004 |
| eMT | 4 | 0.07 | 0.3 | -7 | -0.09 | -0.5 | -21* | -0.23 | -1.6 | -31* | -0.31 | -3.9 | .001 |
| iMT | 1 | 0.02 | 0.1 | -4 | -0.06 | -0.2 | -7 | -0.11 | -0.4 | -27* | -0.33 | -1.4 | .020 |
| aMT | 10 | 0.15 | 0.7 | 3 | 0.05 | 0.2 | 3 | 0.04 | 0.2 | -12 | -0.13 | -0.8 | .153 |
| pMT | -6 | -0.10 | -0.4 | 1 | 0.02 | 0.1 | -7 | -0.12 | -0.5 | -15 | -0.24 | -1.2 | .037 |
| ccMF | -2 | -0.03 | -0.1 | -23 | -0.19 | -1.0 | -49* | -0.33 | -2.5 | -40* | -0.30 | -3.0 | .003 |
| ecMF | 5 | 0.08 | 0.4 | -4 | -0.04 | -0.3 | -22* | -0.21 | -1.7 | -8 | -0.09 | -1.0 | .013 |
| icMF | -2 | -0.03 | -0.1 | -7 | -0.10 | -0.4 | -22 | -0.25 | -1.2 | -35* | -0.29 | -2.1 | .007 |
| cLT | 11 | 0.10 | 0.3 | -20* | -0.20 | -0.6 | -49* | -0.35 | -1.8 | -54* | -0.47 | -2.3 | 1.19E-005 |
| eLT | 6 | 0.11 | 0.4 | -6 | -0.09 | -0.4 | -17 | -0.21 | -1.1 | -26* | -0.38 | -2.0 | .003 |
| iLT | 0 | 0.00 | 0.0 | -16 | -0.22 | -0.8 | -29* | -0.29 | -1.7 | -23 | -0.29 | -1.6 | .053 |
| aLT | -7 | -0.07 | -0.4 | 0 | 0.00 | 0.0 | -5 | -0.07 | -0.3 | -23 | -0.28 | -1.4 | .049 |
| pLT | 2 | 0.02 | 0.1 | -11 | -0.12 | -0.6 | -11 | -0.10 | -0.6 | -15 | -0.19 | -1.0 | .740 |
| ccLF | 10 | 0.11 | 0.5 | 1 | 0.01 | 0.0 | -14 | -0.11 | -0.6 | -31 | -0.23 | -1.5 | .073 |
| ecLF | 4 | 0.06 | 0.2 | 4 | 0.05 | 0.2 | -6 | -0.06 | -0.4 | -22 | -0.23 | -1.5 | .083 |
| icLF | 3 | 0.06 | 0.2 | 0 | 0.00 | 0.0 | -4 | -0.05 | -0.3 | -16 | -0.15 | -0.9 | .337 |
cKLG = calculated Kellgren-Lawrence grade at baseline; MC = mean change in μm, SRM = standardized response mean (= mean change devided by the standard deviation of the change), MC% = mean change in % (expressed as ratio of mean change in μm and average cartilage thickness at baseline in each group. The Kruskal-Wallis was used to test whether or not there were any significant differences in the rate of change between the different subcohorts;
rate of change significantly different from non-exposed healthy controls when applying a Mann-Whitney-U test at p<0.0167 (global error level = 5%,since 3 parallel tests are applied). MFTC = medial femorotibial compartment (=MT+cMF), LFTC = lateral femorotibial compartment (=LT+cLF); MT/LT = medial/lateral tibia, cMF/cLF = medial/lateral central (weight-bearing) femoral condyle; c = central, e = external, i = internal, a = anterior, p = posterior subregion.
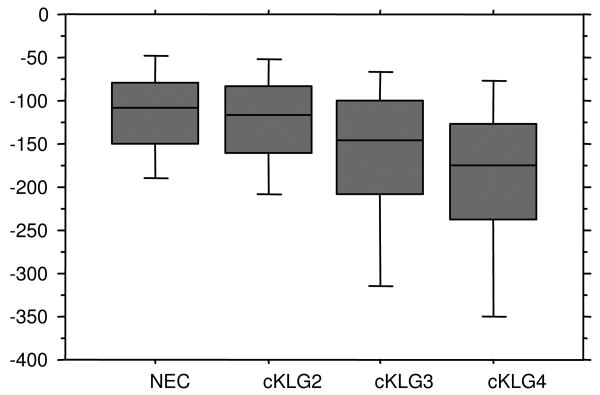
The results in OV1 (the subregion with the largest reduction in cartilage thickness in each knee) were significantly different between non-exposed healthy and ROA knees (p=5.5×10-14; Figure 1). The OV1 changes were -137μm in cKLG2, -180μm in cKLG3, and -200μm in cKLG4 knees. The Mann-Whitney-U tests revealed that changes in OV1 were significantly greater in cKLG3 and cKLG4 knees than in non-exposed healthy knees, respectively, but not significantly greater in cKLG2 than in non-exposed control knees (Table 2, Figure 1). Note that OV1 may refer to different subregions across persons (and KL grades).
Figure 1.
Box plots showing the rate of change in cartilage thickness for ordered value 1 (in μm) in non exposed control (NEC) knees, and in knees with calculated Kellgren-Lawrence grade (cKLG) 2, 3 and 4, respectively.
Exploration of other OVs revealed that cKLG4 knees displayed significantly greater cartilage loss than non-exposed healthy knees in OVs1-13, cKLG3 knees in OVs1-10, and cKLG2 knees only in OV11 (Table 2). Results in OV16 (the subregion with the smallest loss or largest increase in cartilage thickness) did not display significant differences between groups (Kruskal-Wallis; p=0.48). Results obtained with the Welch's test were very similar to those reported above for the Mann-Whitney-U.
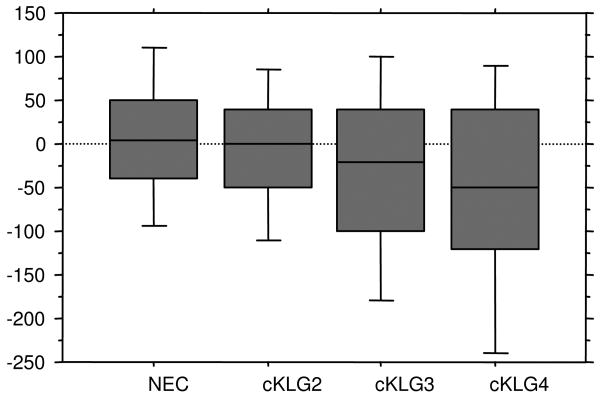
Cartilage compartments, plates and subregions (Table 3; Figure 2) in cKLG2 knees showed only small losses in thickness (up to -0.6% in plates and up to -1.0% in subregions). The most negative SRM was -0.22 both at the plate (LT) and at the subregional level (iLT); these changes were not significantly different from those in the non-exposed healthy sample, except for cLT (Table 3). In cKLG3 knees, the largest loss at the plate level was observed in cMF (-1.8%, SRM -0.32), and that at subregional level in ccMF (-2.5%); the most negative SRM (-0.35) was seen in cLT (Table 3), indicating that the most homogeneous change occurred in this subregion. In cKLG4 knees, the largest reduction at plate level (-2.1%) was observed in cMF, and the smallest SRM (-0.42) in MT. At subregional level the largest reduction in cKLG4 knees was observed in eMT (-3.9%) and the most negative SRM (-0.47) in cLT (Table 3). cKLG4 knees generally displayed the largest rates of (and sensitivity to change) compared with all other groups (Table 3). The OV approach was more sensitive in revealing differences in progression between cKLG and non-exposed healthy knees than the region-based approach.
Figure 2.
Box plots showing the rate of change in cartilage thickness for the medial femorotibial compartment (in μm) in non exposed control (NEC) knees, and in knees with calculated Kellgren-Lawrence grade (cKLG) 2, 3 and 4, respectively.
Discussion
In this study we explored the one-year rate of (and sensitivity to) change in femorotibial cartilage thickness in a large subset of OAI knees. Systematic comparisons were made across all radiographic disease stages, ranging from non-exposed healthy reference to end-stage ROA. We tested the hypotheses that knees with end-stage ROA (cKLG4) have a rate of change similar to those with mild to moderate ROA (cKLG2 or 3), and that ROA knees display larger rates of change than non-exposed healthy control knees. Our results show, for the first time, that knees with end-stage ROA display a similar or even higher rate of and sensitivity to change compared with knees with less severe JSN (cKLG3), when MRI-based measures of subregional cartilage thickness changes are used. In keeping with another (but smaller) longitudinal study involving radiography and MRI (30), knees with definite osteophytes but without JSN (cKLG2) did not show significantly greater rates of change than non-exposed healthy reference knees.
One study reported that an MRI-based measure of cartilage loss was a better predictor of future TKA than radiography (16); however, the clinical relevance of cartilage thickness change still remains to be established. This will occur through a variety of studies qualifying a biomarker such as “MRI-based cartilage thickness change”: Such studies should describe and establish the performance of the marker in patients with various stages and phenotypes of disease (as done in the current study) and then further need to link the biomarker performance with clinical outcome. Finally, clinical trials will have to establish whether treatment effects on the biomarker are reflected in clinical improvement.
A limitation of the study is that ROA disease stages were defined based on the site rather than central readings of fixed flexion radiographs, but central readings were only available for a small part of the cohort examined here. The approach taken here, however, is similar to many clinical trials, where participants are included (excluded) based on a site reading of the baseline radiograph. Nevertheless, future studies may explore to what extent rates of cartilage loss in different ROA stages are affected by the type of reading (i.e. central versus sites; cKLG versus original KLG). Another limitation of the approach taken here is that precision errors reported for MRI-based measures of cartilage thickness (13,36-38) are relatively large when compared with changes observed over one year. Yet, one year follow up periods are highly preferable from a clinical trial perspective. The symmetric distribution of the OVs in the healthy reference knees (-121μm for OV1 to +124 μm for OV16), in whom no change in cartilage thickness is expected (and in whom no such change was observed at regional level) can be viewed as an expression of random reading errors at subregion level. These values are used as a reference here, to test whether the changes observed in ROA knees are significantly greater than those occurring from random error.
A strength of the current study is the inclusion of knees from the non-exposed healthy reference cohort without symptoms, radiographic signs, or risk factors of knee OA. This group displayed no relevant change over one year, and this provides assurance that the rates of change of cartilage thickness measured in ROA knees in the OAI are real and not due to scanner drift or other systematic biases. The specific strength of the OV approach is the separation of the rate of change from its specific location in an individual knee. It was shown that the location of maximal change varies substantially among knees, depending on knee alignment (23,25), the location of meniscal damage (41). Although the OV approach is not suited for testing whether or not a given change is significantly different from zero (specifically when looking at OV1), the approach was shown to be superior to a region-specific comparison in identifying differences in the rate of cartilage thickness changes between knees with and without JSN (39,40). This observation is confirmed by the current study, in that p-values for differences in rates of change between cKLG groups were much smaller for OV1 than for any given femorotibial cartilage compartment, plate, or subregion. This superiority is further enhanced by eliminating the need to adjust for testing multiple regions in parallel, if the location of the largest change is not known “a priori”. For these reasons, OV1 was defined as the primary outcome. Nevertheless, results in compartments, plates and subregions point in the same direction, confirming the OV results, but, as stated above, these did not attain statistical significance.
Our results show that knees with end-stage ROA (cKLG4 and OARSI JSN grade 3) display rates of (and sensitivity to) change that tend to be larger than in cKLG3 or 2 knees. Although previous studies reported higher rates of change in knees with JSN compared to those without JSN (29,30,39,40), this has not been shown for end-stage ROA (cKLG4 knees). Similar observations were previously made based on semi-quantitative readings of cartilage changes (43) using the whole organ MRI scoring system (WORMS) (44). The authors (43) reported that the proportion of knees with an increase in cartilage lesions at follow-up was similar or larger for end-stage ROA knees compared with knees with less severe radiographic disease. These findings suggest that, at least from a perspective of sensitivity to change, cKLG4 knees need not to be excluded from longitudinal studies, and that these knees can still be followed in terms of “progression” when MRI-based measures of cartilage morphology (i.e. thickness) are used. Whether or not a drug will be able to modify progression of OA at such a late stage of disease, and whether differences in subsequent cartilage loss among knees with end-stage ROA are related to differences in clinical outcome, however will have to be explored in future studies. A potential advantage of a drug effect occurring at this late disease stage is that it may be easier to show a translation of the structural treatment effect into an improvement of an accepted clinical outcome such as the delay of TKA, whereas much longer follow-up periods would be required for “earlier” disease stages in this context. Even if drug effects at this late stage are deemed unlikely, cKLG4 knees need not to be excluded from epidemiological studies investigating risk factors of OA progression, when MRI is used as an outcome. In contrast, using radiographs, there is little chance to measure further progression in JSW reductions longitudinally in cKLG4 knees with a 67% to 100% reduction in JSW at baseline.
The observation of lower rates of change in cKLG2 knees (without JSN) than in cKLG3 knees (with JSN) have been previously reported using radiographic outcomes (30,45-47) and MRI-based measures of cartilage thickness (29,30,39,40). It is surprising that the rate of change in cKLG2 knees was only marginally larger than (and not significantly different from) that in the non-exposed healthy control knees. However, similar observations had been made in a previous (albeit much smaller) study (30). Another recent study suggested that, whereas the mean rate of change was not significantly different between cKLG2 and healthy knees (30), the distribution of subregional changes was, i.e. some cKLG2 knees displayed significant cartilage increase (hypertrophy or swelling) whereas others showed cartilage loss (48). Comparison of OV16 in the current study, however, did not indicate that there is a difference in the rate of cartilage thickening between cKLG2 and non-exposed healthy knees in this cohort.
In conclusion, we find that MRI-based cartilage thickness measures display high rates of loss and sensitivity to change in cartilage thickness at end-stage ROA, which are similar to slightly higher than those in knees with less severe baseline JSN, and small rates of change (statistically indistinguishable from non-exposed healthy control knees) in mild ROA (knees with osteophytes, but without JSN at baseline). From the perspective of sensitivity to change, therefore, cKLG4 knees need not be excluded from longitudinal studies that use MRI-based cartilage thickness as an endpoint, in particular when an ordered value (OV) approach is employed. A potential benefit of studying knees at this late stage of the disease is that the potential translation of structural changes or treatment effects into improvements of clinical outcomes (i.e. delay in TKA) can be tested over relatively short time intervals.
Acknowledgments
We would like to thank the following readers: Gudrun Goldmann, Linda Jakobi, Manuela Kunz, Dr. Susanne Maschek, Jana Matthes, Sabine Mühlsimer, Annette Thebis, and Dr. Barbara Wehr for dedicated data segmentation.
The image analysis of this study was funded by an industry consortium consisting of Pfizer Inc, Eli Lilly & Co, MerckSerono SA, Glaxo Smith Kline Inc., Wyeth Research, Centocor Research and Development Inc., and Novartis Pharma AG, and by the coordinating center of the OAI at UCSF and Chondrometrics GmbH.
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Funding Source: The study and image acquisition was funded by the Osteoarthritis Initiative, a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262). The image analysis of this study was funded by an industry consortium consisting of Pfizer Inc., Eli Lilly & Co, Merck Serono SA, Glaxo Smith Kline Inc., Wyeth Research, Centocor Research and Development, Inc, and Novartis Pharma AG and by the OAI coordinating center (UCSF).
Footnotes
Competing interests: Felix Eckstein is CEO of Chondrometrics GmbH, a company providing MR image analysis services. He provides consulting services to Pfizer, MerckSerono, and Novartis. Susanne Maschek, Wolfgang Wirth, and Martin Hudelmaier have part time appointments with Chondrometrics GmbH. Alberto Gimona has a full time appointment with Novartis, Kristen Picha with Centocor, Jennifer H Lee with Pfizer (Wyeth), Richard Y Davies with Glaxo Smith Kline, Donatus Dreher with MerckSerono, Olivier Benichou with Eli Lilly, and Marie-Pierre Hellio Le Graverand with Pfizer.
Reference List
- 1.Buckland-Wright JC, Macfarlane DG, Lynch JA, Jasani MK, Bradshaw CR. Joint space width measures cartilage thickness in osteoarthritis of the knee: high resolution plain film and double contrast macroradiographic investigation. Ann Rheum Dis. 1995;54:263–268. doi: 10.1136/ard.54.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piperno M, Hellio Le Graverand MP, Conrozier T, Bochu M, Mathieu P, Vignon E. Quantitative evaluation of joint space width in femorotibial osteoarthritis: comparison of three radiographic views. Osteoarthritis Cartilage. 1998;6:252–259. doi: 10.1053/joca.1998.0118. [DOI] [PubMed] [Google Scholar]
- 3.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32:128–132. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 4.Felson DT, Goggins J, Niu J, Zhang Y, Hunter DJ. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004;50:3904–3909. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 5.Niu J, Zhang YQ, Torner J, Nevitt M, Lewis CE, Aliabadi P, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329–335. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA. 2001;286:188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 7.Brandt KD, Mazzuca SA, Katz BP, Lane KA, Buckwalter KA, Yocum DE, et al. Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2005;52:2015–2025. doi: 10.1002/art.21122. [DOI] [PubMed] [Google Scholar]
- 8.Dougados M, Hawker G, Lohmander S, Davis AM, Dieppe P, Maillefert JF, et al. OARSI/OMERACT criteria of being considered a candidate for total joint replacement in knee/hip osteoarthritis as an endpoint in clinical trials evaluating potential disease modifying osteoarthritic drugs. J Rheumatol. 2009;36:2097–2099. doi: 10.3899/jrheum.090365. [DOI] [PubMed] [Google Scholar]
- 9.Nevitt MC, Peterfy C, Guermazi A, Felson DT, Duryea J, Woodworth T, et al. Longitudinal performance evaluation and validation of fixed-flexion radiography of the knee for detection of joint space loss. Arthritis Rheum. 2007;56:1512–1520. doi: 10.1002/art.22557. [DOI] [PubMed] [Google Scholar]
- 10.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgkart R, Glaser C, Hyhlik-Durr A, Englmeier KH, Reiser M, Eckstein F. Magnetic resonance imaging-based assessment of cartilage loss in severe osteoarthritis: accuracy, precision, and diagnostic value. Arthritis Rheum. 2001;44:2072–2077. doi: 10.1002/1529-0131(200109)44:9<2072::AID-ART357>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Graichen H, Eisenhart-Rothe R, Vogl T, Englmeier KH, Eckstein F. Quantitative assessment of cartilage status in osteoarthritis by quantitative magnetic resonance imaging: technical validation for use in analysis of cartilage volume and further morphologic parameters. Arthritis Rheum. 2004;50:811–816. doi: 10.1002/art.20191. [DOI] [PubMed] [Google Scholar]
- 13.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27:737–744. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 14.Eckstein F, Burstein D, Link TM. Quantitative MRI of cartilage and bone: degenerative changes in osteoarthritis. NMR Biomed. 2006;19:822–854. doi: 10.1002/nbm.1063. [DOI] [PubMed] [Google Scholar]
- 15.Guermazi A, Burstein D, Conaghan P, Eckstein F, Hellio Le Graverand-Gastineau MP, Keen H, et al. Imaging in osteoarthritis. Rheum Dis Clin North Am. 2008;34:645–687. doi: 10.1016/j.rdc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Cicuttini FM, Jones G, Forbes A, Wluka AE. Rate of cartilage loss at two years predicts subsequent total knee arthroplasty: a prospective study. Ann Rheum Dis. 2004;63:1124–1127. doi: 10.1136/ard.2004.021253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frobell RB, Nevitt MC, Hudelmaier M, Wirth W, Wyman BT, Benichou O, et al. Femorotibial subchondral bone area and regional cartilage thickness - a cross-sectional description in healthy reference cases and various radiographic stages of osteoarthritis in 1003 knees from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2010 doi: 10.1002/acr.20262. [DOI] [PubMed] [Google Scholar]
- 18.Burgkart R, Glaser C, Hinterwimmer S, Hudelmaier M, Englmeier KH, Reiser M, et al. Feasibility of T and Z scores from magnetic resonance imaging data for quantification of cartilage loss in osteoarthritis. Arthritis Rheum. 2003;48:2829–2835. doi: 10.1002/art.11259. [DOI] [PubMed] [Google Scholar]
- 19.Wluka AE, Stuckey S, Snaddon J, Cicuttini FM. The determinants of change in tibial cartilage volume in osteoarthritic knees. Arthritis Rheum. 2002;46:2065–2072. doi: 10.1002/art.10460. [DOI] [PubMed] [Google Scholar]
- 20.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Labonte F, Beaudoin G, de Guise JA, et al. Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum. 2004;50:476–487. doi: 10.1002/art.20000. [DOI] [PubMed] [Google Scholar]
- 21.Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9:R74. doi: 10.1186/ar2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding C, Cicuttini F, Scott F, Boon C, Jones G. Association of prevalent and incident knee cartilage defects with loss of tibial and patellar cartilage: a longitudinal study. Arthritis Rheum. 2005;52:3918–3927. doi: 10.1002/art.21474. [DOI] [PubMed] [Google Scholar]
- 23.Sharma L, Eckstein F, Song J, Guermazi A, Prasad P, Kapoor D, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58:1716–1726. doi: 10.1002/art.23462. [DOI] [PubMed] [Google Scholar]
- 24.Raynauld JP, Martel-Pelletier J, Bias P, Laufer S, Haraoui B, Choquette D, et al. Protective effects of licofelone, a 5-lipoxygenase and cyclooxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: a first Multi-Centre Clinical Trial using quantitative MRI. Ann Rheum Dis. 2008 doi: 10.1136/ard.2008.088732. [DOI] [PubMed] [Google Scholar]
- 25.Eckstein F, Wirth W, Hudelmaier M, Stein V, Lengfelder V, Cahue S, et al. Patterns of femorotibial cartilage loss in knees with neutral, varus, and valgus alignment. Arthritis Rheum. 2008;59:1563–1570. doi: 10.1002/art.24208. [DOI] [PubMed] [Google Scholar]
- 26.Eckstein F, Maschek S, Wirth W, Hudelmaier M, Hitzl W, Wyman B, et al. One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann Rheum Dis. 2009;68:674–679. doi: 10.1136/ard.2008.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunter DJ, Niu J, Zhang Y, Totterman S, Tamez J, Dabrowski C, et al. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. Ann Rheum Dis. 2009;68:349–356. doi: 10.1136/ard.2007.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wirth W, Hellio Le Graverand MP, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, et al. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage. 2009;17:291–297. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckstein F, Benichou O, Wirth W, Nelson DR, Maschek S, Hudelmaier M, et al. Direct comparison of cartilage loss in painful contra-lateral knees with and without joint space narrowing - data from the Osteoarthritis Initiative (OAI) Arthritis Care Res. 2009;61:1218–1225. doi: 10.1002/art.24791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Graverand MP, Buck RJ, Wyman BT, Vignon E, Mazzuca SA, Brandt KD, et al. Change in regional cartilage morphology and joint space width in osteoarthritis participants versus healthy controls: a multicentre study using 3.0 Tesla MRI and Lyon-Schuss radiography. Ann Rheum Dis. 2010;69:155–162. doi: 10.1136/ard.2008.099762. [DOI] [PubMed] [Google Scholar]
- 31.Schneider E, NessAiver M, White D, Purdy D, Martin L, Fanella L, et al. The osteoarthritis initiative (OAI) magnetic resonance imaging quality assurance methods and results. Osteoarthritis Cartilage. 2008;16:994–1004. doi: 10.1016/j.joca.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altman RD, Hochberg M, Murphy WA, Jr, Wolfe F, Lequesne M. Atlas of individual radiographic features in osteoarthritis. Osteoarthritis Cartilage. 1995;3(A):3–70. [PubMed] [Google Scholar]
- 33.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(A):1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Spector TD, Hart DJ, Byrne J, Harris PA, Dacre JE, Doyle DV. Definition of osteoarthritis of the knee for epidemiological studies. Ann Rheum Dis. 1993;52:790–794. doi: 10.1136/ard.52.11.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spector TD, Cooper C. Radiographic assessment of osteoarthritis in population studies: whither Kellgren and Lawrence? Osteoarthritis Cartilage. 1993;1:203–206. doi: 10.1016/s1063-4584(05)80325-5. [DOI] [PubMed] [Google Scholar]
- 36.Eckstein F, Hudelmaier M, Wirth W, Kiefer B, Jackson R, Yu J, et al. Double echo steady state magnetic resonance imaging of knee articular cartilage at 3 Tesla: a pilot study for the Osteoarthritis Initiative. Ann Rheum Dis. 2006;65:433–441. doi: 10.1136/ard.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14(1):46–75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 38.Eckstein F, Buck RJ, Burstein D, Charles HC, Crim J, Hudelmaier M, et al. Precision of 3.0 Tesla quantitative magnetic resonance imaging of cartilage morphology in a multicentre clinical trial. Ann Rheum Dis. 2008;67:1683–1688. doi: 10.1136/ard.2007.076919. [DOI] [PubMed] [Google Scholar]
- 39.Buck RJ, Wyman BT, Le Graverand MP, Hudelmaier M, Wirth W, Eckstein F. Does the use of ordered values of subregional change in cartilage thickness improve the detection of disease progression in longitudinal studies of osteoarthritis? Arthritis Rheum. 2009;61:917–924. doi: 10.1002/art.24613. [DOI] [PubMed] [Google Scholar]
- 40.Wirth W, Buck R, Hellio Le, Graverand-Gastineau MP, Benichou O, Dreher D, Davies RY, et al. Ordered values of cartilage loss in knee subregions more efficiently differentiate progression at different radiographic stages of OA: Data from the OA Initiative. Osteoarthritis Cartilage. 2009;17(Suppl. 1):S203. abstract. [Google Scholar]
- 41.Chang A, Moisio K, Chmiel JS, Eckstein F, Guermazi A, Almagor O, et al. Subregional effects of meniscal tears on cartilage loss over 2 years in knee osteoarthritis. Ann Rheum Dis. 2010 doi: 10.1136/ard.2010.130278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eckstein F, Wirth W, Hudelmaier MI, Maschek S, Hitzl W, Wyman BT, et al. Relationship of compartment-specific structural knee status at baseline with change in cartilage morphology: a prospective observational study using data from the osteoarthritis initiative. Arthritis Res Ther. 2009;11:R90. doi: 10.1186/ar2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amin S, Niu J, Guermazi A, Grigorian M, Hunter D, Felson DT. Cartilage loss in men and women with x-ray defined end-stage knee osteoarthritis. Arthritis and Rheumatism. 2007;56:S127. doi: 10.1002/art.21724. abstract. [DOI] [PubMed] [Google Scholar]
- 44.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Wolfe F, Lane NE. The longterm outcome of osteoarthritis: rates and predictors of joint space narrowing in symptomatic patients with knee osteoarthritis. J Rheumatol. 2002;29:139–146. [PubMed] [Google Scholar]
- 46.Mazzuca SA, Brandt KD, Katz BP, Ding Y, Lane KA, Buckwalter KA. Risk factors for progression of tibiofemoral osteoarthritis: an analysis based on fluoroscopically standardised knee radiography. Ann Rheum Dis. 2006;65:515–519. doi: 10.1136/ard.2005.039115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Graverand MP, Vignon EP, Brandt KD, Mazzuca SA, Piperno M, Buck R, et al. Head-to-head comparison of the Lyon Schuss and fixed flexion radiographic techniques. Long-term reproducibility in normal knees and sensitivity to change in osteoarthritic knees. Ann Rheum Dis. 2008;67:1562–1566. doi: 10.1136/ard.2007.077834. [DOI] [PubMed] [Google Scholar]
- 48.Buck RJ, Wyman BT, Le Graverand MP, Hudelmaier M, Wirth W, Eckstein F. Osteoarthritis may not be a one-way-road of cartilage loss - comparison of spatial patterns of cartilage change between osteoarthritic and healthy knees. Osteoarthritis Cartilage. 2010;18:329–335. doi: 10.1016/j.joca.2009.11.009. [DOI] [PubMed] [Google Scholar]