Abstract
Objective
To assess whether onset of rheumatoid arthritis (RA) prior to conception is associated with a delayed time to pregnancy (TTP).
Methods
The study included pregnant women from across Denmark enrolled in the Danish National Birth Cohort (DNBC) between 1996 and 2002, who had planned or partly planned the cohort pregnancy. RA diagnosis was identified using the Danish National Hospital Discharge Registry. Self-reported data including TTP, maternal age, parity, pre-pregnancy height and weight, maternal occupational status, smoking and alcohol consumption were collected using a detailed computer-assisted telephone interview at approximately 16 weeks of gestation. We used logistic regression analyses as well as a complementary log regression model to examine whether TTP was influenced by RA, adjusting for the above-mentioned variables.
Results
Overall, compared to women with no recorded RA (n=68,170), women with prevalent RA (onset prior to conception) (n=112) were slightly older (30.8±4.3 vs. 29.7±4.1 years), were more likely to have been treated for infertility (9.8% vs. 7.6%) and were more likely to have taken longer than 12 months to conceive (25.0% vs. 15.6%). The association between RA and TTP was borderline significant after adjusting for covariates in the regression analyses (OR=1.6, 95% CI: 1.0, 2.4). Similar results were obtained after restricting the analyses to women who had planned the pregnancy or those who were nulliparous before the cohort pregnancy.
Conclusion
Women with RA onset prior to conception had a slightly longer TTP compared to those who did not have RA, indicating a slight reduction in fecundity.
Introduction
Although rheumatoid arthritis (RA) tends to have a late onset, the disease also occurs during child-bearing years and may interfere with procreation (1). It is, however, not entirely clear whether fecundity, i.e. probability of conception, is affected by the disease or its treatment. Investigations on fecundity and RA have been few as shown by the sparse reports in the literature spanning the last several decades. A few case control studies have found RA cases to have reduced fecundity prior to disease onset (2, 3). Women with prevalent RA at the time of conception were also found to have a longer waiting time to pregnancy (TTP) compared to women without RA (2), perhaps reflecting a reduced fecundity. Given the practical difficulties in assembling datasets of RA patients to investigate associations with fecundity, this topic has not been further investigated. In the present study, we used a nationwide cohort of pregnant women in Denmark to assess whether prevalent RA prior to conception was associated with a longer TTP, i.e. the time it takes to obtain a clinically recognized pregnancy.
Subjects and Methods
Study subjects
The initial study population consisted of 92,892 pregnancies from the Danish National Birth Cohort (DNBC) – pregnant women from across Denmark had been enrolled into DNBC between 1996 and 2002, with the goal of exploring the potential importance of social, environmental, and lifestyle factors during pregnancy and early childhood on health and development of children and adults (4). Only coded data were available for the present analyses, and hence the study was human subjects research exempt. Approval for the study was obtained from the Danish Data Protection Agency and the DNBC Steering Committee.
RA Diagnosis
To identify women with a diagnosis of RA, the DNBC database was linked to the Danish National Hospital Discharge Registry, in which clinical diagnoses for in-patients have been recorded since 1977, and also for out-patients since 1995. The Danish National Hospital Discharge Registry allows for up to 20 diagnoses other than the reason for hospitalization to be recorded (for each hospitalization), in addition to the primary diagnosis for which the patient was hospitalized. Thus, prior to 1995, we identified women who had a recorded diagnosis of RA irrespective of whether RA was the primary reason of hospitalization or was one of the other conditions they were suffering from at the time of hospitalization. From 1995 onwards, the RA patients we identified also included out-patients attending the hospital for RA treatment. RA diagnoses were identified using ICD-8 codes 712.19, 712.39 and 712.59, and ICD-10 codes M05 and M06 (except M06.1 – adult Still’s disease). ICD-9 codes have not been used in Denmark. Subjects with a diagnosis of other rheumatic diseases (ICD-8 codes 712.09, 712.29, 712.49 and ICD-10 codes M06.1, M07-M14.8), including juvenile-onset RA, were excluded (n=521).
For each RA patient identified from the Danish National Hospital Discharge Registry, we determined whether there was a recorded diagnosis of RA prior to the DNBC cohort pregnancy – this group constituted our primary patient group for our data analyses, i.e. patients who had prevalent RA before pregnancy. For women who only had a recorded diagnosis of RA after the computed conception date, we assumed that they developed the disease after conception, and used the date of hospitalization when RA diagnosis was first recorded in the National Hospital Discharge Registry as the date of RA onset, assuming that diagnosis occurred close to the time of disease onset.
Pregnancy-related data
Self-reported information on socio-demographic, environmental, and lifestyle factors were collected through a computer-assisted telephone interview at approximately 16 weeks of gestation. Women were asked whether their pregnancy was planned, if they had been treated for infertility, and for how long they had tried to become pregnant before succeeding. Data collected on maternal age, parity, pre-pregnancy height and weight, maternal occupational status, smoking in early pregnancy, and alcohol consumption (number of units/week) before pregnancy, were also used in the present analyses. Subjects with missing data for any of these variables were excluded from the analyses (n=4,269).
Time to pregnancy
Since “time to pregnancy” was the main outcome of interest in the present study, subjects for whom the time to pregnancy could not be determined because they had not planned the pregnancy or “did not try to get pregnant” were excluded from the analyses (n=13,575). For the remaining 74,527 subjects whose pregnancy had been “planned” or “partly planned”, time to pregnancy was recorded as 4 categories: 0–2, 3–5, 6–12, or more than 12 months. In the analyses, TTP was dichotomized as ≤12 months and >12 months, ≤6 months and >6 months or used as recorded.
Statistical Analyses
We used logistic regression analyses to examine whether TTP (≤12 months vs. >12 months) was influenced by RA status, with our primary focus on RA onset prior to conception. Hence, for these analyses, subjects who had a diagnosis of RA during or after the documented pregnancy were excluded. Covariates that were adjusted for in the regression models included maternal age, parity, pre-pregnancy body mass index (BMI), maternal occupational status, smoking in early pregnancy and alcohol consumption before pregnancy. Occupational status was categorized as follows for the regression analyses: high (managers, professionals and technicians), medium (clerks, service and sales workers, skilled agricultural workers and craft workers), low (unskilled workers), students or unemployed or unknown, and alcohol consumption was categorized as 0–1, 1–2, 2–3 or >3 units. Variables such as oral contraceptive use prior to conception, number of previous spontaneous abortions, infertility treatment, age at menarche, and having irregular periods, which could themselves directly affect time to pregnancy, were not included as covariates in the main model, but we checked whether they affected the association.
In addition to the logistic regression models, we used a complementary log regression model to make use of the recorded TTP distribution (all 4 categories). We also repeated all analyses using subjects who had an RA onset during or after the pregnancy, instead of the prevalent cases, to determine whether future onset of RA was associated with TTP.
Results
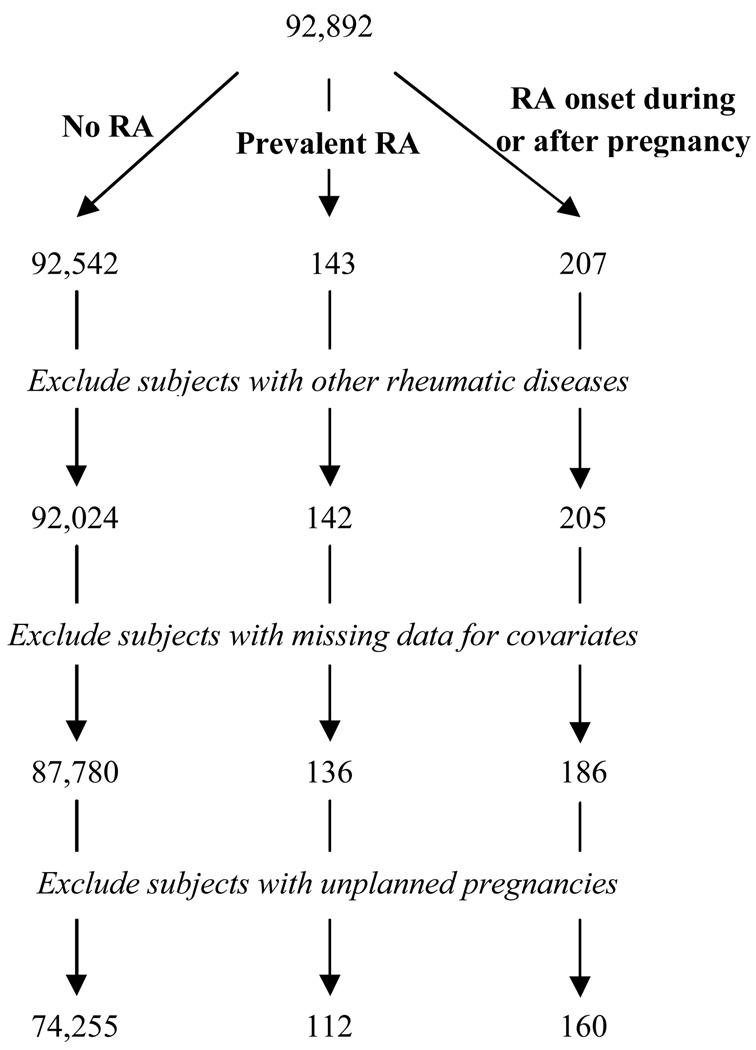
A total of 74,527 women were included in the present study, after subjects who had a diagnosis of other rheumatic diseases and those who had not planned the pregnancy or had missing data for key variables had been excluded (Figure 1). Of those, 112 women had been hospitalized with a diagnosis of RA prior to the documented pregnancy, i.e. at the time when they were trying to conceive, and 160 had a recorded diagnosis date up to 9 years after the pregnancy. The remaining 68,170 subjects did not have a recorded diagnosis of RA within our follow-up time.
Figure 1.
Selection of Study Subjects from the DNBC Cohort
The characteristics of the women with and without prevalent RA at conception (excluding those who subsequently developed the disease) are summarized in Table 1. Overall, a higher proportion of women with RA took longer than 12 months to conceive, compared to those who did not have RA (25.0% vs. 15.6%). Women with prevalent RA were also slightly older than those who did not have RA (30.8±4.3 vs. 29.7±4.1), and were more likely to have been treated for infertility (9.8% vs. 7.6%).
Table 1.
Baseline characteristics for women with and without RA in the Danish National Birth Cohort
| Women with prevalent RA at conception (n=112) |
Women without RA* (n=74,255) |
|
|---|---|---|
| Time to pregnancy, n (%) | ||
| 0–2 months | 46 (41.1) | 35,434 (47.7) |
| 3–5 months | 21 (18.8) | 15,234 (20.5) |
| 6–12 months | 17 (15.2) | 12,035 (16.2) |
| >12 months | 28 (25.0) | 11,552 (15.6) |
| Time since RA diagnosis (years), median (IQR¥) |
4.9 (2.3 – 9.0) | - |
| Age at conception (years), mean±SD | 30.8±4.3 | 29.7±4.1 |
| Had infertility treatment, n (%) | 11 (9.8) | 5,615 (7.6) |
| Parity, % | ||
| 0 | 52 (46.4) | 35,560 (47.9) |
| 1 | 45 (40.2) | 28,394 (38.2) |
| ≥2 | 15 (13.4) | 10,301 (13.9) |
| Pre-pregnancy BMI, mean±SD | 23.6±4.7 | 23.6±4.2 |
| Smoked during pregnancy, n (%) | 27 (24.1) | 17,638 (23.8) |
No RA diagnosis before or after conception
IQR = Inter-quartile range
As shown in Table 2, prevalent RA was associated with longer TTP (>12 months vs. ≤12 months) (crude odds ratio (OR) = 1.8). After adjusting for covariates, the association between RA and TTP was borderline significant (adjusted OR=1.6, 95% CI: 1.0, 2.4). When the analyses were restricted to those women who had planned the pregnancy (100 women with prevalent RA and 66,118 women without RA), after excluding those who “partly planned”, the results were very similar to those described above for the entire cohort (crude OR=1.9; adjusted OR=1.7, 95% CI: 1.1, 2.7). Similarly, restricting the analyses to women who were nulliparous at the time of conception (52 women with prevalent RA and 35,560 women without RA), the results were very similar to those described for the entire cohort (crude OR=1.9; adjusted OR=1.7, 95% CI: 0.9, 3.2).
Table 2.
Sub-fecundity among Women with RA Compared to Women with No Recorded RA
| TTP: | Crude Odds Ratio (OR)¶ /Fecundity Ratio (FR)§ |
Adjusted* OR /FR | 95% Confidence Interval (for adjusted OR/FR) |
|---|---|---|---|
| >6 months | 1.4 | 1.4 | 0.9, 2.0 |
| >12 months | 1.8 | 1.6 | 1.0, 2.4 |
| 4 categories: (0–2, 3–5, 6–12 or >12 months) |
0.8 | 0.8 | 0.6, 1.0 |
OR’s are shown for the logistic regression models with TTP >6 months or >12 months as the outcome variables.
FR’s are shown for the complementary log regression model with TTP as 4 categories.
Covariates that were adjusted for in the model were maternal age (as a continuous variable), parity (0, 1, 2, ≥3), BMI (continuous), socio-occupational status (categorical – 5 levels), alcohol consumption (categorical – 4 levels), and smoking (binary).
A weaker association was found when using TTP>6 months vs. ≤6 months as the outcome variable, after adjusting for the same covariates (Table 2 – adjusted OR=1.4, 95% CI: 0.9, 2.0). When using the entire TTP distribution (0–2, 3–5, 6–12, or >12 months) in the complementary log regression analysis, similar results were found for fecundity (adjusted fecundity ratio (FR)=0.8, 95% CI: 0.6, 1.0), indicating as before, a longer waiting time to pregnancy among women with RA. Similar results were found for women who had planned the pregnancy, and also for nulliparous women.
When we considered “onset of RA during or after pregnancy” as our exposure of interest (i.e. subject population of 160 women who later developed RA and 74,255 women without RA), we found a lower and non-significant association between this exposure and TTP (>12 months vs. ≤12 months) (crude OR=1.3; adjusted OR=1.2, 95% CI: 0.8, 1.8).
Discussion
We found that women who had RA at the time of trying to become pregnant had slightly longer waiting time to pregnancy compared to those who did not have RA, irrespective of whether they were experiencing a first pregnancy or were multiparous. Previous investigations on the topic of fecundity and RA are rare in the existing literature (2, 3) and have examined associations between future onset of RA and fecundity. The case-control study by del Junco et. al. also examined a possible association between prevalent RA and fecundity and showed a reduced fecundity among women with RA compared to controls (2). Thus, the results from our nationwide DNBC cohort support these previous findings by Del Junco et. al. (2). The exact mechanism by which RA may influence fecundity is not clear, especially since the underlying disease mechanisms are largely unknown. A reduced fecundity could be caused by the disease, its treatment or something else that correlates with RA. Since it is current practice that women with RA who are planning a pregnancy are advised by their physicians to stop taking disease modifying anti-rheumatic drugs (DMARDs) at least 3 months prior to trying to conceive (1), it is possible that they may experience flares of disease activity while trying to conceive which may contribute to delayed conception.
The mean maternal age was slightly higher among the women with RA compared to those without the disease (mean±S.D. (years): 30.8±4.3 vs. 29.8±4.1), and since increasing maternal age has been associated with reduced fecundity (5), the unadjusted association between RA and TTP was confounded by maternal age. The other variables in the model, i.e. parity, smoking, BMI, occupational status and alcohol consumption, did not appear to confound the observed association between prevalent RA and longer TTP once the analysis was adjusted for age, although they were all independently associated with TTP as expected (6–9).
A total of 420 women in the DNBC cohort had a self-reported diagnosis of RA during the interview when specifically asked if they have ever been diagnosed with the disease; of those 313 remained in the final analysis dataset, and only 73 of the 313 also had a diagnosis of RA from the Danish National Hospital discharge Registry. We do not know if the self-reported data on RA diagnosis are reliable, and whether the 240 women who did not have a recorded diagnosis in the registry were mild cases who did not get hospitalized. Including these 240 women in the analyses did not change the results (data not shown). Further, when we examined the subset of 160 women who did not have RA at conception but subsequently developed the disease, we did not find prolonged TTP to be a strong marker of future onset of RA. All of these women had a recorded diagnosis of RA between 1999 and 2008, when out-patient records were being included in the registry and thus we assumed that these correctly represented the dates of diagnosis as being after conception. The results, however, did not agree with previous reports of reduced fecundity prior to onset of RA (2, 3), but differences in methods as well as sources of data make the studies difficult to compare.
The identification of RA cases in our cohort through linkage with the Hospital Discharge Registry is likely to have introduced over-sampling of more severe cases of RA, although we did not have data on severity of RA for these patients. It is possible that the association with longer TTP may be with the severity of the disease rather than with RA itself, although it has been reported that fecundity is not associated with joint damage over time (10). We did consider the possibility that duration of RA (time since diagnosis) may influence TTP, since the disease may progress into more severe forms over time; however, within the subset of women with RA in our cohort, we did not find any correlation between RA duration and TTP (data not shown).
This study has a number of limitations that should be recognized. First, data on RA diagnosis was available from the Danish National Hospital Discharge Registry, and it is possible that some of the women in our “no RA” group had been diagnosed with the disease outside the hospital system. Nonetheless, we do not expect to have many such misclassified cases. Second, we may have under-estimated the association between fecundity and RA since any women with RA who had planned a pregnancy but did not get pregnant would not be represented in our dataset. Third, although TTP data were collected after the end of the waiting time, and may have been subject to recall bias, we believe that any such misclassification will be small since the recall time was short and non-differential since this specific study was not mentioned at recruitment.
In summary, our results show that women with RA had longer waiting time to pregnancy compared to those who did not have RA, indicating a slight reduction in fecundity.
Acknowledgements
“The Danish National Research Foundation has established the Danish Epidemiology Science Centre that initiated and created the Danish National Birth Cohort. The cohort is furthermore a result of a major grant from this Foundation. Additional support for the Danish National Birth Cohort is obtained from the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Augustinus Foundation, and the Health Foundation”
We would like to thank Ms. Inge Eisensee for data extraction. Dr. Jawaheer is supported by a Career Development Award (K01AR053496) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS).
References
- 1.Golding A, Haque UJ, Giles JT. Rheumatoid arthritis and reproduction. Rheum Dis Clin North Am. 2007;33(2):319–343. vi–vii. doi: 10.1016/j.rdc.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Del Junco DJ, Annegers JF, Coulam CB, Luthra HS. The relationship between rheumatoid arthritis and reproductive function. Br J Rheumatol. 1989;28 Suppl 1:33. doi: 10.1093/rheumatology/xxviii.suppl_1.33. [DOI] [PubMed] [Google Scholar]
- 3.Nelson JL, Koepsell TD, Dugowson CE, Voigt LF, Daling JR, Hansen JA. Fecundity before disease onset in women with rheumatoid arthritis. Arthritis Rheum. 1993;36(1):7–14. doi: 10.1002/art.1780360103. [DOI] [PubMed] [Google Scholar]
- 4.Olsen J, Melbye M, Olsen SF, Sorensen TI, Aaby P, Andersen AM, et al. The Danish National Birth Cohort--its background, structure and aim. Scand J Public Health. 2001;29(4):300–307. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- 5.Nugent D, Balen AH. The effects of female age on fecundity and pregnancy outcome. Hum Fertil (Camb) 2001;4(1):43–48. doi: 10.1080/1464727012000199251. [DOI] [PubMed] [Google Scholar]
- 6.Olsen J, Rachootin P, Schiodt AV, Damsbo N. Tobacco use, alcohol consumption and infertility. Int J Epidemiol. 1983;12(2):179–184. doi: 10.1093/ije/12.2.179. [DOI] [PubMed] [Google Scholar]
- 7.Ramlau-Hansen CH, Thulstrup AM, Nohr EA, Bonde JP, Sorensen TI, Olsen J. Subfecundity in overweight and obese couples. Hum Reprod. 2007;22(6):1634–1637. doi: 10.1093/humrep/dem035. [DOI] [PubMed] [Google Scholar]
- 8.Zaadstra BM, Seidell JC, Van Noord PA, te Velde ER, Habbema JD, Vrieswijk B, et al. Fat and female fecundity: prospective study of effect of body fat distribution on conception rates. BMJ. 1993;306(6876):484–487. doi: 10.1136/bmj.306.6876.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vikat A. Women’s labor force attachment and childbearing in Finland. Demographic Research. 2004;S3(8):177–212. [Google Scholar]
- 10.van Dunne FM, Lard LR, Rook D, Helmerhorst FM, Huizinga TW. Miscarriage but not fecundity is associated with progression of joint destruction in rheumatoid arthritis. Ann Rheum Dis. 2004;63(8):956–960. doi: 10.1136/ard.2002.004291. [DOI] [PMC free article] [PubMed] [Google Scholar]