Abstract
The purpose of this study was to examine ethnic differences in the metabolic responses to a 16-week intervention designed to improve insulin sensitivity (SI), adiposity, and inflammation in obese African-American and Latino adolescents. A total of 100 participants (African Americans: n = 48, Latino: n = 52; age: 15.4 ± 1.1 years, BMI percentile: 97.3 ± 3.3) were randomly assigned to interventions: control (C; n = 30), nutrition (N; n = 39, 1×/week focused on decreasing sugar and increasing fiber intake), or nutrition + strength training (N+ST; n = 31, 2×/week). The following were measured at pre- and postintervention: strength, dietary intake, body composition (dual-energy X-ray absorptiometry/magnetic resonance imaging) and glucose/insulin indexes (oral glucose tolerance test (OGTT)/intravenous glucose tolerance test (IVGTT)) and inflammatory markers. Overall, N compared to C and N+ST reported significant improvements in SI (+16.5% vs. −32.3% vs. −6.9% respectively, P < 0.01) and disposition index (DI: +15.5% vs. −14.2% vs. −13.7% respectively, P < 0.01). N+ST compared to C and N reported significant reductions in hepatic fat fraction (HFF: −27.3% vs. −4.3% vs. 0% respectively, P < 0.01). Compared to N, N+ST reported reductions in plasminogen activator inhibitor-1 (PAI-1) (−38.3% vs. +1.0%, P < 0.01) and resistin (−18.7% vs. +11.3%, P = 0.02). There were no intervention effects for all other measures of adiposity or inflammation. Significant intervention by ethnicity interactions were found for African Americans in the N group who reported increases in total fat mass, 2-h glucose and glucose incremental areas under the curve (IAUC) compared to Latinos (P’s < 0.05). These interventions yielded differential effects with N reporting favorable improvements in SI and DI and N+ST reporting marked reductions in HFF and inflammation. Both ethnic groups had significant improvements in metabolic health; however some improvements were not seen in African Americans.
INTRODUCTION
Impaired fasting glucose and future risk of type 2 diabetes have emerged as significant health issues in obese African-American and Latino youth (1). The highest rates of pediatric type 2 diabetes are documented among 15- to 19-year-old ethnic minority adolescents (1). This ethnic disparity in diabetes risk may result from greater rates of obesity, low-grade inflammation and insulin resistance in African-American and Latino youth relative to non-Latino whites (2,3).
Intervention strategies that target eating behavior and physical activity may be effective ways of reducing metabolic risk factors for type 2 diabetes in minority youth. Our group recently completed a 16-week intervention in obese African-American and Latino adolescents that assessed the incremental effects of the following three intervention groups on reducing adiposity, low-grade inflammation, and improving insulin sensitivity (SI): (i) control; (ii) a once per week modified carbohydrate nutrition education program that focused on decreasing sugar intake and increasing fiber intake; and (iii) same nutrition education program with twice per week strength training (4). In the Latino participants, there were significant improvements in the glycemic response to oral glucose, with significant decreases in oral glucose response by 18% and 6% in the nutrition (N) and nutrition with strength training (N+ST) groups respectively, compared with a 32% increase in the control group (C) (4).
Baseline comparisons between the African-American and Latino participants showed distinct differences in obesity-related metabolic risk factors by ethnicity. Specifically, African Americans had higher volumes of subcutaneous fat and tended to have lower volumes of visceral fat and hepatic fat fraction compared to Latino adolescents (5). Data from the intravenous glucose tolerance test (IVGTT) demonstrated that African-American adolescents were more insulin resistant compared to Latino adolescents and this difference was independent of total fat mass as well as visceral fat mass (5). As a result of this lower SI, African-American adolescents had a compensatory higher acute insulin response to glucose (AIR), which further exacerbated their inherently higher AIR responses to glucose (5). However, β-cell function (as reflected by a greater disposition index (DI)) was enhanced in African Americans compared to Latinos, resulting in greater glucose tolerance as measured by an oral glucose tolerance test (OGTT).
Given these ethnic differences previously reported in adiposity and insulin action between African-American and Latino adolescents (5), we were interested in assessing whether these two ethnic groups would respond similarly to a randomized controlled trial intervention. Therefore, the purpose of this study was to examine ethnic differences in the metabolic responses to a 16-week intervention designed to reduce adiposity and low-grade inflammation as well as improve SI in obese African-American and Latino adolescents. It was hypothesized that changes in carbohydrate quality along with strength gains in the N+ST group would produce the largest improvements in adiposity, low-grade inflammation and SI compared to the C and N groups. Because our African-American participants were more insulin resistant with greater volumes of subcutaneous fat compared to Latino participants at the start of the intervention, we hypothesized that African Americans would demonstrate a greater improvement in metabolic health (i.e., SI, adiposity, and inflammation) compared to their Latino counterparts.
METHODS AND PROCEDURES
Participant characteristics, description of intervention, and general procedures used in this study have been previously reported (4,5), however the most relevant information is described below.
Participants
One hundred obese, but otherwise healthy African-American (n = 48) and Latino (n = 52) boys and girls volunteered to participate in this study. Participants and parents were provided with a full description of the study and all participants signed an informed assent document while consent was obtained from their parents. The University institutional review board approved this study.
Pretesting visit at the General Clinical Research Center (GCRC)
Outpatient visit
Participants arrived at the GCRC at 7:30 AM after an overnight fast to complete a detailed medical history and physical exam where Tanner staging (6,7) was determined as well as weight and height measured to calculate BMI and BMI percentiles (8). Total fat mass and total lean mass were measured by dual energy X-ray absorptiometry using a Hologic QDR 4500W (Hologic, Bedford, MA). Abdominal fat (visceral, subcutaneous, and hepatic fat fraction (HFF)) were assessed by magnetic resonance imaging using a General Electric 1.5-Tesla magnet (GE Healthcare, Waukesha, WI). Multiple-slice axial TR 400/16 view of the abdomen at the level of the umbilicus was analyzed for cross-sectional area of adipose tissue. The standard body transmit and receive coil was used, along with a rectangular field-of-view of 420 mm (right/left) by 315 mm (anterior/posterior). The slice thickness was 10 mm with no inter-slice gaps. The fat-only data set was used in the subsequent quantification of subcutaneous and visceral adipose tissue volume, while the fat fraction data set was used to assess percent hepatic fat content. A commercially available image segmentation and quantification software (SliceOmatic, Tomovision, Magog, Quebec) was used. Subcutaneous and visceral volumes were computed across all 19 image slices in each participant. Hepatic fat fraction was computed as the mean fat fraction in all imaging slices within which the liver was present. Following the exam, a 3-h OGTT was conducted (4,5).
In-patient visit
Approximately 7–14 days following the outpatient visit, participants were admitted to the GCRC in the evening hours and served a standardized dinner and evening snack. After a 12-h fast, participants completed an IVGTT the following morning (4,5).
Randomization
All eligible participants were randomized to one of three groups: (i) a control group, (ii) a once per week modified carbohydrate nutrition education program, and (iii) same nutrition education program with twice per week strength training. Within each ethnicity, randomization was blocked by sex to achieve balance in randomization between sexes. A detailed description of the interventions employed in the present study have been previously reported (4,5).
Dietary intake and strength assessment
During the outpatient visit both before and after the intervention, participants were given 3-day dietary records to complete at home. During the in-patient visit, participants were instructed to bring their completed dietary records to be reviewed and clarified by research staff, who were trained and supervised by a registered dietitian. Nutrition data were analyzed using the Nutrition Data System for Research (NDS-R ver 5.0_35).
Using established procedures (9), upper- and lower-body strength assessments were completed before and after the intervention by one-repetition maximum in the bench press and leg press, respectively.
Post-testing procedures
At the conclusion of the 16-week control/intervention period, participants returned to the GCRC for follow-up testing. The procedures were identical to those conducted at the pretest visits.
Assays
Assays for glucose and insulin blood samples have been previously reported (4,5). Inflammatory markers measured during the IVGTT including: interleukin-8, leptin, tumor necrosis factor-α, monocyte chemotactic protein-1, hepatocyte growth factor, nerve growth factor, adiponectin, plasminogen activator inhibitor (PAI)-1, and resistin were assayed in duplicate using a specific human insulin enzyme-linked immunosorbent assay kit from Linco (St Charles, MO).
Outcome measures
Fasting and 2-h OGTT glucose concentrations were used to determine normal or impaired glucose tolerance as defined by the American Diabetes Association (10). Three-hour glucose and insulin incremental areas under the curve (IAUC) were calculated from the OGTT data, in nmol/l/min. Plasma collected during the IVGTT was analyzed for glucose and insulin, and concentrations were entered into the MINMOD Millenium 2003 computer program (Ver 6.02, Bergman, University of Southern California, Los Angeles, CA) to determine SI, AIR, DI, and glucose effectiveness.
Statistical analysis
Across-group comparisons of baseline characteristics were conducted for evaluable participants using ANOVA to identify possible randomization imbalance. Student’s t-tests and χ2 tests were used to compare ethnic differences in continuous and categorical variables at baseline. An analysis of covariance (ANCOVA) was used to compare ethnic differences in SI and markers of inflammation at baseline, after controlling for preplanned covariates. The main effects and interactions of intervention groups and ethnicity were assessed using a two-way ANCOVA on the change score (post-pre), after controlling for pretest values and preplanned covariates. Covariates included Tanner stage, sex, total fat mass, and total lean mass. Change scores were evaluated for normality and log transformations were made when needed. When significant differences across intervention group were identified, post hoc pairwise comparisons with Bonferroni adjustments were conducted. Residual analyses were performed for all main outcome variables to test for assumption of normality and subsequent outliers greater than two standard deviations (n = 2) were removed from the analyses. For all analyses, α < 0.05.
RESULTS
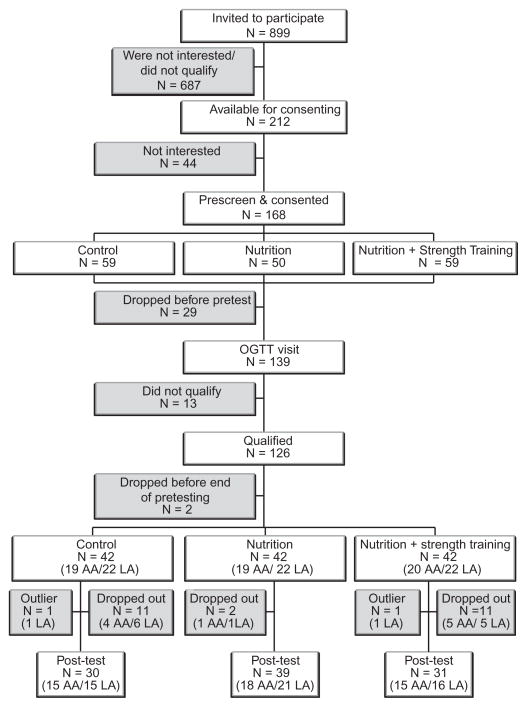
Consort diagram for the African-American and Latino participants is presented in Figure 1. Of the 212 potential participants (aged 14–18 years) who were available for consenting, 126 qualified participants were randomized to one of the three groups. Of these 102 completed the intervention but because two Latino participants were identified as outliers (insulin parameters were >2 s.d. above the mean), they were removed from the analysis. Hence, the evaluable cohort consisted of 100 participants, (African Americans, n = 48; Latinos, n = 52). There were no statistically significant differences in baseline demographics, anthropometrics, or body composition measures between the 24 participants who dropped out of the program and the 100 participants who completed the program. Evaluable participants (n = 100) included 30 in the C group, 39 in the N group, and 31 in the N+ST group.
Figure 1.
Consort diagram of participant flow for African-American (n = 48) and Latino (n = 52) participants. OGTT, oral glucose tolerance test.
Participant characteristics at baseline
The overall mean age of participants (n = 100) was 15.4 ± 1.1 years and the mean Tanner stage was 4.5 ± 0.8 with 41% of the participants’ male (Table 1). Similar to previous findings (5), African-American participants had significantly greater volumes of subcutaneous fat (P < 0.01) as well as lower volumes of visceral fat (P = 0.07) and HFF (P = 0.02) compared to Latino participants. Furthermore, African Americans had significantly lower SI derived from the IVGTT (P < 0.01) and markers of inflammation (P < 0.05; with the exception of adiponectin) compared to their Latino counterparts. When comparing the intervention groups within each group, there were no significant differences in any of the baseline characteristics (data not shown).
Table 1.
Participant characteristics at baseline
| African Americans |
Latinos |
P value | |
|---|---|---|---|
| (n = 48) | (n = 52) | ||
| Tanner stage | 4.6 ± 0.1 | 4.4 ± 0.1 | 0.17 |
| Tanner stage, n (%) | |||
| 1 | 1 (2.1) | 0 (0.0) | – |
| 2 | 0 (0.0) | 1 (1.9) | – |
| 3 | 5 (10.4) | 4 (7.7) | – |
| 4 | 15 (31.3) | 6 (11.5) | – |
| 5 | 31 (64.6) | 37 (71.2) | – |
| Gender, n female (%) | 34 (70.8) | 27 (48.1) | 0.03 |
| Age (years) | 15.3 ± 1.2 | 15.5 ± 1.0 | 0.53 |
| Weight (kg) | 100.0 ± 26.1 | 92.6 ± 22.6 | 0.13 |
| BMI (kg/m2) | 36.0 ± 7.5 | 33.9 ± 6.9 | 0.15 |
| BMI Z-score | 2.2 ± 0.5 | 2.1 ± 0.5 | 0.27 |
| BMI percentile | 97.8 ± 2.5 | 96.8 ± 3.9 | 0.11 |
| Fat-free mass (kg) | 55.6 ± 8.4 | 53.8 ± 9.1 | 0.33 |
| Fat mass (kg) | 35.7 ± 11.4 | 32.8 ± 11.5 | 0.23 |
| Subcutaneous adiposity (liters) | 15.2 ± 6.3 | 9.1 ± 4.0 | <0.01 |
| Visceral adiposity (liters) | 1.3 ± 0.9 | 1.7 ± 0.8 | 0.07 |
| Hepatic fat fraction (%) | 4.1 ± 0.3 | 7.9 ± 1.5 | <0.01 |
| Insulin sensitivity (× 10−4 min−1/μU/ml) | 1.2 ± 0.7 | 1.8 ± 1.4 | 0.01 |
| IL-8 (pg/ml) | 2.6 ± 0.3 | 4.1 ± 0.5 | 0.03 |
| Leptin (ng/ml) | 66.2 ± 4.8 | 46.0 ± 3.8 | <0.05 |
| TNF-α (pg/ml) | 6.4 ± 0.5 | 13.5 ±1.1 | <0.01 |
| MCP-1 (pg/ml) | 191.9 ± 11.6 | 293.5 ± 19.9 | <0.01 |
| HGF (pg/ml) | 996.7 ± 75.2 | 1,388.4 ± 122.1 | 0.02 |
| NGF (μg/ml) | 7.1 ± 1.0 | 19.0 ± 1.5 | <0.01 |
| Adiponectin (μg/ml) | 17.2 ± 1.3 | 18.9 ± 11.1 | 0.69 |
| PAI-1 (μg/ml) | 61.6 ± 4.7 | 122.2 ± 9.8 | <0.01 |
| Resistin (μg/ml) | 27.0 ± 2.2 | 45.2 ± 3.8 | <0.01 |
Data are presented as mean ± s.d. Boldface values represent significant difference by ethnicity (P < 0.05).
HGF, hepatocyte growth factor, IL-8, interleukin-8; MCP-1, monocyte chemotactic protein; NGF, nerve growth factor; PAI-1, plasminogen activator inhibitor; TNF-α, tumor necrosis factor-α.
Changes in strength and dietary variables
For the combined ethnic groups, there was a significant intervention group effect for bench press (P < 0.01; data not shown). The N+ST group had a greater increase in upper body strength compared to the N group (29.7% vs. 2.9%, P < 0.01). There was also a significant intervention group effect for leg press (P < 0.01). The N+ST group increased lower body strength by 24.1% compared to a 9.4% and 2.7% increase in the C and N group, respectively. In addition, there was a significant intervention by ethnicity interaction with African-American participants demonstrating a smaller increase in lower body strength compared to Latino participants (21.0% vs. 26.2%, P < 0.01, Table 2).
Table 2.
Strength and dietary outcomes: intervention group effects for evaluable participants (n = 100)
| Outcomesa | Control (C) (N = 30) |
Nutrition education (N) (N = 39) |
Nutrition + strength training (N+ST) (N = 31) |
Significant effectb | |||
|---|---|---|---|---|---|---|---|
| African Americans (n = 15) | Latinos (n = 15) | African Americans (n = 18) | Latinos (n = 21) | African Americans (n = 15) | Latinos (n = 16) | ||
| Strength | |||||||
| Bench press (lb) | 9.6 (12.8) | 11.7 (9.0) | 5.9 (13.4) | 0.5 (10.7) | 28.3 (14.7)** | 27.2 (14.4)** | I (< 0.01) |
| Leg press (lb) | 51.1 (107.6) | 20.9 (299.1) | 81.6 (70.3)* | −36.4 (114.9)* | 77.0 (80.2)*,** | 127.2 (123.8)*,**,*** | I (<0.01) I × E (<0.01) |
| Dietary | |||||||
| Energy (kcal/d) | 99.6 (710.5) | 352.2 (880.9) | 203.7 (605.9)* | −202.4 (459.5)* | −155.8 (760.6)* | −287.4 (587.0)* | I (<0.01) |
| Carbohydrate (g/d) | 22.5 (81.8) | 46.2 (130.6) | −15.2 (106.6) | −22.3 (94.7) | −19.3 (103.8)* | −38.3 (69.5)* | I (0.02) |
| Protein (g/d) | 7.4 (33.9) | 14.7 (31.6) | 9.0 (40.1) | −4.8 (20.3) | −2.5 (33.7) | −4.7 (22.1) | – |
| Fat (g/d) | −3.5 (35.4) | 12.2 (35.6) | −8.9 (27.8) | −10.2 (20.5) | −6.5 (37.5) | −12.5 (27.5) | – |
| Total sugar (g/d) | −9.3 (38.9) | 11.4 (64.4) | −31.6 (56.7) | −10.1 (51.4) | −18.4 (60.7) | −13.3 (40.4) | I (0.09) |
| Added sugar (g/d) | −9.7 (43.9) | 4.3 (53.9) | −19.3 (41.1)* | −17.6 (51.5)* | −26.6 (45.8)* | −10.4 (40.9)* | I (0.04) |
| Fiber (g/1,000 kcal) | −0.3 (7.0) | 3.8 (7.5) | 4.6 (14.4) | 1.0 (9.5) | 6.6 (10.1) | 1.2 (9.9) | – |
Data are unadjusted change scores: mean (s.d.). Sample sizes for dietary variables are as follows: C (n = 28); N (n = 37); N+ST (n = 29).
P-values were calculated using two-way ANCOVA. Covariates included: Tanner stage, sex, DEXA fat and lean tissue mass. Analyses were based on log scores for the following variables: bench press, leg press, carbohydrate, protein, fat, total sugar, added sugar, and fiber. Mean differences and interactions across intervention (I) and ethnic (E) groups are significantly different from one another using Bonferroni multiple comparisons (P < 0.05).
Denotes significantly different from C group at (P < 0.05).
Denotes significantly different from N group at (P < 0.05).
Denotes significantly smaller improvement in leg press in African Americans compared to Latinos at P < 0.05.
For the combined ethnic groups, there was a significant intervention group effect for total energy intake (P < 0.01; data not shown). The N and N+ST groups decreased their energy intake by 9.2% and 13.7%, respectively, compared to a 15.7% increase in the C group. There was also a significant intervention group effect for carbohydrate intake (P = 0.02). The N+ST group decreased carbohydrate intake by 13.0% compared to a 6.7% decrease in the N group and 17.2% increase in the C group. In addition, there was also a significant intervention group effect for added sugar intake (P = 0.04). The N and N+ST groups decreased added sugar intake by 26.0% and 32.5% respectively, compared to a 1.3% increase in the C group. Furthermore, there was a trend toward decreased total sugar intake in the N and N+ST group (19.6% and 15.9%, respectively) compared to an increase in the C group (4.6%, P = 0.09). There were no significant intervention group effects for dietary protein, fat, or fiber intake. There were also no significant intervention by ethnicity interactions for changes in dietary variables.
Changes in anthropometry and body composition
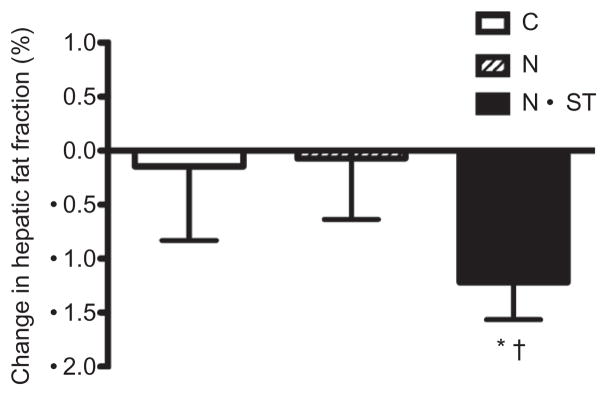
For the combined ethnic groups, there was a significant intervention group effect for HFF (P < 0.01, Figure 2). The N+ST group had a 27.3% decrease in HFF compared to 4.3% decrease in the C group and no change in the N group. There were no significant intervention group differences for BMI, BMI z-score, BMI percentile, or body weight, total lean mass, subcutaneous and visceral fat. There was however a significant intervention group by ethnicity interaction for change in total fat mass with African Americans in the N group demonstrating a greater increase in total fat mass compared to Latinos (+6.4% vs. 1.1%, P = 0.03, data not shown).
Figure 2.
Unadjusted mean ± s.e. change scores for hepatic fat fraction. C, control; N, nutrition education; N+ST, nutrition + strength training. *Significantly different from C; †Significantly different from N at P < 0.05.
Changes in glucose and insulin indexes
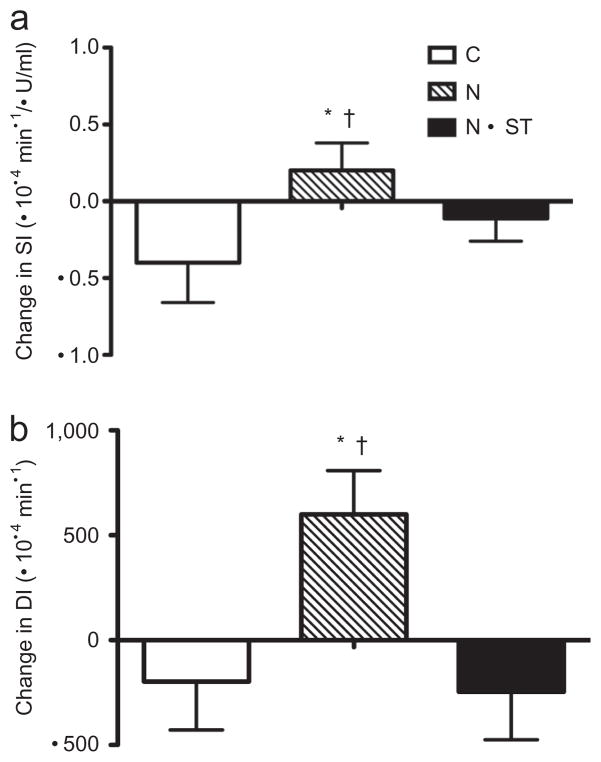
For the combined ethnic groups, there was a significant intervention group effect for SI (P < 0.01, Figure 3). The N group increased by 16.5% compared to the N+ST and C group, which decreased by 6.9% and 32.3%, respectively. Subsequently, there was also a significant intervention group effect for DI (P < 0.01). The N group increased by 15.5% compared to the N+ST and C groups, which decreased by 13.7% and 14.2%, respectively (Figure 3). There were no significant intervention group effects for fasting and 2-h glucose and insulin, glucose and insulin IAUC measured by the OGTT, or for AIR and glucose effectiveness measured by the IVGTT.
Figure 3.
Unadjusted mean ± s.e. change scores for (a) insulin sensitivity (SI) and (b) disposition index (DI). C, control; N, nutrition education; N+ST, nutrition + strength training. *Significantly different from C; †Significantly different from N + ST at P < 0.05.
There were significant group by ethnicity interactions for 2-h glucose (P < 0.01) and glucose IAUC (P = 0.02, Table 3). In the N group, African-American participants had significant increases in 2-h glucose (7.1% vs. 14.5%, P < 0.01) and glucose IAUC derived from the OGTT (14.5% vs. 20.4%, P = 0.02), compared to decreases in Latino participants. In the N+ST group, there was a trend for AIR to decrease in African Americans compared to Latinos (18.0% vs. 2.9%, respectively, P = 0.05).
Table 3.
Glucose and insulin dynamics from oral glucose tolerance test (OGTT) and frequently sampled intravenous glucose tolerance test (FSIVGTT): intervention group effects for evaluable participants (n = 100)
| Outcomesa | Control (C) (N = 30) |
Nutrition education (N) (N = 39) |
Nutrition + strength training (N+ST) (N = 31) |
Significant effectb | |||
|---|---|---|---|---|---|---|---|
| African Americans (n = 15) | Latinos (n = 15) | African Americans (n = 18) | Latinos (n = 21) | African Americans (n = 15) | Latinos (n = 16) | ||
| OGTT (3-h) | |||||||
| Fasting glucose (mg/dl) | −2.7 (3.6) | −5.3 (6.6) | −2.8 (5.9) | −0.9 (7.6) | −2.7 (4.8) | −2.5 (4.8) | – |
| 2–hglucose (mg/dl) | −6.9 (22.3) | 0.7 (20.0) | 7.9 (13.6)*** | −17.0 (26.1) | −0.1 (22.7) | −10.7 (23.6) | I×E (<0.01) |
| Fasting insulin (μU/ml) | 2.2 (6.3) | −1.0 (13.7) | −1.3 (7.5) | −2.6 (7.1) | −2.3 (6.9) | −4.0 (8.6) | – |
| 2-h Insulin (μU/ml) | −1.2 (82.7) | 35.1 (133.0) | 21.8 (55.0) | −80.9 (246.8) | −34.9 (79.3) | −34.1 (110.8) | – |
| Glucose IAUC (nmol/min/l) | 1.9 (36.1) | 22.1 (41.5) | 9.8 (31.5)*** | −19.3 (35.8) | −15.8 (29.0) | −4.1 (37.9) | I×E (0.02) |
| Insulin IAUC (nmol/min/l) | 18.5 (148.6) | −8.1 (156.5) | 33.8 (184.4) | −78.9 (127.2) | −49.1 (100.4) | −43.9 (137.3) | – |
| HOMA | 0.4 (1.7) | −1.1 (1.8) | −0.1 (1.4) | −0.6 (1.9) | −0.7 (1.5) | −1.0 (1.9) | – |
| FSIVGTT | |||||||
| SI (×10−4 min−1/μU/ml) | −0.4 (0.7) | −0.4 (1.8) | 0.1 (0.6)* | 0.3 (1.3)* | 0.0 (0.7) | −0.2 (0.9) | I (<0.01) |
| AIR (μU/ml × 10 min) | 132.3 (753.8) | 199.7 (1,433.5) | 384.3 (400.3) | 29.4 (1,153.1) | −96.8 (569.1) | −40.4 (985.3) | I×E (0.05) |
| DI (×10−4 min−1) | −468.3 (1,003.7) | 53.3 (1,344.3) | 342.8 (1,081.1)*,** | 582.9 (1,090.5)*,** | −209.1 (1,287.6) | −277.3 (1,271.9) | I (<0.01) |
| SG (% per min) | 0.0 (0.02) | 0.0 (0.04) | 0.0 (0.01) | 0.0 (0.04) | 0.0 (0.01) | 0.0 (0.02) | – |
Data are unadjusted change scores: mean (s.d.).
P-values were calculated using two-way ANCOVA. Covariates included: Tanner stage, sex, DEXA fat and lean tissue mass. AIR included SI as a covariate. While unadjusted scores are reported here for all variables, analyses were based on log scores for fasting insulin, 2-hr insulin, insulin IAUC, HOMA, SI, AIR, DI, and SG. Means differences and interactions across intervention (I) and ethnic (E) groups are significantly different from one another using Bonferroni multiple comparisons (P < 0.05).
Denotes significantly different from C group at (P < 0.05).
Denotes significantly different from N+ST group at (P < 0.05).
Denotes significant intervention by ethnicity interaction at P < 0.05.
AIR, acute insulin response to glucose; DI, disposition index; HOMA, homeostasis model assessment; IAUC, incremental areas under the curve; SG, glucose effectiveness; SI, insulin sensitivity.
Changes in markers of inflammation
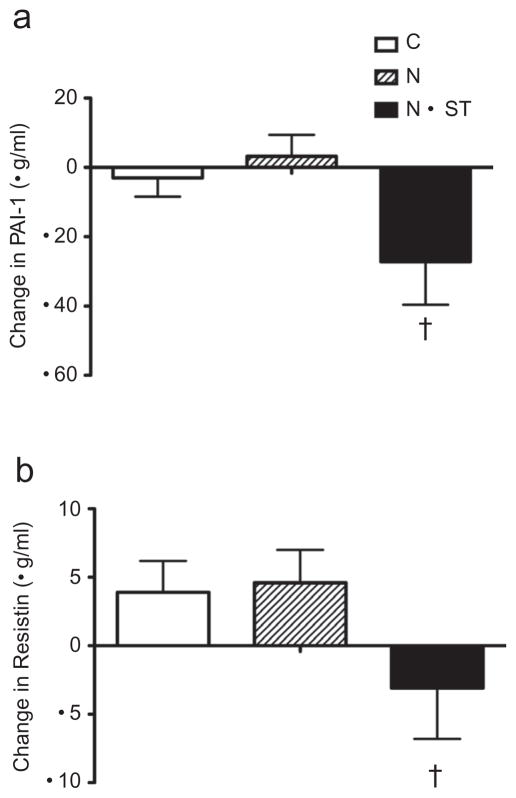
For the combined ethnic groups, there was a significant intervention group effect for PAI-1 (P < 0.01, Figure 4). The N+ST group decreased by 38.3% compared to the N group, which increased by 1.0%. There was also a significant intervention group effect for resistin (P = 0.02). The N+ST group decreased by 18.7% compared to the N group, which increased by 11.3% (Figure 4). There were no other significant intervention group effects for any other markers of inflammation. There was a significant intervention group by ethnicity interaction for nerve growth factor (P < 0.01, Table 4). Specifically, African Americans in the N+ST group had a significant decrease in nerve growth factor compared to an increase in Latinos (−14.9% vs. +20.9%).
Figure 4.
Unadjusted mean ± s.e. change scores for (a) plasminogen activator inhibitor-1 (PAI-1) and (b) resistin. C, control; N, nutrition education; N+ST, nutrition + strength training. †denotes significantly differently from N at P < 0.05.
Table 4.
Markers of inflammation: intervention group effects for evaluable participants (n = 100)
| Outcomesa | Control (C) (N = 30) |
Nutrition education (N) (N = 39) |
Nutrition + strength training (N+ST) (N = 31) |
Significant effectb | |||
|---|---|---|---|---|---|---|---|
| African Americans (n = 15) | Latinos (n = 15) | African Americans (n = 18) | Latinos (n = 21) | African Americans (n = 15) | Latinos (n = 16) | ||
| IL-8 (pg/ml) | −0.2 (0.9) | 0.4 (1.5) | −0.1 (0.5) | −0.6 (3.4) | 0.0 (0.8) | −0.1 (1.3) | – |
| Leptin (ng/ml) | 7.6 (34.6) | −6.6 (15.7) | 0.3 (16.1) | −1.7 (20.2) | 1.3 (17.4) | −4.4 (9.6) | – |
| TNF-α (pg/ml) | 0.3 (2.2) | −0.7 (2.7) | −0.4 (1.9) | −1.1 (5.4) | 0.2 (1.3) | −0.1 (2.9) | – |
| MCP-1 (pg/ml) | −23.3 (59.7) | −36.9 (38.9) | 13.9 (60.4) | −8.3 (78.8) | 16.9 (26.7) | −12.9 (59.7) | – |
| HGF (pg/ml) | 106.6 (272.7) | −102.8 (640.0) | −39.4 (378.3) | −81.6 (741.0) | 53.6 (343.7) | −117.8 (383.3) | – |
| NGF (μg/ml) | 2.2 (2.9) | −1.6 (5.9) | −1.9 (3.1) | −2.9 (19.2) | −1.4 (4.8) | 1.9 (4.1)** | I×E (<0.01) |
| Adiponectin (μg/ml) | 0.4 (5.2) | −0.6 (6.5) | −1.9 (4.3) | 0.8 (7.8) | −4.3 (8.6) | 0.5 (7.8) | – |
| PAI-1 (μg/ml) | 1.6 (14.5) | −7.3 (34.8) | −2.3 (22.6) | 7.9 (45.1) | −18.8 (47.7)* | −35.0 (85.7)*,** | I (<0.01) I× E (0.06) |
| Resistin (μg/ml) | 0.8 (10.4) | 7.0 (12.3) | 0.8 (10.7) | 8.1 (16.1) | −7.5 (21.0)* | 1.4 (19.1)* | I (0.02) |
Data are mean (s.d.).
P-values were calculated using two-way ANCOVA. Covariates included: Tanner stage, sex, DEXA fat and lean tissue mass. Analyses were based on log scores for all variables. Means differences and interactions across intervention (I) and ethnic (E) groups are significantly different from one another using Bonferroni multiple comparisons (P < 0.05).
Denotes significantly different from N group at (P < 0.05).
Denotes significant intervention group by ethnicity interaction at (P < 0.05).
HGF, hepatocyte growth factor, IL-8, interleukin-8; MCP-1, monocyte chemotactic protein; NGF, nerve growth factor; PAI-1, plasminogen activator inhibitor; TNF-α, tumor necrosis factor-α.
DISCUSSION
The primary purpose of this study was to examine ethnic differences in the metabolic responses to a 16-week intervention designed to reduce adiposity and low-grade inflammation and improve SI in obese African-American and Latino adolescents. We hypothesized that changes in carbohydrate quality along with strength gains in the N+ST group would produce the largest improvements in adiposity, inflammation, and insulin action compared to the C and N groups. Because our African-American cohort was more insulin resistant with greater volumes of subcutaneous fat (5), we also hypothesized that the African-American adolescents would have a greater improvement in metabolic health compared to Latino adolescents. Our findings demonstrate that this intervention had differential outcomes, with the N group demonstrating significant improvements in SI and β-cell function, while the N+ST group exhibited marked reductions in HFF and markers of inflammation. These improvements were reported in both African-American and Latino adolescents, however ethnic differences were present in some of the metabolic responses to the interventions. Specifically, in the nutrition intervention group, 2-h glucose concentrations and glucose IAUC during the OGTT as well as total fat mass increased in the African-American cohort compared to general improvements for these outcomes in the Latino cohort. In the N+ST group however, African Americans experienced greater decreases in AIR, nerve growth factor, and PAI-1 compared to Latinos. To our knowledge this is one of the first studies to examine ethnic differences in the metabolic responses to a nutrition and physical activity intervention.
Improvements in insulinemia and glycemia in the N group
Few intervention studies have been conducted that focus on reducing sugar and increasing fiber intake however our findings are in agreement with Ebbeling et al. (11) who reported that a low glycemic load diet improved insulin resistance compared to a conventional low-fat diet in 16 overweight adolescents (aged 13–21). In this study, reductions in dietary energy intake, and more specifically added sugar intake resulted in significant improvements in SI and β-cell function. Specifically, SI and β-cell function improved by 16.5% and 15.5%, respectively, in the N group. These glycemic improvements may be related to the change in fiber-to-carbohydrate ratio noted in the N group only (12). Post hoc analyses reported a greater postintervention fiber-to-carbohydrate ratio in the N group compared to the C group (P = 0.05). This change was not associated with improvements in 2-h glucose, glucose IAUC, AIR, DI, the improvement in SI was no longer significant when fiber-to-carbohydrate ratio was included as covariate. Hence, the maintenance of fiber intake while reducing carbohydrate intake may have mediated the improvement in SI in the N group. Surprisingly, there were no significant reductions in adiposity in the N group however, it is possible that the significant reductions in total caloric intake and carbohydrate intake in this group were insufficient to reduce fat mass, particularly HFF.
It is important to note that the improvements in SI and β-cell function previously published in the Latino cohort did not reach statistical significance (4). The differences in the results reported by Davis et al. (4) and this study are likely explained by a larger sample size employed (n = 54 vs. n = 100), which gave us more power to detect a significant difference in SI and β-cell function. This larger sample size however, was not powered to detect sex differences. An even larger sample size would have allowed for better exploration of sex differences across intervention and ethnic groups.
Reductions in adiposity and inflammation in the N+ST group
In this study, strength training paired with dietary changes resulted in significant reductions in HFF in both ethnic groups. Specifically, HFF decreased by 27.3% in the N+ST group compared to a 4.3% decrease in the C group and no change in the N group. This reduction in HFF may be related to reductions in inflammatory markers reported in this intervention group (13). PAI-1 decreased 38.3% in the N+ST group compared to a 1.0% increase in the N group and resistin decreased by 18.7% compared to an 11.3% increase in the N group. Post hoc analyses also revealed reductions in monocyte chemotactic protein-1 when subcutaneous and visceral fat were included as covariates (P = 0.01). A cause and effect mechanism cannot be deduced from this study, nevertheless these findings suggest that combination therapy that targets both exercise and eating behaviors attenuate potential increases in fat mass previously reported with strength training alone (14) and reduces HFF and markers of inflammation in obese African-American and Latino adolescents.
Reductions in added sugar intake along with reductions in HFF and inflammation did not result in improved SI and β-cell function in the N+ST group. One explanation for this null finding is the possibility of energy compensation in response to the strength training. Little is known about the effects of energy compensation in response to exercise in children, however research in adults have demonstrated that when individuals exercise they often compensate with increased energy intake and this compensation is even more pronounced in females. Thus, the participants in the N+ST group may have altered their dietary intake or habitual levels of physical activity in response to the exercise throughout the program and the diet records collected after completion of the intervention might not reflect this acute compensatory intake.
Ethnic disparity in diabetes risk
This intervention was successful at retaining minority youth throughout the course of the study (African-American participant retention: 81.0% and Latino participant retention: 81.8%). In addition, adherence rates were similar across both intervention groups with all evaluable participants attending a minimum of 12 of the 16-week nutrition classes and a minimum of 28 of the 32 exercise classes. As a result, this randomized controlled trial resulted in significant improvements in select aspects of metabolic health in both African-American and Latino adolescents at increased risk for type 2 diabetes. This intervention was however insufficient to eliminate the ethnic disparities in adiposity, SI, and inflammation reported at baseline. At postintervention, African Americans in the N group were still more insulin resistant compared to Latinos (1.2 vs. 2.0 × 10−4·min−1·μU/ml, respectively) and Latinos in the N+ST group still had greater volumes of HFF compared to African Americans (6.3% vs. 3.6%, respectively). In addition, Latinos in the N+ST group also had greater concentrations of PAI-1 and resistin compared to African Americans at the conclusion of the intervention (PAI-1: 90.1 vs. 50.7 μg/ml, respectively; resistin: 36.7 vs. 20.4 μg/ml, respectively). In response to the nutrition intervention, African Americans tended to gain weight, fat mass in particular, relative to the Latinos and exhibited no improvement in glycemic control. Post hoc analyses also revealed that African Americans in the N group also had a significant increase in 2-h insulin and monocyte chemotactic protein-1 compared to Latinos, when subcutaneous and visceral fat were used as covariates. Taken together these findings suggest that metabolic responses to dietary interventions may vary by ethnicity and further research is warranted to better identify appropriate intervention strategies aimed at reducing the disparity in adiposity, inflammation, and SI between African-American and Latino adolescents.
In summary, this 16-week randomized controlled trial, designed specifically for obese African-American and Latino adolescents living in the greater Los Angeles area resulted in differing outcomes based on intervention group. The intervention focused on changing the quality of carbohydrate intake resulted in significant improvements in SI (16.5%) and β-cell function (15.5%). When the dietary intervention was combined with strength training, these improvements were ameliorated but marked reductions were seen in other outcomes, including HFF (27.3%), PAI-1 (38.3%), and resistin (18.7%). Despite the expected ethnic differences in baseline measures of SI, adiposity, and markers of inflammation, similar improvements in metabolic outcomes were reported in both ethnic groups. However, not all improvements were noted in the African Americans whose outcomes related to glucose tolerance and total fat mass worsened. These results emphasize the importance of promoting healthy eating and physical activity to target underlying metabolic abnormalities that increase risk for type 2 diabetes. Furthermore, these findings also highlight the possibility that response to dietary interventions may vary between African-American and Latino adolescents.
Acknowledgments
This work was supported by the USC Transdisciplinary Research on Energetics and Cancer (U54 CA 116848), the National Institute of Child Health and Human Development (RO1 HD/HL 33064), the Dr Robert C. and Veronica Atkins Foundation, the National Cancer Institute (T32 CA 09492). C.K.R. was supported during this project by a National Institute on Aging Training Award (5 T32 AG000093-24). We would like to thank the SANO LA (Strength and Nutrition Outcomes for Latino Adolescents) and STAND (Strength Training and Nutrition Development in African American Youth) team as well as the nursing staff at the GCRC. In addition, we are grateful for our study participants and their families for their involvement.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Dabelea D, Bell RA, D’Agostino RB, Jr, et al. Writing Group for the SEARCH for Diabetes in Youth Study Group. Incidence of diabetes in youth in the United States. JAMA. 2007;297:2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- 2.Carroll JF, Fulda KG, Chiapa AL, et al. Impact of race/ethnicity on the relationship between visceral fat and inflammatory biomarkers. Obesity (Silver Spring) 2009;17:1420–1427. doi: 10.1038/oby.2008.657. [DOI] [PubMed] [Google Scholar]
- 3.Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care. 2002;25:2184–2190. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 4.Davis JN, Kelly LA, Lane CJ, et al. Randomized control trial to improve adiposity and insulin resistance in overweight Latino adolescents. Obesity (Silver Spring) 2009;17:1542–1548. doi: 10.1038/oby.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasson RE, Adam TA, Davis JN, et al. Ethnic differences in insulin action in obese African American and Latino adolescents. J Clin Endocrinol Metab. 2010;95:4048–51. doi: 10.1210/jc.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mei Z, Grummer-Strawn LM, Pietrobelli A, et al. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr. 2002;75:978–985. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- 9.Faigenbaum AD, Milliken LA, Westcott WL. Maximal strength testing in healthy children. J Strength Cond Res. 2003;17:162–166. doi: 10.1519/1533-4287(2003)017<0162:mstihc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Association AD. Standards of medical care in diabetes-2009. Diabetes Care. 2009;32:S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebbeling CB, Leidig MM, Sinclair KB, Hangen JP, Ludwig DS. A reduced-glycemic load diet in the treatment of adolescent obesity. Arch Pediatr Adolesc Med. 2003;157:773–779. doi: 10.1001/archpedi.157.8.773. [DOI] [PubMed] [Google Scholar]
- 12.Aleixandre A, Miguel M. Dietary fiber in the prevention and treatment of metabolic syndrome: a review. Crit Rev Food Sci Nutr. 2008;48:905–912. doi: 10.1080/10408390701761886. [DOI] [PubMed] [Google Scholar]
- 13.Tsochatzis EA, Papatheodoridis GV, Archimandritis AJ. Adipokines in nonalcoholic steatohepatitis: from pathogenesis to implications in diagnosis and therapy. Mediators Inflamm. 2009;2009:831670. doi: 10.1155/2009/831670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benson AC, Torode ME, Fiatarone Singh MA. The effect of high-intensity progressive resistance training on adiposity in children: a randomized controlled trial. Int J Obes (Lond) 2008;32:1016–1027. doi: 10.1038/ijo.2008.5. [DOI] [PubMed] [Google Scholar]