Abstract
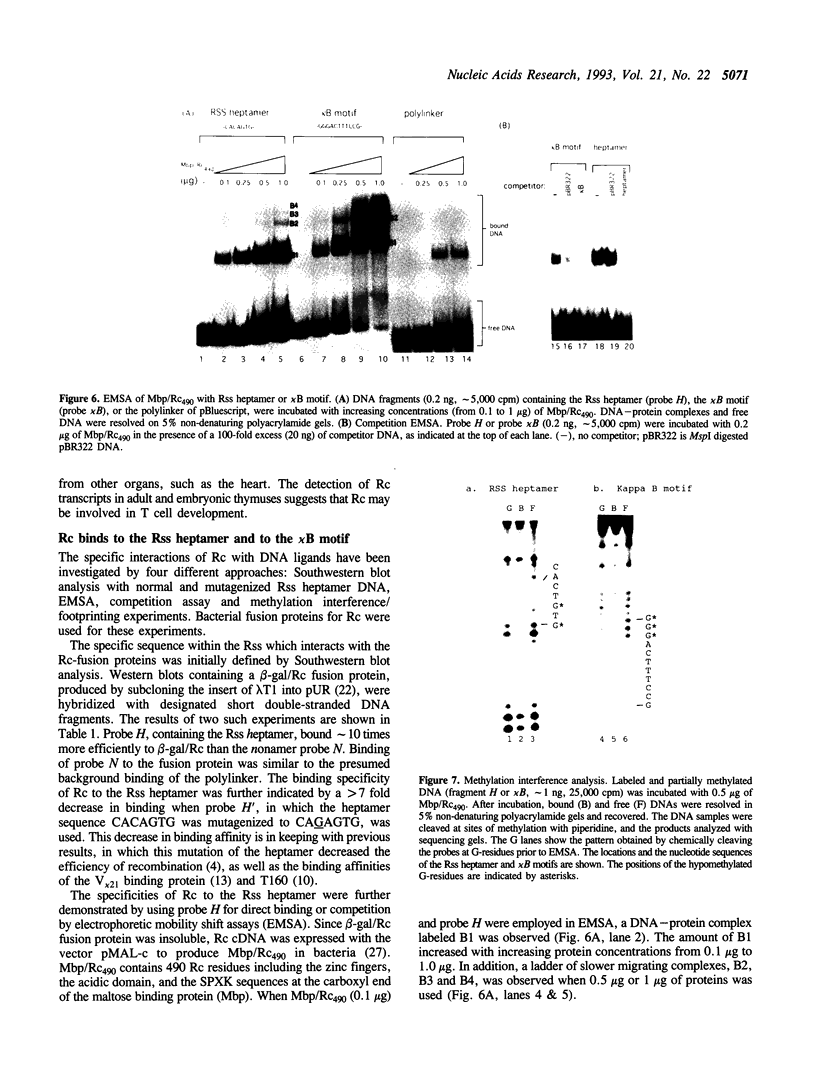
The somatic V(D)J recombination for the assembly of the Ig and TCR genes is mediated by the recombination signal sequences (Rss) and the V(D)J recombinase. A cDNA clone was isolated from a lambda gt11 expression library made from mouse thymocyte poly(A)+ RNA, using the Rss as a ligand. The deduced amino acid sequence of the putative protein, designated Recognition component (Rc), reveals a pair of Cys2-His2 zinc fingers followed by a Glu- and Asp-rich acidic domain. In addition, there are five copies of the Ser/Thr-Pro-X-Arg/Lys sequence, which are putative DNA binding units. The zinc finger-acidic domain structures present in Rc are also found in several enhancer binding proteins, such as those for the kappa B motif of the Ig kappa light chain enhancer or related sequences. Bacterial fusion proteins for Rc bind preferentially to the Rss heptamer and to the kappa B motif. The dual affinities of Rc for the Rss heptamer and the kappa B motif suggest a possible link between Ig transcription and somatic recombination. The formation of multiple 'gel-shifted' DNA-protein complexes for Rc and its DNA ligand suggests that these complexes tend to multimerize.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilera R. J., Akira S., Okazaki K., Sakano H. A pre-B cell nuclear protein that specifically interacts with the immunoglobulin V-J recombination sequences. Cell. 1987 Dec 24;51(6):909–917. doi: 10.1016/0092-8674(87)90578-2. [DOI] [PubMed] [Google Scholar]
- Akira S., Okazaki K., Sakano H. Two pairs of recombination signals are sufficient to cause immunoglobulin V-(D)-J joining. Science. 1987 Nov 20;238(4830):1134–1138. doi: 10.1126/science.3120312. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Atchison M. L., Perry R. P. The role of the kappa enhancer and its binding factor NF-kappa B in the developmental regulation of kappa gene transcription. Cell. 1987 Jan 16;48(1):121–128. doi: 10.1016/0092-8674(87)90362-x. [DOI] [PubMed] [Google Scholar]
- Boehm T., Gonzalez-Sarmiento R., Kennedy M., Rabbitts T. H. A simple technique for generating probes for RNA in situ hybridization: an adjunct to genome mapping exemplified by the RAG-1/RAG-2 gene cluster. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3927–3931. doi: 10.1073/pnas.88.9.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Churchill M. E., Suzuki M. 'SPKK' motifs prefer to bind to DNA at A/T-rich sites. EMBO J. 1989 Dec 20;8(13):4189–4195. doi: 10.1002/j.1460-2075.1989.tb08604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. E. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Fan C. M., Maniatis T. A DNA-binding protein containing two widely separated zinc finger motifs that recognize the same DNA sequence. Genes Dev. 1990 Jan;4(1):29–42. doi: 10.1101/gad.4.1.29. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- Kleinfield R., Hardy R. R., Tarlinton D., Dangl J., Herzenberg L. A., Weigert M. Recombination between an expressed immunoglobulin heavy-chain gene and a germline variable gene segment in a Ly 1+ B-cell lymphoma. 1986 Aug 28-Sep 3Nature. 322(6082):843–846. doi: 10.1038/322843a0. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Kuang A., Gifford A., Baltimore D. NF-kappa B protein purification from bovine spleen: nucleotide stimulation and binding site specificity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8825–8829. doi: 10.1073/pnas.85.23.8825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Morzycka-Wroblewska E., Desiderio S. V. NBP, a protein that specifically binds an enhancer of immunoglobulin gene rearrangement: purification and characterization. Genes Dev. 1989 Nov;3(11):1801–1813. doi: 10.1101/gad.3.11.1801. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Developmental stage specificity of the lymphoid V(D)J recombination activity. Genes Dev. 1987 Oct;1(8):751–761. doi: 10.1101/gad.1.8.751. [DOI] [PubMed] [Google Scholar]
- Maekawa T., Sakura H., Sudo T., Ishii S. Putative metal finger structure of the human immunodeficiency virus type 1 enhancer binding protein HIV-EP1. J Biol Chem. 1989 Sep 5;264(25):14591–14593. [PubMed] [Google Scholar]
- Matsunami N., Hamaguchi Y., Yamamoto Y., Kuze K., Kangawa K., Matsuo H., Kawaichi M., Honjo T. A protein binding to the J kappa recombination sequence of immunoglobulin genes contains a sequence related to the integrase motif. Nature. 1989 Dec 21;342(6252):934–937. doi: 10.1038/342934a0. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Nagawa F., Okazaki K., Kingsbury L., Yoshida K., Müller U., Larue D. T., Winer J. A., Sakano H. Detection of somatic DNA recombination in the transgenic mouse brain. Science. 1991 Oct 4;254(5028):81–86. doi: 10.1126/science.1925563. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mitchelmore C., Traboni C., Cortese R. Isolation of two cDNAs encoding zinc finger proteins which bind to the alpha 1-antitrypsin promoter and to the major histocompatibility complex class I enhancer. Nucleic Acids Res. 1991 Jan 11;19(1):141–147. doi: 10.1093/nar/19.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muegge K., West M., Durum S. K. Recombination sequence-binding protein in thymocytes undergoing T-cell receptor gene rearrangement. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4151–4155. doi: 10.1073/pnas.90.9.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Donovan D. M., Hamada K., Sax C. M., Norman B., Flanagan J. R., Ozato K., Westphal H., Piatigorsky J. Regulation of the mouse alpha A-crystallin gene: isolation of a cDNA encoding a protein that binds to a cis sequence motif shared with the major histocompatibility complex class I gene and other genes. Mol Cell Biol. 1990 Jul;10(7):3700–3708. doi: 10.1128/mcb.10.7.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Rustgi A. K., Van 't Veer L. J., Bernards R. Two genes encode factors with NF-kappa B- and H2TF1-like DNA-binding properties. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8707–8710. doi: 10.1073/pnas.87.22.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Nau M. M., Norman B., Kwan S. P., Scharff M., Leder P. Immunoglobulin V/J recombination is accompanied by deletion of joining site and variable region segments. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6022–6026. doi: 10.1073/pnas.77.10.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Shirakata M., Hüppi K., Usuda S., Okazaki K., Yoshida K., Sakano H. HMG1-related DNA-binding protein isolated with V-(D)-J recombination signal probes. Mol Cell Biol. 1991 Sep;11(9):4528–4536. doi: 10.1128/mcb.11.9.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., LeBowitz J. H., Baldwin A. S., Jr, Sharp P. A. Molecular cloning of an enhancer binding protein: isolation by screening of an expression library with a recognition site DNA. Cell. 1988 Feb 12;52(3):415–423. doi: 10.1016/s0092-8674(88)80034-5. [DOI] [PubMed] [Google Scholar]
- Staden R. Automation of the computer handling of gel reading data produced by the shotgun method of DNA sequencing. Nucleic Acids Res. 1982 Aug 11;10(15):4731–4751. doi: 10.1093/nar/10.15.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M. SPXX, a frequent sequence motif in gene regulatory proteins. J Mol Biol. 1989 May 5;207(1):61–84. doi: 10.1016/0022-2836(89)90441-5. [DOI] [PubMed] [Google Scholar]
- Takeda S., Zou Y. R., Bluethmann H., Kitamura D., Muller U., Rajewsky K. Deletion of the immunoglobulin kappa chain intron enhancer abolishes kappa chain gene rearrangement in cis but not lambda chain gene rearrangement in trans. EMBO J. 1993 Jun;12(6):2329–2336. doi: 10.1002/j.1460-2075.1993.tb05887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson C. R., LaMarco K. L., Johnson P. F., Landschulz W. H., McKnight S. L. In situ detection of sequence-specific DNA binding activity specified by a recombinant bacteriophage. Genes Dev. 1988 Jul;2(7):801–806. doi: 10.1101/gad.2.7.801. [DOI] [PubMed] [Google Scholar]
- van 't Veer L. J., Lutz P. M., Isselbacher K. J., Bernards R. Structure and expression of major histocompatibility complex-binding protein 2, a 275-kDa zinc finger protein that binds to an enhancer of major histocompatibility complex class I genes. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8971–8975. doi: 10.1073/pnas.89.19.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]