Abstract
Purpose
The aim of this study was to evaluate the prognostic factors for survival in patients treated surgically for transitional cell carcinoma of the upper urinary tract (UUT-TCC).
Materials and Methods
We retrospectively reviewed the medical records of 87 patients (64 men and 23 women, mean age of 62.2 years) with UUT-TCC who had undergone radical nephroureterectomy at our institution between June 1994 and June 2009. The median follow-up period was 32 months. The prognostic significance of various clinicopathological variables for recurrence-free and cancer-specific survival was analyzed by using univariate and multivariate analysis.
Results
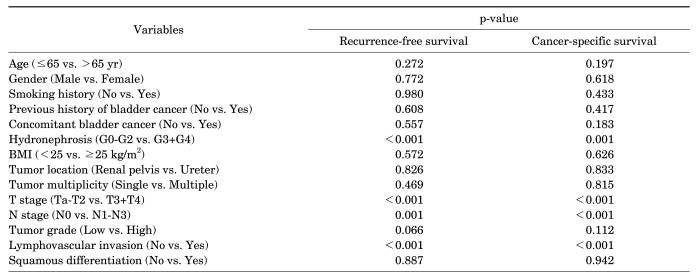
Of the total 87 patients, 21 patients (24.1%) developed local recurrence or distant metastasis and 16 patients (18.4%) died of disease during the follow-up period. The 5-year recurrence-free and cancer-specific survival rates were 74.6% and 75.2%, respectively. In the univariate analysis, hydronephrosis, T stage, N stage, and lymphovascular invasion (LVI) were significant prognostic factors for recurrence-free and cancer-specific survival. In the multivariate analysis, T stage and LVI were independent prognostic factors for recurrence-free and cancer-specific survival.
Conclusions
The T stage and LVI are independent prognostic factors for recurrence-free and cancer-specific survival in patients with UUT-TCC treated by radical nephroureterectomy. These findings would be helpful for guiding decisions about adjuvant therapies and the surveillance interval.
Keywords: Prognosis, Transitional cell carcinoma, Urologic neoplasms
INTRODUCTION
Transitional cell carcinoma of the upper urinary tract (UUT-TCC) is a relatively uncommon disease, accounting for 5% to 6% of all urothelial carcinomas. Radical nephroureterectomy with bladder cuff excision has been considered as the standard treatment for UUT-TCC [1-3]. However, UUT-TCC is known to have a higher recurrence rate even after radical surgery, which may be due in part to the difficulty in early detection of the tumor, the thinner muscular and submucosal layers, and the absence of serosa in the upper urinary tract [4,5].
Tumor stage, grade, and surgical procedure performed have been documented as the major prognostic factors in patients with UUT-TCC [6-9]. In addition, patient age, tumor size and architecture, tumor location, tumor multiplicity, lymphovascular invasion (LVI), and a previous history of bladder cancer have been suggested as potential prognostic factors [1,9-11]. However, the influence of these potential prognostic factors for UUT-TCC remains less clear. A clear knowledge of these prognostic indicators of tumor recurrence and progression at the time of surgery would allow better prognostic evaluation and approach.
In this study, we retrospectively reviewed our single-center experience in patients with UUT-TCC to identify the prognostic factors that predict recurrence-free and cancer-specific survival in patients with UUT-TCC after radical nephroureterectomy.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of 87 patients (64 men and 23 women) who had undergone radical nephroureterectomy with bladder cuff excision for UUT-TCC at our institution between June 1994 and June 2009. Mean patient age was 62.2 years (range, 33-85 years). None of the patients included in this study had distant metastasis at diagnosis. Regional lymph node dissection was performed in patients with clinically apparent lymphadenopathy on a preoperative radiologic imaging or in those who were suspected of having enlarged lymph nodes intraoperatively. Tumors were staged by using the 2002 TNM staging system [12] and were graded according to the World Health Organization (WHO)/International Society of Urological Pathology (ISUP) grading criteria [13].
Hydronephrosis was assessed by preoperative imaging, and grade of hydronephrosis was estimated according to the methods described by Cho et al [14]. The tumors without caliceal or pelvic dilation were classified as grade 0, tumors with pelvic dilation only as grade 1, tumors with mild caliceal dilation as grade 2, tumors with severe caliceal dilation as grade 3, and tumors with caliceal dilation accompanied by renal parenchymal atrophy as grade 4.
Patient follow-up was relatively uniform and included basic laboratory examinations, chest x-ray, cystoscopy, and urine cytology every 3 months for the first 2 years, every 6 months for the subsequent 2 years, and then yearly thereafter. Abdominopelvic computed tomography was performed annually during follow-up or when clinically indicated.
The 5-year recurrence-free and cancer-specific survival rates were analyzed. Disease recurrence was defined as local failure in the tumor bed or regional lymph nodes or distant metastasis. Because bladder recurrence did not affect survival in the patients with UUT-TCC, it was not considered in the analysis of the recurrence-free survival rate in this study.
Univariate and multivariate analyses were performed by using the log-rank test and the Cox's proportional hazards regression model, respectively. The prognostic factors assessed were age, gender, smoking history, previous history of bladder TCC, concomitant bladder TCC, grade of hydronephrosis, body mass index (BMI), tumor location, tumor multiplicity, T stage, N stage, tumor grade, LVI, and squamous differentiation. All statistical analyses were performed by using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA). Values of p<0.05 were considered to be statistically significant in all of the analyses.
RESULTS
The clinicopathological characteristics of the 87 patients with UUT-TCC are summarized in Table 1. The primary tumor was located in the renal pelvis, ureter, or both in 48 (55.2%), 33 (37.9%), and 6 patients (6.9%), respectively. Six patients (6.9%) had a previous history of bladder TCC and 9 patients (10.3%) had concomitant bladder TCC when the UUT-TCC was diagnosed. In the preoperative imaging studies, 60 patients (69.0%) presented with ipsilateral hydronephrosis. The grade of hydronephrosis was 1, 2, 3, and 4 in 1 (1.1%), 25 (28.7%), 19 (21.8%), and 15 patients (17.2%), respectively. Regional lymph node dissection was performed in 49 patients (56.3%) with enlarged lymph nodes, and 10 revealed lymph node metastasis on pathological examination. The median number of lymph nodes removed was 7 (mean, 8.2; range, 1 to 24). A positive surgical margin was found in 6 patients (6.9%) on pathological examination. Chemotherapy was administered to 19 patients and radiotherapy at the surgical bed was performed in 5 patients.
TABLE 1.
Clinicopathological data of the 87 patients included in the study
The median follow-up period was 32 months (mean, 42.9; range, 1 to 131 months). Of the total 87 patients, 21 patients (24.1%) experienced recurrence, including 7 with local recurrence and 14 with distant metastasis. The site of local recurrence was retroperitoneal or in the pelvic lymph nodes, and distant metastasis occurred in the lung, liver, bone, contralateral adrenal gland, sigmoid colon, or pelvic side wall. Sixteen patients (18.4%) died of disease during the follow-up period. Two of them had local recurrence and 14 had distant metastasis. Among 21 patients with local recurrence or distant metastasis after surgery, 12 patients received adjuvant chemotherapy. Bladder recurrence was found in 16 patients (18.4%), and none of them developed local recurrence or distant metastasis or died during the follow-up period.
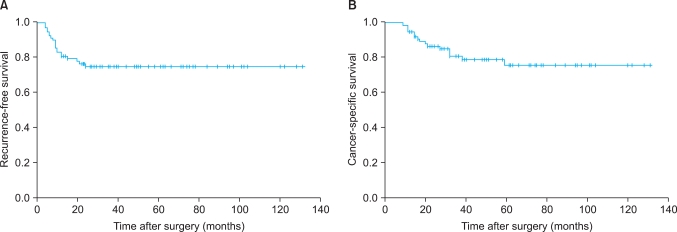
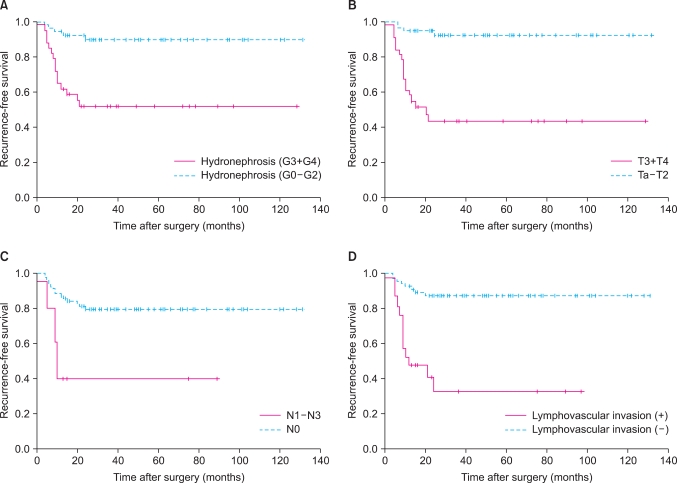
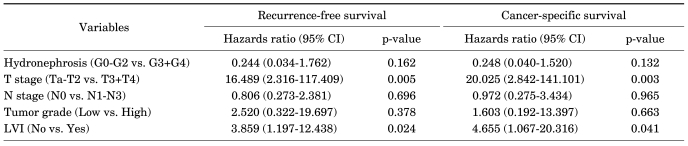
The 5-year recurrence-free survival rate was 74.6% (Fig. 1A). The recurrence-free survival rate was lower in patients with severe hydronephrosis, higher T stage, lymph node metastasis, and lymphovascular invasion (Fig. 2). The univariate analysis identified that grade of hydronephrosis (p<0.001), T stage (p<0.001), N stage (p=0.001), and LVI (p<0.001) were significant prognostic factors for recurrence-free survival (Table 2), whereas the multivariate analysis indicated that T stage (p=0.005) and LVI (p=0.024) were independent prognostic factors (Table 3).
FIG. 1.
Recurrence-free (A) and cancer-specific (B) survival is illustrated for the 87 patients who underwent radical nephroureterectomy for transitional cell carcinoma of the upper urinary tract.
FIG. 2.
Kaplan-Meier recurrence-free survival curves according to hydronephrosis grade (A), T stage (B), N stage (C), and lymphovascular invasion (D).
TABLE 2.
Univariate analysis of potential prognostic factors for recurrence-free and cancer-specific survival
BMI: body mass index
TABLE 3.
Multivariate analysis of potential prognostic factors for recurrence-free and cancer-specific survival
LVI: lymphovascular invasion, CI: confidence interval
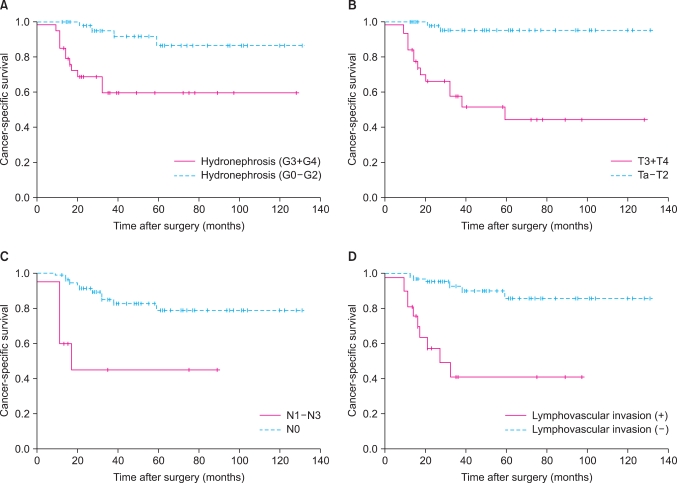
The 5-year cancer-specific survival rate was 75.2% (Fig. 1B). The cancer-specific survival rate was lower in patients with severe hydronephrosis, higher T stage, lymph node metastasis, and lymphovascular invasion (Fig. 3). The univariate analysis revealed that grade of hydronephrosis (p=0.001), T stage (p<0.001), N stage (p<0.001), and LVI (p<0.001) were significant prognostic factors for cancer-specific survival (Table 2), whereas the multivariate analysis demonstrated that T stage (p=0.003) and LVI (p=0.041) were independent prognostic factors (Table 3).
FIG. 3.
Kaplan-Meier cancer-specific survival curves according to hydronephrosis grade (A), T stage (B), N stage (C), and lymphovascular invasion (D).
DISCUSSION
Although recent literature suggests that endourological techniques for UUT-TCC have been associated with the preservation of renal function in selected cases, nephroureterectomy with bladder cuff excision is still the standard treatment with the highest cancer-specific survival [9]. However, UUT-TCC shows a significantly high local failure even after radical surgery, especially in patients with high stage and grade tumors [6]. Because the location of the UUT-TCC may hinder early detection, UUT-TCC is more likely to be diagnosed as invasive cancers than as bladder cancers. Anatomical differences between the bladder and ureter or renal pelvis wall also account for differences in the higher incidence of invasive UUT-TCC. The thickness of the bladder wall is estimated to exceed that of the ureteral wall by 2- to 3-fold [15]. The distal ureter is covered by 3 muscular layers, whereas the more proximal part of the ureter contains only 2 relatively thin interlacing layers [16]. Urothelial cancer invasion may be correlated with muscular wall thickness [5]. This fact highlights the importance of surgical competence and adequate adjuvant therapy in patients with a high risk for failure of UUT-TCC. Other potential prognostic factors for predicting high risk in UUT-TCC should be identified.
It has been suggested that factors such as T stage, tumor grade, tumor location, lymph node involvement, LVI, and surgical procedure performed are associated with prognosis [6,9-11,17-19]. T stage and tumor grade probably were the best established major prognostic factors in UUT-TCC. Hall et al reported 5-year, cancer-specific survival probabilities of 100% for patients with Ta/Tis tumors, 92% for patients with T1 tumors, 73% for patients with T2 tumors, and 41% for patients with T3 tumors [9]. In the present study, T stage was an independent prognostic factor for recurrence-free and cancer-specific survival, supporting that T stage is one of the most important prognostic factors in UUT-TCC.
With regard to the tumor grade, the 5-year cancer-specific survival rates are reported to range from 90% to 100% for low-grade UUT-TCC and from 28% to 46% for high-grade UUT-TCC [20,21]. Moreover, a few studies have indicated the independent prognostic role of tumor grade [4,21], suggesting that patients with high-grade cancers had at least a 2-fold increased risk of cancer-related death compared with those with low-grade tumors. In the present study, tumor grade showed marginal significance (p=0.066) only for recurrence-free survival. A larger study population and longer follow-up period might be needed to clarify the prognostic role of tumor grade.
Several other putative prognostic factors have also been proposed with sometimes conflicting results. One of the major issues in UUT-TCC is the prognostic role of a previous history of bladder cancer and concurrent bladder cancer. A previous history of bladder cancer was identified in approximately 7% of our cases and from 9% to 20% of patients in other studies [9,21]. In our analysis, a previous history of bladder cancer did not show any prognostic significance for UUT-TCC. However, Mullerad et al reported that a history of bladder cancer was an independent predictor of cancer-specific survival in multivariate analysis [22]. Conversely, Rabbani et al reported that de novo UUT-TCC had a 1.67-fold increased risk of cancer-related death compared with UUT-TCC after bladder cancer in the multivariate analysis and they emphasized the prognostic importance of concurrent bladder cancer more than previous bladder cancer history [23]. Kang et al reported that the presence of concurrent bladder cancer at the time of treatment for UUT-TCC, mostly by nephroureterectomy, was an independent predictor of cancer-specific survival [4]. However, our study did not show any relationship between concurrent bladder cancer and recurrence-free or cancer-specific survival.
With regard to the tumor location, some studies reported that patients with cancers arising from the renal pelvis have a better cancer-related survival than do those with cancers in the ureter, and tumor site is an independent predictor of cancer-specific survival [21,24]. Such results may be explained by the presence of a thin layer of adventitia surrounding the ureter, which contains an extensive plexus of ureteral blood vessels and lymphatics that makes tumor invasion easier. The other possible reason is that the renal parenchyma and perihilar adipose tissue surrounding the renal pelvis may act as a barrier against early spread [15,25]. Conversely, van der Poel et al demonstrated that patients with tumors in the renal pelvis and proximal ureter were 2.5 times as likely to die of disease as were those with tumors in the distal ureter [5]. In our series, there was no significant difference in recurrence-free or cancer-specific survival between renal pelvis and ureteral cancers.
Novara et al demonstrated that tumor multiplicity within the UUT-TCC was an independent predictor of cancer-specific survival [26]. The authors suggested that these features strongly reconfirmed the panurothelial nature of the disease along with the prognostic role of prior or concomitant bladder cancers. In our series, however, tumor multiplicity was predictive of neither recurrence-free nor cancer-specific survival, which was in agreement with other reports that failed to demonstrate a significant prognostic role of multiplicity in UUT-TCC [4,22].
The presence of LVI has been reported to be an important prognostic factor in UUT-TCC [11,18,19]. Kim et al demonstrated that LVI was an independent prognostic factor for recurrence-free and cancer-specific survival in patients with localized UUT-TCC after nephroureterectomy [11]. Hong et al also suggested LVI as an independent prognostic factor for recurrence-free survival in patients with UUT-TCC [19]. LVI was also an independent predictor of recurrence-free and cancer-specific survival in our series.
Preoperative hydronephrosis has also been reported as an important predictor of UUT-TCC. Brien et al demonstrated that preoperative evaluation for hydronephrosis could identify patients at risk for advanced UUT-TCC, and such knowledge might impact the extent of surgery as well as the need for perioperative chemotherapy regimens [27]. Cho et al found that the grade of hydronephrosis and the tumor diameter had a significant influence on the prognosis of UUT-TCC [14,28]. They suggested that ureteral tumors cause gradual ureteral obstruction, resulting in the development of hydronephrosis, and that there was a correlation between the hydronephrosis grade and T stage. Consequently, severe hydronephrosis may be a poor prognostic factor in UUT-TCC. In the present study, hydronephrosis was associated with recurrence-free and cancer-specific survival but failed to have an independent prognostic role.
Our study had several limitations. It was retrospective in nature and the size of the study population was smaller than a recently reported multicenter study, because we collected our single-center experience of an uncommon disease. The follow-up period might not have been long enough. In addition, lymph node dissection was not performed in all patients. Nevertheless, we believe that the results of our study support the already established T stage and LVI as independent prognostic factors for recurrence-free and cancer-specific survival in patients with UUT-TCC treated by radical nephroureterectomy.
CONCLUSIONS
The results of this study have demonstrated that T stage and LVI are independent prognostic factors for recurrence-free and cancer-specific survival in patients with UUT-TCC treated by radical nephroureterectomy. These findings will be helpful for guiding decisions about adjuvant therapies and the surveillance interval.
Footnotes
The authors have nothing to disclose.
References
- 1.Oosterlinck W, Solsona E, van der Meijden AP, Sylvester R, Böhle A, Rintala E, et al. EAU guidelines on diagnosis and treatment of upper urinary tract transitional cell carcinoma. Eur Urol. 2004;46:147–154. doi: 10.1016/j.eururo.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Cho KS, Cho NH, Choi YD. Pattern of recurrence and the prognostic factors of upper urinary tract transitional cell carcinoma. Korean J Urol. 2006;47:124–130. [Google Scholar]
- 3.Park S, Hong B, Kim Y, Kim CS, Ahn H. Prognostic factors for survival in the transitional cell carcinoma of the upper urinary tract. Korean J Urol. 2003;44:1087–1092. [Google Scholar]
- 4.Kang CH, Yu TJ, Hsieh HH, Yang JW, Shu K, Huang CC, et al. The development of bladder tumors and contralateral upper urinary tract tumors after primary transitional cell carcinoma of the upper urinary tract. Cancer. 2003;98:1620–1626. doi: 10.1002/cncr.11691. [DOI] [PubMed] [Google Scholar]
- 5.van der Poel HG, Antonini N, van Tinteren H, Horenblas S. Upper urinary tract cancer: location is correlated with prognosis. Eur Urol. 2005;48:438–444. doi: 10.1016/j.eururo.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Heney NM, Nocks BN, Daly JJ, Blitzer PH, Parkhurst EC. Prognostic factors in carcinoma of the ureter. J Urol. 1981;125:632–636. doi: 10.1016/s0022-5347(17)55143-5. [DOI] [PubMed] [Google Scholar]
- 7.Corrado F, Ferri C, Mannini D, Corrado G, Bertoni F, Bacchini P, et al. Transitional cell carcinoma of the upper urinary tract: evaluation of prognostic factors by histopathology and flow cytometric analysis. J Urol. 1991;145:1159–1163. doi: 10.1016/s0022-5347(17)38562-2. [DOI] [PubMed] [Google Scholar]
- 8.Hisataki T, Miyao N, Masumori N, Takahashi A, Sasai M, Yanase M, et al. Risk factors for the development of bladder cancer after upper tract urothelial cancer. Urology. 2000;55:663–667. doi: 10.1016/s0090-4295(99)00563-4. [DOI] [PubMed] [Google Scholar]
- 9.Hall MC, Womack S, Sagalowsky AI, Carmody T, Erickstad MD, Roehrborn CG. Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: a 30-year experience in 252 patients. Urology. 1998;52:594–601. doi: 10.1016/s0090-4295(98)00295-7. [DOI] [PubMed] [Google Scholar]
- 10.Krogh J, Kvist E, Rye B. Transitional cell carcinoma of the upper urinary tract: prognostic variables and post-operative recurrences. Br J Urol. 1991;67:32–36. doi: 10.1111/j.1464-410x.1991.tb15064.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim DS, Lee YH, Cho KS, Cho NH, Chung BH, Hong SJ. Lymphovascular invasion and pT stage are prognostic factors in patients treated with radical nephroureterectomy for localized upper urinary tract transitional cell carcinoma. Urology. 2010;75:328–332. doi: 10.1016/j.urology.2009.07.1350. [DOI] [PubMed] [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG. AJCC cancer staging manual. 6th ed. New York: Springer-Verlag; 2002. pp. 329–331. [Google Scholar]
- 13.Epstein JI, Amin MB, Reuter VR, Mostofi FK Bladder Consensus Conference Committee. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Am J Surg Pathol. 1998;22:1435–1448. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Cho KS, Hong SJ, Cho NH, Choi YD. Grade of hydronephrosis and tumor diameter as preoperative prognostic factors in ureteral transitional cell carcinoma. Urology. 2007;70:662–666. doi: 10.1016/j.urology.2007.06.1106. [DOI] [PubMed] [Google Scholar]
- 15.Yang JM, Huang WC. Bladder wall thickness on ultrasonographic cystourethrography: affecting factors and their implications. J Ultrasound Med. 2003;22:777–782. doi: 10.7863/jum.2003.22.8.777. [DOI] [PubMed] [Google Scholar]
- 16.Hanna MK, Jeffs RD, Sturgess JM, Barkin M. Ureteral structure and ultrastructure. Part I. The normal human ureter. J Urol. 1976;116:718–724. doi: 10.1016/s0022-5347(17)58986-7. [DOI] [PubMed] [Google Scholar]
- 17.Roscigno M, Cozzarini C, Bertini R, Scattoni V, Freschi M, Da Pozzo LF, et al. Prognostic value of lymph node dissection in patients with muscle-invasive transitional cell carcinoma of the upper urinary tract. Eur Urol. 2008;53:794–802. doi: 10.1016/j.eururo.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Kikuchi E, Horiguchi Y, Nakashima J, Hatakeyama N, Matsumoto M, Nishiyama T, et al. Lymphovascular invasion independently predicts increased disease specific survival in patients with transitional cell carcinoma of the upper urinary tract. J Urol. 2005;174:2120–2123. doi: 10.1097/01.ju.0000181801.22474.8b. [DOI] [PubMed] [Google Scholar]
- 19.Hong B, Park S, Hong JH, Kim CS, Ro JY, Ahn H. Prognostic value of lymphovascular invasion in transitional cell carcinoma of upper urinary tract. Urology. 2005;65:692–696. doi: 10.1016/j.urology.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Lee SH, Lin JS, Tzai TS, Chow NH, Tong YC, Yang WH, et al. Prognostic factors of primary transitional cell carcinoma of the upper urinary tract. Eur Urol. 1996;29:266–270. doi: 10.1159/000473758. [DOI] [PubMed] [Google Scholar]
- 21.Akdogan B, Dogan HS, Eskicorapci SY, Sahin A, Erkan I, Ozen H. Prognostic significance of bladder tumor history and tumor location in upper tract transitional cell carcinoma. J Urol. 2006;176:48–52. doi: 10.1016/S0022-5347(06)00511-8. [DOI] [PubMed] [Google Scholar]
- 22.Mullerad M, Russo P, Golijanin D, Chen HN, Tsai HH, Donat SM, et al. Bladder cancer as a prognostic factor for upper tract transitional cell carcinoma. J Urol. 2004;172:2177–2181. doi: 10.1097/01.ju.0000144505.40915.98. [DOI] [PubMed] [Google Scholar]
- 23.Rabbani F, Perrotti M, Russo P, Herr HW. Upper-tract tumors after an initial diagnosis of bladder cancer: argument for long-term surveillance. J Clin Oncol. 2001;19:94–100. doi: 10.1200/JCO.2001.19.1.94. [DOI] [PubMed] [Google Scholar]
- 24.Park S, Hong B, Kim CS, Ahn H. The impact of tumor location on prognosis of transitional cell carcinoma of the upper urinary tract. J Urol. 2004;171:621–625. doi: 10.1097/01.ju.0000107767.56680.f7. [DOI] [PubMed] [Google Scholar]
- 25.Miyake H, Hara I, Gohji K, Arakawa S, Kamidono S. The significance of lymphadenectomy in transitional cell carcinoma of the upper urinary tract. Br J Urol. 1998;82:494–498. doi: 10.1046/j.1464-410x.1998.00800.x. [DOI] [PubMed] [Google Scholar]
- 26.Novara G, De Marco V, Gottardo F, Dalpiaz O, Bouygues V, Galfano A, et al. Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract: multi-institutional dataset from 3 European centers. Cancer. 2007;110:1715–1722. doi: 10.1002/cncr.22970. [DOI] [PubMed] [Google Scholar]
- 27.Brien JC, Shariat SF, Herman MP, Ng CK, Scherr DS, Scoll B, et al. Preoperative hydronephrosis, ureteroscopic biopsy grade and urinary cytology can improve prediction of advanced upper tract urothelial carcinoma. J Urol. 2010;184:69–73. doi: 10.1016/j.juro.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 28.Cho KS, Cho NH, Choi YD. The clinical significance of hydronephrosis and the tumor diameter in ureteral transitional cell carcinoma. Korean J Urol. 2006;47:131–136. [Google Scholar]