Abstract
Previous comparisons of territorial and gregarious finches (family Estrildidae) suggest the hypothesis that arginine vasotocin (VT) neurons in the medial bed nucleus of the stria terminalis (BSTm) and V1a-like receptors in the lateral septum (LS) promote flocking behavior. Consistent with this hypothesis, we now show that intraseptal infusions of a V1a antagonist in male zebra finches (Taeniopygia guttata) reduce gregariousness (preference for a group of 10 versus 2 conspecific males), but have no effect on the amount of time that subjects spend in close proximity to other birds (“contact time”). The antagonist also produces a profound increase in anxiety-like behavior, as exhibited by an increased latency to feed in a novelty-suppressed feeding test. Bilateral knockdown of VT production in the BSTm using LNA-modified antisense oligonucleotides likewise produces increases in anxiety-like behavior and a potent reduction in gregariousness, relative to subjects receiving scrambled oligonucleotides. The antisense oligonucleotides also produced a modest increase in contact time (irrespective of group size). Together, these combined experiments provide clear evidence that endogenous VT promotes preferences for larger flock sizes, and does so in a manner that is coupled to general anxiolysis. Given that homologous peptide circuitry of the BSTm-LS is found across all tetrapod vertebrate classes, these findings may be predictive for other highly gregarious species.
Central nonapeptide circuits play phylogenetically widespread roles in the modulation of social behaviors and stress responses and often exert their effects in a species-specific manner (Engelmann et al., 2004; De Vries and Panzica, 2006; Donaldson and Young, 2008; Veenema and Neumann, 2008; Choleris et al., 2009; Goodson and Thompson, 2010). These circuits arise primarily from magnocellular and parvocellular neurons in the preoptic area and hypothalamus that produce either arginine vasotocin (VT; in nonmammalian vertebrates) or arginine vasopressin (VP; in mammals), plus a single oxytocin-like peptide form in any given species. In addition to these cell groups, virtually all tetrapods, including humans, exhibit VT/VP neurons in the medial extended amygdala, primarily within the medial bed nucleus of the stria terminalis (BSTm) (De Vries and Panzica, 2006; Goodson and Thompson, 2010). Unlike the various hypothalamic VT/VP cell groups, the BSTm neurons and their projections to basal forebrain sites such as the lateral septum (LS), medial preoptic area and habenula (De Vries et al., 1983; Absil et al., 2002) are typically sexually dimorphic, with males expressing more cells and a higher density of projections than females, are strongly regulated by sex steroids in most seasonally breeding species, and virtually disappear in animals that are in non-reproductive condition (De Vries and Panzica, 2006). Interestingly, these latter two features are not exhibited by several finch species that breed opportunistically based upon unpredictable rainfall, including the zebra finch (Estrildidae: Taeniopygia guttata) (Kabelik et al., 2010).
Another notable characteristic of the BSTm VT/VP neurons is their sensitivity to the valence of social stimuli, such that these neurons increase their transcriptional (Fos) activity in response to positive, affiliation-related stimuli, and show no response or even a decreased response to aversive stimuli (Goodson and Wang, 2006; Goodson et al., 2009b). This functional profile has now been demonstrated in C57BL/6J mice (Ho et al., 2010) and also in five estrildid finch species that are all monogamous and biparental, but that differ selectively in their species-typical group sizes (ranging from territorial pairs to flocks of hundreds; (Goodson and Wang, 2006). Simple exposure to a same-sex conspecific through a wire partition significantly increases Fos expression in the BSTm VT cells of highly gregarious finches that naturally affiliate with same-sex individuals, but decreases VT-Fos colocalization (i.e., the percent of VT neurons that colocalize Fos) in territorial finch species that typically attack or avoid same-sex individuals. In addition, VT-Fos colocalization in the BSTm is increased in the territorial finches following exposure to the subject’s pair bond partner (a presumably positive stimulus), whereas in the highly gregarious zebra finch, courtship-induced increases in VT-Fos colocalization are blocked by intense subjugation (a presumably negative stimulus) (Goodson and Wang, 2006). Similarly, in male C57BL/6J mice, BSTm VP neurons exhibit a robust Fos response to copulation and a modest Fos response to nonaggressive same-sex interaction, but no specific response to male-male fights (Ho et al., 2010).
In addition to the species differences in VT neuronal responses, signaling from the BSTm VT neurons may be essentially magnified in the gregarious finch species, relative to the territorial species, in a few different ways. First, the flocking species exhibit significantly higher levels of constitutive VT-Fos colocalization than do the two territorial species. If this constitutive transcriptional activity is associated with actual VT release, which remains to be demonstrated, VT derived from the BSTm may play a relatively greater role in tonic brain modulation in gregarious finches as compared to territorial finches. Tonic release may be further compounded in the two most gregarious species given that they exhibit approximately 10 times the number of VT neurons in the BSTm than do the less social species (Goodson and Wang, 2006). Finally, all three flocking species exhibit significantly higher densities of V1a-like VT binding sites in the LS than do the territorial species (Goodson et al., 2006).
These findings strongly suggest the hypothesis that VT circuitry of the BSTm and LS promotes gregariousness, which we explicitly test in the present experiments, first using intra-LS infusions of a V1a receptor antagonist in male zebra finches and a recently developed behavioral assay of group size preference (Goodson et al., 2009c). However, approaches such as this do not directly determine the contributions of VT cells in the BSTm. In fact, despite three decades of research into the anatomy and physiology of the BSTm VT/VP cell group, the specific contributions of these cells to behavior have not been directly demonstrated and are difficult to infer from pharmacological manipulations given that 1) VT/VP projections from the BSTm appear to partially overlap with those from hypothalamic populations, 2) magnocellular hypothalamic neurons may bathe much of the brain in peptide released volumetrically from dendrites and soma, and 3) paracrine modes of action appear to be widespread (Landgraf and Neumann, 2004; Ludwig and Leng, 2006; Goodson and Kabelik, 2009). Thus, a major goal of the second experiment was to determine the direct contribution of the BSTm VT cells to gregariousness, which we achieved through the use of VT antisense oligonucleotide infusions into the BSTm.
Given the importance of septal VP for anxiety modulation in rodents (Landgraf et al., 1995; Everts and Koolhaas, 1999; Bielsky et al., 2005), we additionally examined the effects of antisense and receptor antagonism on two measures of anxiety-like behavior – exploration of a novel environment and novelty-suppressed feeding. All together, the present experiments provide clear evidence that endogenous VT titrates gregariousness, and does so in a manner that is coupled with unexpected anxiolytic behavioral effects.
Methods
Subjects
A total of 97 adult male zebra finches were used as subjects in the behavioral experiments, 68 of which exhibited accurate cannula placement and were retained for analyses. Of those 97 males, 15 were used exclusively for antisense validation tests, as were 5 females. Validation tests were also conducted using tissue from experimental subjects, as described below. Subjects were housed in groups of 6–10 same-sex individuals on a 14L:10D photoperiod with full spectrum lighting and were provided finch seed mix, cuttlebone, grit, and water ad libitum. Experiments were conducted in a humane manner and in full compliance with all federal and institutional regulations.
Vasotocin antisense production and validation
Antisense production
RNA was collected from zebra finch brains, and 5′ RACE techniques were used to generate cDNA (5′ RACE System for Rapid Amplification of cDNA Ends; Invitrogen, Carlsbad, CA). The 5′ end of the VT gene was PCR amplified from multiple brains using gene-specific downstream primers to determine if there were any polymorphisms surrounding the start codon. An antisense, LNA-modified 15-mer antisense oligonucleotide was synthesized for consensus sequence beginning 9 nucleotides upstream from the start codon, as well as an LNA-modified scrambled control using the same 15 nucleotides (Exiqon, Woburn, MA). Sequences were searched on BLAST (National Center of Biotechnology, Bethesda, MD) to ensure no significant alignment with other known transcripts. The sequence for the zebra finch VT LNA antisense was CT+CTGC CAT GG+C T+CA, and the sequence for the zebra finch VT LNA scrambled oligonucleotide was AG+C GTA TCT TG+C C+CC.
Antisense validation
Zebra finches exhibit very large individual variation in the number of BSTm VT-ir cells, with the standard deviation in cell number being only slightly less than the mean (e.g., present validation data from hemispheres infused with scrambled oligonucleotides, and data from normal zebra finches; (Goodson et al., 2009b), suggesting that between-subjects comparisons of birds infused with scrambled and antisense oligonucleotides would produce a very imprecise estimate of knockdown. Thus, in order to accurately quantify antisense efficacy, 15 bilaterally cannulated male birds (8 of which exhibited accurate bilateral cannula placement and were retained for analysis) were infused with VT antisense oligonucleotides into one side of the BSTm and scrambled oligonucleotides into the contralateral side (each 1 μg in 0.25 μl of isotonic saline) at 12 h intervals for 3 days. An additional 5 animals were used for a similar assay of saline versus scrambled oligonucleotides in order to determine whether the scrambled oligonucleotides had unanticipated effects on transcription. Finally, in order to determine whether the antisense may have produced nonspecific knockdown of other proteins, we also labeled BSTm tissue for calbindin and aromatase using tissue from 7 randomly selected antisense subjects and tissue from 7 scrambled control subjects.
Similar to mammals (Moore and Lowry, 1998), birds exhibit many VT cell groups, including populations in the preoptic area; and suprachiasmatic, paraventricular (PVN), and supraoptic nuclei of the hypothalamus; plus numerous accessory cell clusters in the anterior and lateral hypothalamus (Aste et al., 1996; Panzica et al., 1999). Given that our present hypotheses are focused on the BSTm and LS, we did not target these many other cell groups, although they may also be involved in similar functions (but see Goodson and Kabelik, 2009). However, in order to ensure that our infusions were selectively targeting the BSTm and not diffusing to other structures, we additionally conducted VT cell counts in the PVN, the closest hypothalamic population to the BSTm.
Surgeries and infusions
In order to determine the functions of the VT circuitry in the BSTm and LS, we first infused a V1a receptor antagonist into the LS, which is a primary target of VT projections from the BSTm, but not a site of VT production. In a second experiment, we then knocked down VT production in the BSTm using antisense oligonucleotides.
For the antagonist experiments, subjects were stereotaxically fitted with a unilateral 26-ga cannula directed at the right LS (Fig. 1A; small animal cannulae from Plastics One, Akron, OH). Subjects were anesthetized with isoflurane vapor delivered at 1.5–3.5% of a compressed air flow. Cannulae were referenced to the anterior pole of the cerebellum, and then moved 2.9 mm rostral to the reference point, 1.7 mm right of the midline, and inserted at a 26° angle to avoid midline vasculature. Guide cannulae were advanced 2.3 mm into the brain. The cannula was mounted to the skull using dental acrylic and veterinary-grade cyanoacrylate glue. The skin was then glued and at least 5 days recovery prior to infusions and behavioral testing were allowed. Approximately 1 h before behavioral testing, subjects were infused with either 250 ng of a selective V1a antagonist [β-Mercapto-β,β-cyclopentamethylenepropionyl1, O-Me-Tyr2, Arg8]-Vasopressin (V2255, Sigma-Aldrich, St. Louis, MO) or saline control delivered in a counterbalanced order with 2 days between tests. Injectors extended 1 mm beyond the tip of the guide cannula. For logistical purposes, not all subjects were tested in each of the three behavioral paradigms.
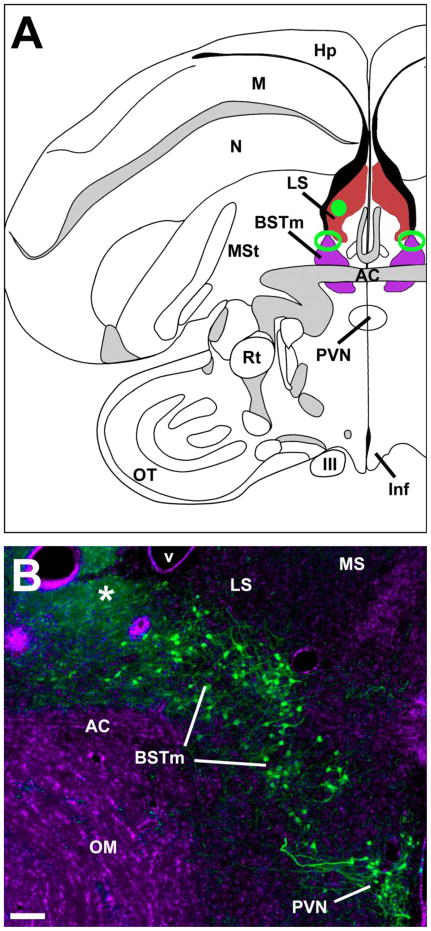
Fig. 1.
(A) Location of the medial bed nucleus of the stria terminalis (BSTm; purple), lateral septum (LS; red), and modal injection sites in each site (green; open and solid, respectively). Most placements for both targets were positioned rostrocaudally at the level of the anterior commissure (AC). For the BSTm, placements were required to be within 120 μm rostral or caudal of the AC, and centered in the area indicated, which spans the dorsolateral BSTm and immediately adjacent portions of the lateral BST (BSTl) and LS. For the LS, we simply required that infusions be centered on the LS, since previous results show that infused substances diffuse to virtually the entire structure (Goodson, 1998). (B) A representative BSTm infusion site (damaged tissue; asterisk) at the caudal margin of the AC. DAPI nuclear stain is shown pseudocolored purple; VT immunoreactivity is labeled green. This photo is from a control subject that received infusions of scrambled oligonucleotides. Note that the tip of the guide cannulae was 1 mm dorsal to this site, and that only the tip of the injector reached the BSTm. Scale bar = 100 μm. Other abbreviations: III, third cranial nerve; Hp, hippocampus; Inf, infundibulum; M, mesopallium; MS, medial septum; MSt, medial striatum; N, nidopallium; OM, occipitomesencephalic tract; OT, optic tectum; PVN, paraventricular nucleus of the hypothalamus; Rt, nucleus rotundus (thalamic).
For the antisense experiments, subjects were stereotaxically fitted with a bilateral 26-ga cannula device (1.5 mm tip separation; Plastics One) aimed at the dorsolateral aspect of the BSTm (Fig. 1). After referencing to the anterior pole of the cerebellum, cannulae were moved 2.8 mm rostral and then advanced 3.0 mm into the brain. Other surgical methods follow those just described. At least 5 days recovery prior to infusions and behavioral testing were allowed. Subjects were bilaterally infused with either 1 μg VT antisense oligonucleotides or scrambled oligonucleotides in 0.25 μl of isotonic saline at 12 h intervals for 3 days. Injectors extended 1 mm beyond the tip of the guide cannula. Group size preference tests were conducted on the afternoon before the last infusion, and other tests were conducted the following morning, approximately 12–15 h after the sixth and final infusion. For logistical purposes, not all subjects were tested in each of the three behavioral paradigms described below. Subjects were excluded from analysis if both injector tips were not accurately placed in the dorsolateral BSTm or immediately adjacent portion of the lateral BST.
Behavioral testing
Gregariousness and social contact
For the antagonist experiment, subjects were tested for group size preference (gregariousness) and social contact in a counterbalanced, within-subjects design with two days between tests. Infusions of the V1a antagonist were administered 30 min prior to testing. For the antisense experiments, these measures were quantified prior to surgery and again after 2.5 days of bilateral infusions, in a between-subjects design. Both of these experimental designs allowed us to substantially control for individual differences in behavior.
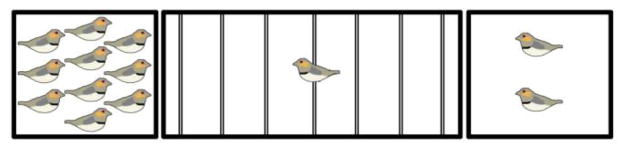
For both the antagonist and antisense experiments, gregariousness and social contact behavior were measured using the group size choice apparatus shown in Fig. 2 (Goodson et al., 2009c). Subjects were placed in a 1m wide central cage (43 cm H × 36 cm D) containing seven perches with two 46 cm cages on the sides, one containing a group of 2 same-sex conspecifics and the other 10 same-sex conspecifics. For the between-subjects antisense experiments, the location of the subject was recorded each 15 sec for 4 min, and then the cages on the side holding 2 or 10 birds were switched in order to control for side biases and the location of the subject was recorded again each 15 sec for 4 min. For the within-subjects antagonist experiments, the location of the subject was recorded each 15 sec for 4 min on each of the two test days, counterbalancing for drug order and side biases across the two days. “Social contact” was operationally defined as the percent of test time that subjects spent on the perches immediately adjacent to conspecifics, both ends combined, and group size preference (“gregariousness”) was operationally defined as the percent of social contact time that subjects spent on the perch directly adjacent to the larger group.
Fig. 2.
Testing apparatus for group size preference and social contact. Subjects are placed in a 1m-wide central cage containing seven perches (shown as thin lines). The subject’s location is recorded each 15 sec for 5 min. The percent of test time spent on the two end perches combined yields a measure of social contact (“contact time”). The percent of contact time that is spent next to the large group yields a measure of group size preference (“gregariousness”).
Anxiety-like behavior
Novelty-suppressed feeding tests and exploration tests were used to measure anxiety-like behaviors. Exploration of a novel environment is a classic anxiety assay in mammals and has been widely employed in birds (Groothuis and Carere, 2005; Biondi et al., 2010), including zebra finches (Martins et al., 2007; Scheutt, 2009). The behavior measured by this kind of assay is replicable and has a strongly heritable component in songbirds (Groothuis and Carere, 2005). The novelty-suppressed feeding test is an increasingly popular anxiety measure (Leuner et al.; Bilkei-Gorzo et al., 2002; Catts et al., 2008; Greene et al., 2009) that is easily adapted to birds.
For both the antagonist and antisense experiments food was removed prior to lights-on in the morning. For the antagonist experiment, subjects received intraseptal infusions of the V1a antagonist or saline vehicle and were tested approximately 1 h later, first in the novelty-suppressed feeding test, and then the exploration test. For the antisense experiments, subjects were tested for novelty-suppressed feeding within approximately 1h of lights-on, followed immediately by the exploration test. These tests were conducted in a between-subjects design for both the antagonist and antisense experiments. For the novelty-suppressed feeding tests, subjects were placed in a small cage (31 cm W × 20 cm H × 36 cm D) containing a food dish with an unfamiliar object (a purple nitrile glove) hanging above it. Testing was conducted in the housing room, although subjects were visually isolated from other birds. Subjects were videotaped for 30 (antisense experiments) or 35 min (antagonist experiments), and the latency to feed was recorded. For exploration tests, subjects were placed in a novel cage (1.3 m W × .43 m H × .36 m D) containing 3 clusters of novel evergreen branches, and the latency to move, number of hops, and the number of visits to evergreen clusters (i.e., zone changes) were recorded during a 3 min test period.
Directed song (antisense experiment only)
Previous experiments using intraventricular and intraseptal administrations of VT and a V1a antagonist strongly suggest that VT does not influence directed courtship singing in male zebra finches (Goodson and Adkins-Regan, 1999; Goodson et al., 2004; Kabelik et al., 2009b). Thus, directed song testing provides a good assay to determine whether antisense treatments produced effects on social behavior that are likely not related to VT knockdown per se. Males were tested pre-surgery and after 2.5 days of bilateral infusions. Subjects were placed in a small cage (31 cm W × 20 cm H × 36 cm D) that was divided into halves by a wire barrier. After 3 min, an unfamiliar female was placed on the other side of the wire barrier and the number of songs directed toward the female was recorded for 2 min.
Statistics
Most behavioral data were not normally distributed and were therefore analyzed using Mann-Whitney tests for between-subjects comparisons and Wilcoxon signed-ranks tests for within-subjects comparisons. Exploration data were normally distributed and analyzed using t-tests.
Histology and immunocytochemistry
Immediately after post-infusion behavioral testing, subjects were killed by isoflurane overdose, and perfused with 0.1M phosphate buffered saline followed by 4% paraformaldehyde. Tissue was sectioned into three 40 μm series, one of which was mounted onto chrom-alum subbed slides to verify cannula placement. For validation assays, other tissue sections through the BSTm and PVN were immunofluorescently labeled for VT, or for calbindin and aromatase. Tissue was rinsed 5x for 10 min in 0.1M phosphate buffered saline (PBS; pH 7.4), incubated for 1 h in block (PBS + 5% normal donkey serum + 0.3% Triton-X-100), and were then incubated for approximately 40 h at 4 C in PBS + 2.5% normal donkey serum + 0.3% Triton-X-100 containing either guinea pig anti-VP (1:1000; Bachem, Torrance, CA) or a monoclonal mouse anti-calbindin (1:100; Abcam, Cambridge, MA) combined with a custom sheep anti-aromatase (1:1000; Bethyl Labs, Montgomery, TX) antibodies (Kabelik et al., 2009a). The primary incubation was followed by two 30 min rinses in PBS. For VT labeling, tissues was incubated for 1 h in a donkey anti-guinea pig secondary conjugated to a donkey anti-guinea pig biotin (8:1000; Jackson Immunoresearch Labs, West Grove, PA). All tissue was then rinsed twice for 15 min rinses in PBS, and incubated for 2 h at room temperature in streptavidin conjugated to Alexa Fluor 488 (3:1000) for VT labeling, or both donkey anti-sheep secondary conjugated to Alexa Fluor 680 (6:1000) for aromatase and donkey anti-mouse conjugated to Alexa Fluor 488 (3:1000) for calbindin (secondaries from Invitrogen, Carlsbad, CA), in PBS + 2.5% normal donkey serum + 0.3% Triton-X-100. Following two 30 min rinses in PBS, the sections were mounted on slides subbed with gelatin and chrome alum and were cover-slipped with ProLong Gold antifade reagent with DAPI nuclear stain (Invitrogen). Images were aquired at 10x using a Zeiss AxioImager microscope outfitted with a Z-drive and an Apotome optical dissector (Carl Zeiss Inc., Göttingen, Germany), and cell counts were conducted blindly from flattened Z-stacks by an observer using Photoshop CS3 (Adobe Systems, San Jose, CA) and Image J (National Institutes of Health, Bethesda, MD). The VT-ir population of the BSTm is segregated from other VT-ir cell groups and thus we simply counted all cells that occurred in the BSTm region. For control counts of aromatase and calbindin, standardized box outlines were superimposed on the photomicrographs (boxes were of a size that easily fit within the BSTm area as defined by VT in control tissue) and all cells in the boxes were counted.
Results
Intraseptal infusions of a V1a antagonist decrease gregariousness (group size preference) but not time in social contact
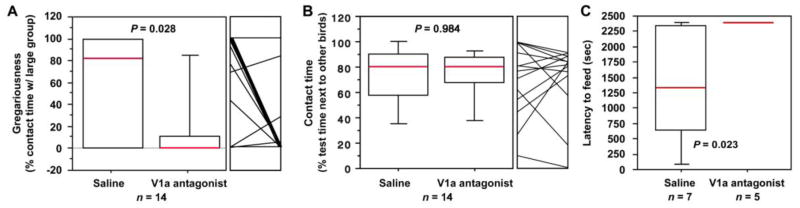
As shown in Fig. 3A, antagonist infusions virtually abolished interest in the large group, reducing the percent of social contact time that subjects spent with the large group from a median of 82% in the saline condition to 0% following infusions of the antagonist (tied P = 0.028; Wilcoxon signed ranks). In contrast, the V1a antagonist had no effect on time spent in social contact (tied P = 0.9839; Fig. 3B).
Fig. 3.
Intraseptal infusions of a VP V1a antagonist reduce gregariousness and increase anxiety-like behavior. Relative to vehicle, antagonist infusions reduce gregariousness (percent of contact time spent with the larger group; see Fig. 1) (A), but not social contact time (B), and increase the latency to feed in the presence of a novel object (C). Social choice tests were run in a counterbalanced within-subjects design; novelty-suppressed feeding tests were conducted in a between-subjects design. Box plots show the median (red line), 75th and 25th percentile (box) and 95% confidence interval (whiskers). For within-subjects tests (A and B), data for each individual are shown in the small panel to the right of the box plot.
Intraseptal infusions of a V1a antagonist increase anxiety-like responses to novelty
Relative to subjects receiving saline, subjects that received intraseptal infusions of the V1a antagonist not only exhibited an increased latency to feed, but failed to feed entirely (tied P = 0.0236; Mann-Whitney; Fig. 3C). In contrast, no significant effect was observed for exploration of a novel environment (saline, 2.25 ± 0.98 zone changes; antagonist, 1.67 ± 1.17; P = 0.7075; unpaired t-test).
VT antisense oligonucleotides produce selective effects on VT production
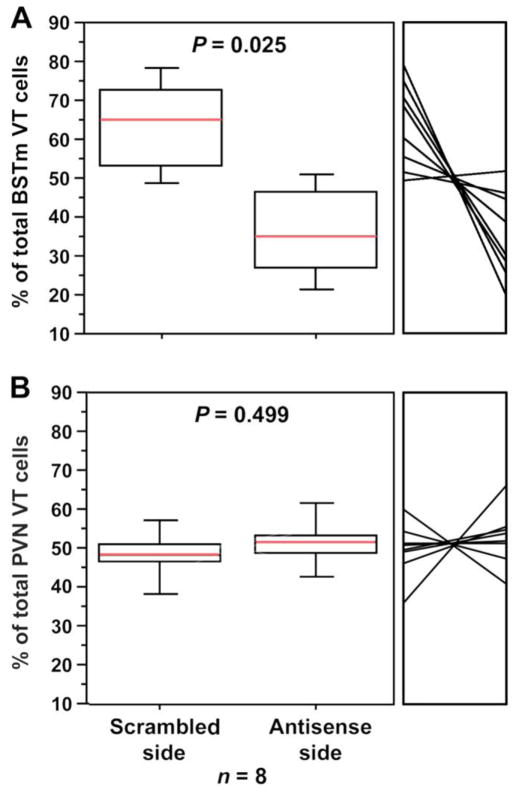
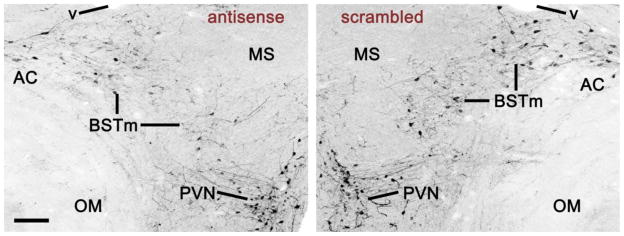
Results from the within-subjects validation experiment demonstrate excellent specificity of the antisense oligonucleotides. Infusions of VT antisense oligonucletotides into the dorsolateral BSTm and/or immediately adjacent portions of the lateral BST reduced VT-ir cell numbers by approximately 54% relative to the scrambled oligonucleotides infused into the contralateral BSTm (tied P = 0.0251; Wilcoxon signed ranks; Fig. 4A). Similar reductions were not observed for the VT population in the PVN, the closest hypothalamic cell group to the BSTm (tied P = 0.6002; Fig. 4B). Photos from a representative validation subject are shown in Fig. 5. Notably, the mean VT-ir cell number in the scrambled hemispheres (26.2 ± 16.4 cells per section; unilateral) is well within the normal range established in other studies (Goodson et al., 2009b; Kabelik et al., 2010), and thus the combined observations suggest that VT antisense effects were specifically localized to the infusion site. Furthermore, as shown in a separate validation experiment, scrambled oligonucleotides produced no effect on VT-ir cell numbers relative to saline (P = 0.8927; Wilcoxon signed ranks).
Fig. 4.
Validation and site-specificity of VT antisense. Bilaterally cannulated zebra finches were given unilateral infusions of VT antisense oligonucleotides into the dorsolateral BSTm and infusions of scrambled oligonucleotides into the contralateral BSTm (A–B). Sides were counterbalanced for treatment. Cell counts are shown as percentages of the total bilateral cell count for each subject. Data for each individual are shown in the small panel to the right of the box plot. Antisense infusions into the BSTm significantly reduced VT-ir neurons locally in the BSTm (A), but not in the PVN (B).
Fig. 5.
VT immunoreactivity in the BSTm and PVN of a bilaterally cannulated validation subject that received scrambled oligonucleotides into the left BSTm and VT antisense oligonucleotides into the right (inverted fluorescence). This section shows a 40% knockdown of VT-ir cells by the antisense, which is slightly less than the median knockdown of 54% (Fig. 3). Scale bar = 100 μm. Abbreviations: AC, anterior commissure; MS, medial septum; OM, occipitomesencephalic tract; v, lateral ventricle.
Finally, neither calbindin-ir cell number nor aromatase-ir optical density in the BSTm was reduced by antisense oligonucleotides relative to scrambled oligonucleotide controls (P = 0.5242 and 0.6755, respectively; unpaired t-tests). Optical density measurements were employed for aromatase rather than cell counts due to the very wide range of detectable aromatase (i.e., density) in individual cells.
Of the 44 subjects that were fitted with bilateral cannulae for the antisense behavioral experiments, 35 showed correct placements. This level of accuracy was possible based on the limited distance between our surgical reference point and the desired infusion site. Of the 9 misses, 4 were too deep and thus destroyed part or all of the BSTm VT cell group. The remainder were variable and distributed across treatment groups, thus we did not conduct comparisons between subjects with cannula placements that were correctly targeted versus those that were incorrectly targeted. Also note that lateral errors in one hemisphere (e.g., targeting the striatum) are associated with medial errors in the other hemisphere, and in such cases the septal region sustained heavy damage.
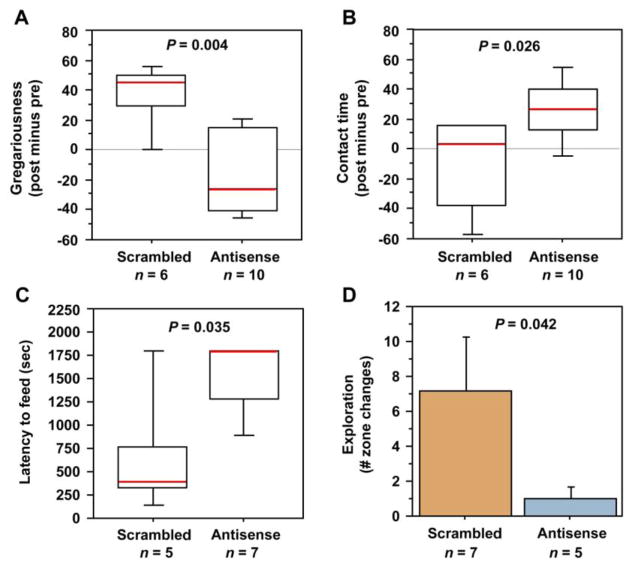
VT antisense oligonucleotide infusions into the BSTm decrease gregariousness but increase social contact
Measures of pre-post effects on gregariousness and social contact were generated for each subject by subtracting presurgical from post-infusion scores, and these values were then compared between groups using Mann-Whitney tests. Subjects that received antisense oligonucleotides exhibited a significant reduction in time spent with the large group relative to scrambled oligo control subjects (tied P = 0.0048; Fig. 6A). Time spent in social contact was not similarly reduced, and in fact antisense modestly increased social contact time (tied P = 0.0258; Fig. 6B).
Fig. 6.
Knockdown of VT production in the BSTm by antisense oligonucleotides reduces gregariousness (preferred group size), increases social contact time and increases anxiety-like behaviors. (A) Relative to scrambled oligonucleotide controls, male zebra finches infused with VT antisense oligonucleotides exhibit a median reduction in gregariousness of approximately 80% in a choice test between 10 and 2 conspecific males. Gregariousness is defined as the percent of contact time spent with the larger group (see Fig. 1). Data are shown as a post-treatment change from pre-surgical testing. (B) The same tests show a more modest but significant increase in social contact time. (C–D) Antisense subjects exhibit an increased latency to feed in the novelty-suppressed feeding test (C) and decreased exploration of a novel environment (D). Box plots show the median (red line), 75th and 25th percentile (box) and 95% confidence interval (whiskers). Data in panel D are shown as means ± SEM.
VT antisense increases anxiety-like responses to novelty
In mammals, septal VP is anxiogenic (Wigger et al., 2004; Beiderbeck et al., 2007; Neumann et al., 2010), but the contributions of the BSTm cells to anxiety effects are unknown. Therefore, in order to determine the involvement of BSTm VT neurons in anxiety-like processes, we tested antisense and scrambled control subjects (after 3 days of infusions) for latency to feed in the presence of a novel object, as well as exploratory behavior in a novel environment. These tests were conducted at a single post-surgical time point. Relative to subjects that received infusions of scrambled oligonucleotides, subjects receiving VT antisense oligos exhibited a longer latency to feed in the presence of a novel object (tied P = 0.0353; Mann-Whitney; Fig. 6C), and visited fewer branches in the exploration test (P = 0.0422; unpaired t-test; Fig. 6D). Novelty-suppressed feeding results were likely not produced by effects on appetite, given that neither scrambled or antisense subjects exhibited any weight change following treatments, and pre-post weight differences were virtually identical in the two groups (P = 0.5952; unpaired t-test; for similar results with chronic antagonist treatments, see Kabelik et al., 2009b). Similarly, exploration effects were likely not produced by non-specific influences on locomotor activity, given that antisense subjects did not differ from controls in the total number of hops exhibited (P = 0.8939; unpaired t-test), and showed only a weak trend towards an increase in their latency to move (P = 0.1339; unpaired t-test).
VT antisense oligonucleotides produce no effect on directed singing
Subjects in both scrambled and antisense oligo groups courted vigorously and pre-post difference measures were nearly identical (scrambled, 8.86 ± 2.00 songs; antisense, 8.00 ± 2.64; P = 0.6547; Mann-Whitney).
Discussion
VT/VP and the evolution of flocking and grouping
Despite the central importance of sociality to many aspects of species-specific behavior and physiology (Krause and Ruxton, 2002), neural variables that titrate group size choice remain almost wholly unknown. This may reflect the fact that in most taxa, species differences in grouping are confounded with other important ecological and behavioral variables such as mating system and patterns of parental care, and thus to date, grouping has been isolated as a quasi-experimental variable only in the avian family Estrildidae (Goodson et al., 2006; Goodson and Wang, 2006; Goodson et al., 2009c). This family offers exceptional opportunities for comparative studies of sociality given that all estrildids exhibit long-term monogamous pair bonds and biparental care, but display a wide range of modal species-typical group sizes, with clear cases of convergent evolution (Goodwin, 1982). Several prior studies in estrildid finches suggest the hypotheses that VT circuitry of the BSTm-LS promotes grouping behavior in flocking species and differentially modulates the social preferences of territorial and gregarious species (Goodson et al., 2006; Goodson and Wang, 2006). Consistent with these hypotheses, the present experiments now directly demonstrate that gregariousness is promoted by BSTm VT neurons and by the binding of endogenous VT to V1a-like receptors in the LS. Antisense effects in the BSTm were complex, given that social contact time was increased concomitantly with decreases in preferred group size (see next section). In contrast, intraseptal infusions of the V1a antagonist titrated gregariousness more selectively, and showed a clear lack of effects on social contact time (see below).
Other findings likewise suggest an important role for LS nonapeptides in the titration of flocking. For example, intraseptal infusions of an OT receptor antagonist significantly reduce gregariousness in female zebra finches, but produce no effect on social contact, and OT-like receptors in the LS (which are also potential binding sites for VT; Leung et al., 2009) exhibit very different distributions between territorial and flocking finch species, such that flocking species exhibit a higher density of receptors dorsally, whereas territorial species exhibit higher densities ventrally (Goodson et al., 2009c). Although relative densities along the dorsoventral axis clearly differentiate territorial from flocking species, the functional significance of this spatial arrangement remains to be specified.
Septal nonapeptides also influence social investigation in rodents
In male prairie voles (Microtus ochrogaster), social investigation of novel females is positively correlated with V1a receptor density in the LS, but negatively correlated with OT receptor density (Ophir et al., 2009), although this latter correlation is difficult to directly compare to finches in the absence of greater spatial resolution along the dorso-ventral axis. Similarly, over-expression of V1a receptors in the LS of male rats improves social discrimination and increases active social interactions (Landgraf et al., 2003), and septal V1a receptors are essential for normal social recognition in both rats and mice (Bluthe et al., 1990; Engelmann et al., 1994; Bielsky et al., 2005). These findings in voles, mice, and rats are not entirely consistent with those in zebra finches, given that we did not here observe an effect of the V1a antagonist on time spent in social contact. However, zebra finches are extraordinarily social (Zann, 1996), especially in comparison to the rodent species mentioned above, and may therefore express redundant neural mechanisms that ensure consistent social contact. In fact, we have observed numerous grouping-related species differences in neuropeptide and catecholamine systems (e.g., receptor densities, cell numbers, and/or Fos responses to social stimuli) that may each promote affiliation in species that flock (Goodson et al., 2006; Goodson and Wang, 2006; Goodson et al., 2009a; Goodson et al., 2009c).
The enigmatic VT/VP neurons of the BSTm
The VT/VP cell group has been indirectly implicated in the control of numerous affiliation and anxiety-like behaviors, largely by virtue of its projections to basal forebrain sites where VT/VP promote social investigation, social recognition, stress coping, parental behavior, and pair bonding (Liu et al., 2001; Engelmann et al., 2004; De Vries and Panzica, 2006; Donaldson and Young, 2008; Veenema and Neumann, 2008; Choleris et al., 2009; Goodson and Thompson, 2010). Lateral septal VT/VP also has complex effects on aggression and agonistic communication, which has often been taken as evidence for an involvement of the BSTm VT/VP neurons (Goodson and Kabelik, 2009). However, septal VP also modulates agonistic behavior in Syrian hamsters (Mesocricetus auratus) (Ferris et al., 1994), a highly asocial species that apparently lacks a VP cell group in the BSTm altogether (Bolborea et al., 2010). Thus, the LS clearly receives behaviorally-active VT/VP from one or more sources outside of the BSTm, underscoring the fact that confident inferences about the functions of the BSTm cell group cannot be made based upon peptide manipulations in BSTm projection targets (e.g., the LS). In fact, although we have learned a great deal about VT/VP functions over the last few decades, we still know very little about the regulation of behavior by the BSTm cell group.
To date, the majority of our knowledge about the functions of the BSTm VT/VP neurons comes from a handful of studies that have examined the responses of these cells to social and non-social stimuli. Overnight cohabitation with a female increases VP mRNA in the BSTm of both monogamous and nonmonogamous male voles (Wang et al., 1994), and recent immediate early gene experiments demonstrate that the BSTm VT/VP neurons have an exquisite sensitivity to the valence of social stimuli. For instance, as just described for finches, these cells increase their Fos activity to affiliation-related stimuli, but not to aversion or aggression-related stimuli (Goodson and Wang, 2006; Goodson et al., 2009b), and similarly, in male mice, VP neurons of the BSTm exhibit an increased Fos response to copulation and nonaggressive same-sex interaction, but not selectively to fights (Ho et al., 2010). This pattern of responses is virtually opposite of the profile of hypothalamic VT/VP cell groups, which are responsive to a wide range of stressors, including aggressive interactions (Wotjak et al., 1996; Briski and Brandt, 2000; Delville et al., 2000; Goodson and Evans, 2004; Ho et al., 2010).
These observations suggested our hypothesis that VT/VP neurons of the BSTm act to generally promote positive social interactions (but note that previous data on courtship singing run counter to this hypothesis; Goodson and Evans, 2004). More specifically, given the pattern of species differences already summarized for territorial and gregarious finch species (see Introduction), we hypothesized that the BSTm VT neurons act to increase gregariousness. This latter hypothesis receives solid support from the present experiments: Knockdown of VT in the BSTm produced a median reduction in gregariousness of 80% relative to scrambled controls, an effect size that is virtually identical to the within-subjects effect size in the antagonist experiment. However, no effect was observed on directed courtship singing to females, and social contact time was actually increased by approximately 25% in antisense subjects relative to birds infused with scrambled oligonucleotides. Given that social contact is not affected by infusions of V1a and OT antagonists into the lateral ventricle and/or LS (Goodson et al., 2009c), the antisense effect on contact time is unlikely to be mediated via BSTm projections to the LS, and may reflect VT actions in areas outside of the forebrain altogether (i.e., in areas distal to the ventricular antagonist infusions). Interestingly, endogenous VT inhibits social approach in goldfish via a cascade that includes descending parvocellular projections to autonomic hindbrain nuclei, activation of substance P vagal efferents, and feedback to the brain from peripheral body state (Thompson et al., 2008a). However, although these descending projections are strongly conserved across all vertebrate taxa (DeVries et al., 1985; Caffe et al., 1989; Panzica et al., 1999; Thompson et al., 2008b; Thompson and Walton, 2009), they appear to primarily arise from parvocellular neurons in the preoptic area (in anamniotes) and homologous neurons in the PVN of amniotes (De Vries and Buijs, 1983; Goodson et al., 2003; Thompson and Walton, 2009), not the BSTm cell group, which is lacking in fish altogether (Goodson et al., 2003; Greenwood et al., 2008). Thus, while the behavioral results in goldfish are consistent with the increased social contact in zebra finches observed here via VT knockdown, it is unlikely that the effects are mediated by similar projection systems. Nonetheless, we cannot exclude the possibility that the VT antisense altered paracrine effects in the brainstem, particularly since VT production was presumably altered for a period of days prior to behavioral testing.
VT/VP circuitry of the BSTm-LS modulates anxiety-like behavior in a species-specific manner: An emotional mechanism for social evolution?
In mammals, VP tends to promote anxiety-like behaviors via activation of both V1a and V1b receptors in the hippocampus (Engin and Treit, 2008), via V1a receptors in the BSTm (Bosch, 2010) and also via actions in multiple targets of the BSTm, such as the LS (Landgraf et al., 1995; Beiderbeck et al., 2007) and ventral pallidum (Pitkow et al., 2001). In contrast, we here provide evidence that endogenous VT circuitry of the BSTm-LS potently reduces anxiety-like responses to novelty in male zebra finches. To some extent, this result is consistent with a small literature showing that septal VP promotes active coping in novel situations such as forced swimming (Ebner et al., 1999; Engelmann et al., 2004), although the behavioral measures employed here are likely more comparable to less stressful exploration measures, such as the elevated plus-maze, which typically support an anxiogenic role for septal VP (Landgraf et al., 1995). However, lateral septal VP has also been shown to decrease anxiety in the elevated plus-maze (Everts and Koolhaas, 1999), suggesting that the context of testing (e.g., in different labs) may be particularly important.
Even accepting that anxiogenic effects of lateral septal VP can be context-dependent, the present results are still strongly at odds with the majority of findings in mammals, given that we obtained atypical results with multiple assays and manipulations, suggesting that there are important species differences in the modulation of anxiety-like behavior by VT/VP. Although we must certainly consider the possibility that VT/VP mechanisms of anxiety-like behavior are simply different in birds and mammals, a more interesting alternative hypothesis is that the extreme sociality of zebra finches has been achieved by adaptive modifications to the anxiety-related VT processes of the BSTm and LS. Further studies in less social finch species or extremely gregarious mammal species would provide good tests of this hypothesis.
Notably, nonapeptides influence social cognition and affiliation behaviors by acting within numerous neural loci where nonapeptides also modulate anxiety (Choleris et al., 2008; Goodson and Thompson, 2010; Labuschagne et al., 2010). In some cases, these actions are clearly coordinated, as in the anxiolytic actions of oxytocin that allow the appropriate display of maternal care (Neumann, 2008). Similarly, nonapeptides may influence gregariousness primarily via modulation of broad emotional states, and indeed, it seems quite possible that gregariousness is titrated largely via anxiety-like mechanisms that are themselves influenced by social context. The VT circuitry of the BSTm and LS may function in just this fashion, given that Fos activity of BSTm VT neurons is strongly modulated by social stimuli and those neurons in turn modulate anxiety-like behaviors. If this view is correct, then species-specific modifications to the links between nonapeptides, anxiety processes and social behavior, as might be produced by species-specific receptor distributions, may support adaptive species-typical behaviors such as mate fidelity, territoriality, or flocking.
But why might socially relevant peptide systems modulate anxiety outside of the context of direct social interaction? And how do we reconcile the observations that BSTm VT neurons increase their Fos responses so selectively to positive social stimuli, and not nonsocial stimuli, and yet still influence anxiety-like behaviors in contexts such as the novelty-suppressed feeding paradigm and exploration of a novel environment? The answer to this second question is perhaps more obvious: Although the BSTm VT neurons exhibit phasic Fos responses to a very restricted range of positive social stimuli, they also exhibit extremely high levels of constitutive Fos expression in zebra finches (Goodson and Wang, 2006; Goodson et al., 2009b), and may therefore exert strong tonic influences on behavioral and emotional states. With regard to the first question – it may well be that for a highly gregarious animal like the zebra finch, there is no such thing as a behavioral or emotional state that is uncoupled from sociality. These animals spend their entire lives in the company of other birds (about 100 on average; Zann, 1996), and thus a zebra finch that is placed in a testing chamber is not an animal that is in some kind of neutral state. Rather, it is an animal that is in the state of social isolation, and peptidergic influences in such tests cannot be assumed to be independent of that particular social context. Considering this perspective, a particularly interesting focus for further research will be to determine whether endogenous VT systems function in the same manner when animals are in familiar social groups and when they are isolated.
Conclusions
The present experiments provide the first direct evidence for specific behavioral functions of VT/VP cells in the BSTm, and show that these neurons potently promote gregariousness and strongly reduce anxiety-like responses to novelty. These behaviors were similarly influenced by V1a antagonist infusions into the LS, but whereas antisense oligonucleotide infusions into the BSTm increased time spent in social contact, the antagonist showed a clear lack of such effects. Treatments did not affect general activity and antisense oligonucleotides did not influence courtship singing, consistent with previous antagonist results (Goodson et al., 2004). Our findings for anxiety-like behavior are not entirely consistent with those in rodents, but given that peptide mechanisms of grouping behavior and anxiety appear to be linked, these differences may reflect evolutionary adaptations that promote the extraordinarily gregarious behavior of zebra finches, a highly colonial species that exhibits a social organization based on biparental nuclear families embedded within much larger social groups.
Acknowledgments
Support provided by NIMH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Absil P, Papello M, Viglietti-Panzica C, Balthazart J, Panzica G. The medial preoptic nucleus receives vasotocinergic inputs in male quail: a tract-tracing and immunocytochemical study. J Chem Neuroanat. 2002;24:27–39. doi: 10.1016/s0891-0618(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Aste N, Muhlbauer E, Grossmann R. Distribution of AVT gene expressing neurons in the prosencephalon of japanese quail and chicken. Cell Tissue Res. 1996;286:365–373. doi: 10.1007/s004410050706. [DOI] [PubMed] [Google Scholar]
- Beiderbeck DI, Neumann ID, Veenema AH. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur J Neurosci. 2007;26:3597–3605. doi: 10.1111/j.1460-9568.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Zimmer A. Diminished Anxiety- and Depression-Related Behaviors in Mice with Selective Deletion of the Tac1 Gene. J Neurosci. 2002;22:10046–10052. doi: 10.1523/JNEUROSCI.22-22-10046.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi LM, Bo MS, Vassallo AI. Inter-individual and age differences in exploration, neophobia and problem-solving ability in a Neotropical raptor (Milvago chimango) Anim Cogn. 2010;13:701–710. doi: 10.1007/s10071-010-0319-8. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Schoenen J, Dantzer R. Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Res. 1990;519:150–157. doi: 10.1016/0006-8993(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Bolborea M, Ansel L, Weinert D, Steinlechner S, Pevet P, Klosen P. The bed nucleus of the stria terminalis in the Syrian hamster (Mesocricetus auratus): absence of vasopressin expression in standard and wild-derived hamsters and galanin regulation by seasonal changes in circulating sex steroids. Neuroscience. 2010;165:819–830. doi: 10.1016/j.neuroscience.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Pfortsch J, Beiderbeck DI, Landgraf R, Neumann ID. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. J Endocrinol. 2010;22:420–429. doi: 10.1111/j.1365-2826.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- Briski KP, Brandt JA. Oxytocin and vasopressin neurones in principal and accessory hypothalamic magnocellular structures express Fos-immunoreactivity in response to acute glucose deprivation. J Neuroendocrinol. 2000;12:409–414. doi: 10.1046/j.1365-2826.2000.00469.x. [DOI] [PubMed] [Google Scholar]
- Caffe AR, Van Ryen PC, Van der Woude TP, Van Leeuwen FW. Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis. J Comp Neurol. 1989;287:302–325. doi: 10.1002/cne.902870304. [DOI] [PubMed] [Google Scholar]
- Catts VS, Al-Menhali N, Burne THJ, Colditz MJ, Coulson EJ. The p75 neurotrophin receptor regulates hippocampal neurogenesis and related behaviours. Eur J Neurosci. 2008;28:883–892. doi: 10.1111/j.1460-9568.2008.06390.x. [DOI] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Kavaliers M. Neuroendocrinology of social information processing in rats and mice. Front Neuroendocrinol. 2009;30:442–459. doi: 10.1016/j.yfrne.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Choleris E, Devidze N, Kavaliers M, Pfaff DW. Steroidal/neuropeptide interactions in hypothalamus and amygdala related to social anxiety. Prog Brain Res. 2008;170:291–303. doi: 10.1016/S0079-6123(08)00424-X. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Best W, Sluiter AA. The influence of androgens on the development of a sex difference in the vasopressinergic innervation of the rat lateral septum. Brain Res. 1983;284:377–380. doi: 10.1016/0165-3806(83)90019-6. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- DeVries GJ, Buijs RM, Van Leeuwen FW, Caffe AR, Swaab DF. The vasopressinergic innervation of the brain in normal and castrated rats. J Comp Neurol. 1985;233:236–254. doi: 10.1002/cne.902330206. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Ebner K, Wotjak CT, Holsboer F, Landgraf R, Engelmann M. Vasopressin released within the septal brain area during swim stress modulates the behavioural stress response in rats. Eur J Neurosci. 1999;11:997–1002. doi: 10.1046/j.1460-9568.1999.00508.x. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ludwig M, Landgraft R. Simultaneous monitoring of intracerebral release and behavior: endogenous vasopressin improves social recognition. J Neuroendocrinol. 1994;6:391–395. doi: 10.1111/j.1365-2826.1994.tb00598.x. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D. Dissociation of the anxiolytic-like effects of Avpr1a and Avpr1b receptor antagonists in the dorsal and ventral hippocampus. Neuropeptides. 2008;42:411–421. doi: 10.1016/j.npep.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Everts HG, Koolhaas JM. Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats. Behav Brain Res. 1999;99:7–16. doi: 10.1016/s0166-4328(98)00004-7. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Delville Y, Irvin RW, Potegal M. Septo-hypothalamic organization of a stereotyped behavior controlled by vasopressin in golden hamsters. Physiol Behav. 1994;55:755–759. doi: 10.1016/0031-9384(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Adkins-Regan E. Effect of intraseptal vasotocin and vasoactive intestinal polypeptide infusions on courtship song and aggression in the male zebra finch (Taeniopygia guttata) J Neuroendocrinol. 1999;11:19–25. doi: 10.1046/j.1365-2826.1999.00284.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK. Neural responses to territorial challenge and nonsocial stress in male song sparrows: segregation, integration, and modulation by a vasopressin V1 antagonist. Horm Behav. 2004;46:371–381. doi: 10.1016/j.yhbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Bass AH. Putative isotocin distributions in sonic fish: relation to vasotocin and vocal-acoustic circuitry. J Comp Neurol. 2003;462:1–14. doi: 10.1002/cne.10679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav. 2006;50:223–236. doi: 10.1016/j.yhbeh.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D. Dynamic limbic networks and social diversity in vertebrates: From neural context to neuromodulatory patterning. Front Neuroendocrinol. 2009:429–441. doi: 10.1016/j.yfrne.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Kabelik D, Kelly AM, Rinaldi J, Klatt JD. Midbrain dopamine neurons reflect affiliation phenotypes in finches and are tightly coupled to courtship. Proc Natl Acad Sci USA. 2009a;106:8737–8742. doi: 10.1073/pnas.0811821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Lindberg L, Johnson P. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm Behav. 2004;45:136–143. doi: 10.1016/j.yhbeh.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Rinaldi J, Kelly AM. Vasotocin neurons in the bed nucleus of the stria terminalis preferentially process social information and exhibit properties that dichotomize courting and non-courting phenotypes. Horm Behav. 2009b;55:197–202. doi: 10.1016/j.yhbeh.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Schrock SE, Klatt JD, Kabelik D, Kingsbury MA. Mesotocin and nonapeptide receptors promote estrildid flocking behavior. Science. 2009c;325:862–866. doi: 10.1126/science.1174929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Curr Opin Neurobiol. 2010 doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Wang Y. Valence-sensitive neurons exhibit divergent functional profiles in gregarious and asocial species. Proc Natl Acad Sci USA. 2006;103:17013–17017. doi: 10.1073/pnas.0606278103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin D. Estrildid Finches of the World. 1. Cornell Univ Press; Ithaca, NY: 1982. [Google Scholar]
- Greene J, Banasr M, Lee B, Warner-Schmidt J, Duman RS. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology. 2009;34:2459–2468. doi: 10.1038/npp.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood AK, Wark AR, Fernald RD, Hofmann HA. Expression of arginine vasotocin in distinct preoptic regions is associated with dominant and subordinate behaviour in an African cichlid fish. Proc Biol Sci. 2008;275:2393–2402. doi: 10.1098/rspb.2008.0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis TG, Carere C. Avian personalities: characterization and epigenesis. Neurosci Biobehav Rev. 2005;29:137–150. doi: 10.1016/j.neubiorev.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Ho JM, Murray JH, Demas GE, Goodson JL. Vasopressin cell groups exhibit strongly divergent responses to copulation and male-male interactions in mice. Horm Behav. 2010:368–377. doi: 10.1016/j.yhbeh.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelik D, Kelly AM, Goodson JL. Dopaminergic regulation of mate competition aggression and aromatase-Fos colocalization in vasotocin neurons. Neuropharmacology. 2009a;58:117–125. doi: 10.1016/j.neuropharm.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelik D, Klatt JD, Kingsbury MA, Goodson JL. Endogenous vasotocin exerts context-dependent behavioral effects in a semi-naturalistic colony environment. Horm Behav. 2009b;56:101–107. doi: 10.1016/j.yhbeh.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabelik D, Morrison JA, Goodson JL. Cryptic regulation of vasotocin neuronal activity but not anatomy by sex steroids and social stimuli in opportunistic desert finches. Brain Behav Evol. 2010;75:71–84. doi: 10.1159/000297522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J, Ruxton GD. Living in Groups. Oxford Univ. Press; Oxford: 2002. [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35:2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X, Terwilliger EF, Niwa M, Wigger A, Young LJ. Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behaviour. Eur J Neurosci. 2003;18:403–411. doi: 10.1046/j.1460-9568.2003.02750.x. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Sexual experience promotes adult neurogenesis in the hippocampus despite an initial elevation in stress hormones. PLoS One. 5:e11597. doi: 10.1371/journal.pone.0011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CH, Goode CT, Young LJ, Maney DL. Neural distribution of nonapeptide binding sites in two species of songbird. J Comp Neurol. 2009;513:197–208. doi: 10.1002/cne.21947. [DOI] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–136. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- Martins TL, Roberts ML, Giblin I, Huxham R, Evans MR. Speed of exploration and risk-taking behavior are linked to corticosterone titres in zebra finches. Horm Behav. 2007;52:445–453. doi: 10.1016/j.yhbeh.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Moore FL, Lowry CA. Comparative neuroanatomy of vasotocin and vasopressin in amphibians and other vertebrates. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;119:251–260. doi: 10.1016/s0742-8413(98)00014-0. [DOI] [PubMed] [Google Scholar]
- Neumann ID. Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J Neuroendocrinol. 2008;20:858–865. doi: 10.1111/j.1365-2826.2008.01726.x. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Veenema AH, Beiderbeck DI. Aggression and anxiety: social context and neurobiological links. Front Behav Neurosci. 2010;4:12. doi: 10.3389/fnbeh.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir AG, Zheng DJ, Eans S, Phelps SM. Social investigation in a memory task relates to natural variation in septal expression of oxytocin receptor and vasopressin receptor 1a in prairie voles (Microtus ochrogaster) Behav Neurosci. 2009;123:979–991. doi: 10.1037/a0016663. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Plumari L, Garcia-Ojeda E, Deviche P. Central vasotocin-immunoreactive system in a male passerine bird (Junco hyemalis) J Comp Neurol. 1999;409:105–117. [PubMed] [Google Scholar]
- Pitkow LJ, Sharer CA, Ren X, Insel TR, Terwilliger EF, Young LJ. Facilitation of affiliation and pair-bond formation by vasopressin receptor gene transfer into the ventral forebrain of a monogamous vole. J Neurosci. 2001;21:7392–7396. doi: 10.1523/JNEUROSCI.21-18-07392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheutt WaDSRX. Sex differences, social context and personality in zebra finches, Taeniopygia guttata. Anim Behav. 2009;77:1041–1050. [Google Scholar]
- Thompson RR, Dickinson PS, Rose JD, Dakin KA, Civiello GM, Segerdahl A, Bartlett R. Pheromones enhance somatosensory processing in newt brains through a vasotocin-dependent mechanism. Proc Biol Sci. 2008a;275:1685–1693. doi: 10.1098/rspb.2008.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RR, Walton JC. Vasotocin immunoreactivity in goldfish brains: characterizing primitive circuits associated with social regulation. Brain Behav Evol. 2009;73:153–164. doi: 10.1159/000219485. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Walton JC, Bhalla R, George KC, Beth EH. A primitive social circuit: vasotocin-substance P interactions modulate social behavior through a peripheral feedback mechanism in goldfish. Eur J Neurosci. 2008b;27:2285–2293. doi: 10.1111/j.1460-9568.2008.06210.x. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Central vasopressin and oxytocin release: regulation of complex social behaviours. Prog Brain Res. 2008;170:261–276. doi: 10.1016/S0079-6123(08)00422-6. [DOI] [PubMed] [Google Scholar]
- Wang Z, Smith W, Major DE, De Vries GJ. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Brain Res. 1994;650:212–218. doi: 10.1016/0006-8993(94)91784-1. [DOI] [PubMed] [Google Scholar]
- Wigger A, Sanchez MM, Mathys KC, Ebner K, Frank E, Liu D, Kresse A, Neumann ID, Holsboer F, Plotsky PM, Landgraf R. Alterations in central neuropeptide expression, release, and receptor binding in rats bred for high anxiety: critical role of vasopressin. Neuropsychopharmacology. 2004;29:1–14. doi: 10.1038/sj.npp.1300290. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Kubota M, Liebsch G, Montkowski A, Holsboer F, Neumann I, Landgraf R. Release of vasopressin within the rat paraventricular nucleus in response to emotional stress: a novel mechanism of regulating adrenocorticotropic hormone secretion? J Neurosci. 1996;16:7725–7732. doi: 10.1523/JNEUROSCI.16-23-07725.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zann RA. The Zebra Finch: A Synthesis of Field and Laboratory Studies. Oxford University Press; USA: 1996. [Google Scholar]