Summary
Background
Temsirolimus is an mTOR inhibitor with single-agent antitumor activity in patients with mantle cell lymphoma (MCL). We therefore tested the efficacy and toxicity of temsirolimus in combination with rituximab in patients with relapsed or refractory MCL.
Methods
Patients received temsirolimus 25 mg intravenously weekly while on study. Four weekly doses of rituximab 375mg/m2 were given during the first cycle followed by a single dose of rituximab every other 28-day cycle thereafter. Responding patients after six cycles could continue treatment for a total of 12 cycles and were then observed without additional maintenance therapy. The primary endpoint was the proportion of patients with either rituximab-sensitive or rituximab-refractory disease who had a partial response or better. The analyses were done on all patients who received treatment. The study was registered with ClinicalTrials.gov, number NCT00109967.
Findings
Seventy-one patients were enrolled between May 2005 and March 2009. Sixty-nine patients are evaluable and are included in the final analysis. Patients had received a median of two prior therapies (range, 1-9), 30·4% (21/69) had received a prior stem cell transplant and 30·4% (21/69) were rituximab-refractory. The overall response rate (ORR) was 59·4% (41/69 patients) with 18·8% (13/69) complete responses and 40·6% (28/69) partial responses. The ORR for rituximab-sensitive patients was 62·5% (30/48; 95% CI 47·4-76·1%) and 52·4% (11/21; 95% CI 29·8 – 74·3%) for rituximab-refractory patients. The most common treatment-related grade 3-4 adverse events were thrombocytopenia in 16 patients (23·2%), neutropenia in 15 (21·7%), fatigue in 10 (14·5%), pneumonia in 7 (10·1%), lymphopenia in 7 (10·1%), pneumonitis in 5 (7·2%), dyspnea in 5 (7·2%) and hypertriglyceridemia in 5 (7·2%).
Interpretation
mTOR inhibitors in combination with rituximab could have a role in the treatment of patients with relapsed and refractory MCL.
Keywords: Mantle Cell Lymphoma, mTOR Inhibitor, Rituximab, Phase 2 Trial
Introduction
Mantle cell lymphoma (MCL) is an aggressive, incurable B-cell malignancy that represents approximately 3-6% of lymphoma cases 1–4. Patients with MCL have a poorer prognosis than those with other indolent lymphomas, despite an improvement in the median survival of particularly younger patients treated with aggressive therapy 5–7. While the majority of patients respond to initial therapy, many patients will subsequently progress and require further therapy 7,8. Patients with relapsed MCL have a poor prognosis with a median survival of less than 2 years 9 and are therefore candidates for treatment with novel agents.
We have previously shown that temsirolimus is an active antitumor agent in MCL10,11. Temsirolimus inhibits signaling via the phosphatidylinosital 3-kinase (PI3K) cellular pathway 12,13 which is important in cell motility and survival 14. PI3K catalyzes the synthesis of phosphatidylinositol-3 phosphate (PIP3) from PIP2 resulting in activation of the serine- threonine kinase AKT 15,16. AKT activates the mammalian target of rapamycin (mTOR), a key cellular regulator of mitosis, survival, and increased cellular size. mTOR activates p70 S6 kinase and inhibits 4EBP1, which enhance and inhibit the translation of mRNAs, respectively 17, and down regulation of p70 S6 kinase and 4EBP1 by mTOR inhibitors has been associated with responses to therapy 18. AKT also induces accumulation of cellular cyclin D1. In a previous phase 2 trial in MCL using temsirolimus as a single agent 10, we observed an overall response rate (ORR) of 38% using a dose of 250mg IV weekly and then found a similar response rate of 41% in a subsequent study using a 10-fold lower dose of 25mg IV weekly 11. A randomized trial was then performed comparing temsirolimus to investigator’s choice of therapy for relapsed MCL19. This study showed a significantly improved PFS and ORR in patients treated with temsirolimus, although the response rate was lower and PFS shorter than what was seen in the phase 2 studies19. In all studies, the predominant toxicities were hematologic and the time to progression (TTP) was approximately 4-6 months in patients with relapsed or refractory disease.
Rituximab, a monoclonal antibody that targets the CD20 antigen, has also been used as a single agent to treat MCL at the time of disease relapse. Reported response rates in these patients ranged from 20-38% 20–22 and response rates were similar whether the standard schedule or prolonged treatment with rituximab was given 21. Although single agent rituximab induced responses in these trials in previously treated patients, complete responses were seen in less than 10% of patients in all but one study 20–22. Furthermore, the TTP after rituximab therapy in relapsed MCL patients has been short – typically 6-8 months. The mechanisms of rituximab action are complex and include induction of apoptosis 23, complement-dependent cytotoxicity24 and antibody-dependent cellular cytotoxicity (ADCC) 25. Interestingly, rituximab has also been shown to inhibit the ERK1/2 pathway and to interact with the PI3K pathway upstream of mTOR at the PIP3 level 26.
There was therefore a strong rationale to combine rituximab with temsirolimus for MCL patients - both agents have single agent activity in MCL; both target the PI3K pathway but at different levels; temsirolimus can mobilize tumor cells into the peripheral circulation and rituximab is known to deplete circulating lymphoma cells 27; and their toxicities as single agents do not overlap. Because similar efficacy with less toxicity had been seen using a lower dose of temsirolimus 11, we tested temsirolimus at the 25mg dose level in combination with rituximab in patients with previously treated MCL with the primary endpoint of evaluating the ORR and assessing the tolerability and adverse events in MCL patients. The secondary endpoint was to assess the duration of response, TTP and overall survival in this patient cohort.
Patients and Methods
Patient Eligibility
In this cooperative group study conducted by the North Central Cancer Treatment Group (NCCTG), patients were required to be 18 years of age or older with relapsed and/or refractory MCL confirmed by central pathology review prior to enrollment. The tumor cells had to be cyclin D1-positive by immunohistochemistry or have the 11;14 translocation by cytogenetic or interphase fluorescence in situ hybridization (FISH) analysis. All patients had to be previously treated and there was no limit on the number of prior treatments. All patients had measurable disease. Patients were required to have adequate organ and marrow function defined as an absolute neutrophil count (ANC) >1,000/mm3, platelet count >75,000/mm3, total bilirubin <1.5 × upper limit of normal (ULN), AST <3 × ULN (<5 × ULN if liver involvement with MCL), serum creatinine <2 × ULN, serum cholesterol <350mg/dL, and fasting triglycerides <400mg/dL. In addition, patients had to have a life expectancy of > 3 months and an Eastern Cooperative Oncology Group performance status of 0, 1, or 2. Women of childbearing potential were required to have a negative pregnancy test done ≤7 days prior to registration.
Exclusion criteria included patients who were HIV positive; patients with central nervous system involvement; investigational agents, corticosteroids, chemotherapy, immunotherapy, biologic therapy and/or radiation therapy in the previous 1 month; failure to fully recover from prior chemotherapy regardless of interval since last treatment; or previous therapy with an mTOR inhibitor. Pregnant and nursing women were not eligible for the study. All patients were required to give informed consent and the Institutional Review Boards of the Mayo Clinic and each NCCTG treatment site approved the study. The study was also registered with the National Cancer Institute (ClinicalTrials.gov Identifier: NCT00109967)
Study Design
Eligible patients received a fixed dose of temsirolimus 25 mg intravenously every week and four weekly doses of rituximab 375mg/m2 intravenously during the first 28-day cycle of treatment. For subsequent 28-day cycles, a fixed dose of temsirolimus 25 mg was given intravenously every week and one dose of rituximab 375mg/m2 was given intravenously on day 1 of every other cycle (cycles 3, 5, 7, 9, and 11). Patients underwent a restaging evaluation after every 3 cycles of therapy that included computerized tomography (CT) scanning of the chest, abdomen and pelvis and flow cytometry of the peripheral blood. A repeat bone marrow biopsy and aspirate was done only if the pretreatment bone marrow showed involvement by mantle cell lymphoma and the patient was in complete remission by CT scanning. Patients with a tumor response after six cycles were eligible to continue treatment for a total of 12 cycles, or two cycles after complete remission, and were then observed without additional maintenance therapy. Patients with stable disease after 6 cycles and those that progressed at any time went off study. Treatment responses as well as disease progression were determined using the “International Workshop to Standardize Response Criteria for NHL” 28.
The patients were stratified by their previous response to rituximab into patients that were rituximab sensitive (group 1) or rituximab refractory (group 2). Rituximab refractory was defined as no response (stable disease or progression) or a response that lasted <6 months the last time the patient received rituximab alone or rituximab with chemotherapy. The study used two separate phase II designs in two different patient populations; the design for group 1 was a two-stage design, while group 2 utilized a one-stage design with an interim analysis. The first stage of the design for group 1 required the enrollment of 24 evaluable patients and they be followed for at least 24 weeks. If 7 or fewer patients had a response in this timeframe, we would consider this early evidence that this treatment regimen was not sufficiently active. If 8 or more patients had a response, then accrual would continue in the second stage of this trial. If 15 or more responses were observed in the first 24 evaluable patients, then this treatment regimen would be considered promising. In the second stage for group 1, an additional 17 evaluable patients would be enrolled. If 20 or fewer successes were observed in the first 41 evaluable patients, we would consider this evidence that this treatment regimen was not sufficiently active in this patient population. If 21 or more successes were observed in the first 41 evaluable patients, then this treatment regimen would be considered promising in this patient population and would be evaluated further in future studies. For group 2, the study regimen would be considered promising in this patient population if 5 or more of the first 25 evaluable patients had a response. Otherwise, it would be considered inactive. When the first 16 patients had been accrued and followed for 24 weeks, an interim analysis would be performed. The regimen would be considered inactive if zero responses were observed.
Toxicity Evaluation and Adverse Event Stopping Rules
As per the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) Version 3, toxicity was defined as adverse events that are classified as either possibly, probably, or definitely related to study treatment by the treating physician. Administration of temsirolimus was held if the ANC was <1,000 or the platelet count was <50,000. Upon recovery to ANC ≥1,000 and platelets to ≥50,000, the dose of temsirolimus was decreased by 5mg. The administration of temsirolimus was also held for any grade 3 or 4 non-hematologic adverse events and restarted once the toxicity had resolved to at least grade 2. There were no dose modifications for rituximab and rituximab was given even if temsirolimus was held for toxicity. Overall (across both groups), if 3 out of the first 22 or if at any time 7 or more patients developed grade 4 non-hematologic toxicity (i.e., adverse events felt to be at least possibly related to treatment), then protocol accrual would be suspended pending review by the study chair and the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute. Infusion reactions related events to rituximab were not considered in the stopping rule. The toxicity data was regularly reviewed by the Data Safety Monitoring Board (DSMB) to ensure compliance with the safety stopping rules.
Immunohistochemistry
Five-micron whole tissue sections were cut from paraffin-embedded tissue from pretreatment biopsies from the patients enrolled in this study. Immunohistochemistry focused on proteins involved in the mTOR pathway and those expressed on cells in the microenvironment. The primary antibodies used for staining recognized the following antigens: GATA3 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), FoxP3 (Abcam, Cambridge, MA), Tbet (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), CD8 (DAKO North America, Inc., Carpinteria, CA), CD11c (Abcam, Cambridge, MA), CD68 (DAKO North America, Inc., Carpinteria, CA), raptor (Novus Biologicals USA, Littleton, CO), rictor (Novus Biologicals USA, Littleton, CO), p4EBP1 (Thr70, Cell Signaling, Danvers, MA), pAkt (Novus Biologicals USA, Littleton, CO) and pS6 ribosomal protein (Ser235/236, Cell Signaling, Danvers, MA). Immunohistochemical staining was considered “high” when >15% of cells demonstrated staining in the appropriate cellular compartment. Slides were viewed with an Olympus BX51 microscope (20x objective) and pictures were taken with an Olympus DP71 camera. Olympus BSW with DP Controller software was used for image acquisition and storage.
Statistical Methods
Duration of response was defined as the time from the date of documented response to the date of progression. Patients who went off treatment due to other reasons (eg, adverse reactions, refusal of further treatment) were censored at that time. Time to progression was defined as the time from registration to the date of progression. Patients who died without disease progression were censored at the date of their last evaluation. Patients who were still receiving treatment at the time of these analyses were censored at the date of their last evaluation. Overall survival (OS) was defined as the time from registration to death resulting from any cause. The distributions of these time-to-event end points were each estimated using the Kaplan-Meier method. Statistical analyses used SAS software (version 9.2).
Role of the funding source
The sponsors of the study were not involved in the collection, analysis, or interpretation of the data. The Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute was involved in the design and monitoring of the trial. The Predolin Foundation and grant funding from the National Cancer Institute funded the correlative studies. The sponsors were not involved in the writing of the report but reviewed the report upon submission. SMA, HT and PAK had access to the raw data. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Patient Characteristics
Seventy-one patients seen at 35 sites were enrolled on this trial between May 6, 2005 and March 6, 2009. Two patients canceled before receiving treatment and 69 patients were therefore included in the analysis. There were 48 patients in group 1 (rituximab-sensitive) and 21 (rituximab-refractory) in group 2. The median age was 67 years (range, 44 to 86 years), with 50 males and 19 females enrolled. Most patients had a performance score of 0 (63·8%; 44/69) or 1 (31·9%; 22/69). Patients had received a median of two prior therapies (range, 1-9) and 30·4% (21/69) had received a prior stem cell transplant. All patients had previously received rituximab and 30·4% (21/69) were rituximab-refractory. Seventy-four percent of patients had initially been treated with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy. Twenty-three patients had previously received cytarabine as part of previous salvage chemotherapy regimens. Patient characteristics by group are shown in Table 1.
Table 1.
Patient Characteristics
| Rituximab Sensitive (N=48) |
Rituximab Refractory (N=21) |
Total (N=69) |
p value | |
|---|---|---|---|---|
| Age | 0·791 | |||
| Median (years) | 67.5 | 66 | 67 | |
| Range (years) | (51-86) | (44-85) | (44-86) | |
| Gender | 0·902 | |||
| female | 13 (27·1%) | 6 (28·6%) | 19 (27·5%) | |
| male | 35 (72·9%) | 15 (71·4%) | 50 (72·5%) | |
| Performance Score | 0·813 | |||
| 0 | 32 (66·7%) | 12 (57·1%) | 44 (63·8%) | |
| 1 | 14 (29·2%) | 8 (38·1%) | 22 (31·9%) | |
| 2 | 2 (4·2%) | 1 (4·8%) | 3 (4·3%) | |
| Number of Previous Treatments | <0·00013 | |||
| Mean (SD) | 2·1 (1·51) | 3·6 (1·66) | 2·5 (1·70) | |
| Median | 2·0 | 3·0 | 2·0 | |
| Range | (1-9) | (1-9) | (1-9) | |
| Previous Initial Therapy | 0·833 | |||
| R-CHOP | 33 (68·8%) | 18 (85·7%) | 51 (73·9%) | |
| R-HyperCVAD | 5 (10·4%) | 1 (4·8%) | 6 (8·7%) | |
| R-DHAP | 1 (2%) | 0 | 1 (1·4%) | |
| Cladribine + rituximab | 5 (10·4%) | 1 (4·8%) | 6 (8·7%) | |
| Other | 4 (8·3%) | 1 (4·8%) | 5 (7·2%) | |
| Previous Stem Cell Transplant | 0·432 | |||
| Yes | 16 (33·3%) | 5 (23·8%) | 21 (30·4%) | |
| No | 32 (66·7%) | 16 (76·2%) | 48 (69·6%) | |
| Previous Radioimmunotherapy | 0·583 | |||
| Yes | 2 (4·2%) | 2 (9·5%) | 4 (5·8%) | |
| No | 46 (95·8%) | 19 (90·5%) | 65 (94·2%) | |
| Previous Radiation Therapy | 0·062 | |||
| Yes | 10 (20·8%) | 9 (42·9%) | 19 (27·5%) | |
| No | 38 (79·2%) | 12 (57·1%) | 50 (72·5%) |
Kruskal Wallis
Chi-Square
Fisher Exact
R-CHOP – rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone. R-HyperCVAD – rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, cytarabine. R-DHAP – rituximab, dexamethasone, cytarabine, cisplatin.
Clinical Responses
During the planned interim analysis, there were 13 responses in the first 24 evaluable patients in group 1. As a result, the trial met the criteria for continuing to the second stage for the rituximab-sensitive cohort and a total of 48 patients were enrolled in this group. In group 2, there were 8 responses in the first 16 evaluable patients. We therefore closed the study early because the accrual goal has been met per the protocol which stated “The study regimen will be considered promising in this patient population if 5 or more of the first 25 evaluable patients have a treatment success”. Once the accrual goals of the study had been met, the ORR was 59·4% (41/69) with 13 complete responses (18·8%) and 28 partial responses (40·6%). There were 8 complete responses in the rituximab-sensitive group and 5 in the rituximab-resistant group (see Table 2). The median duration of response for all patients in complete or partial remission was 10·6 months (95% CI, 6·6 to 12·5 months). The median duration of response in the rituximab-sensitive patients was 11·0 months (95% CI: 7·1-13·2) and 6·6 months (95% CI: 2·0-20·9) in the rituximab-refractory patients (Figure 1). The median duration of complete response in the rituximab-sensitive patients was 12·5 months (95% CI: 10·6-not reached) and 6·6 months (95% CI: 3·5-not reached) in the rituximab-refractory patients. The median OS was 29·5 months (95% CI 23·8 – 32·6) and the median TTP was 9·7 months (95% CI: 5·8 -12·0). The TTP was 10·9 months (95% CI: 8·0-12·8) in the rituximab-sensitive group and 5·4 months (95% CI: 1·8-9·4) in the rituximab-refractory group. The OS was 32·6 months (CI: 24·9-39·7) in the rituximab-sensitive group and 24·2 months (CI: 5·7-30·0) in the rituximab-refractory group.
Table 2.
Response Rate by Treatment Group
| Response | Overall Rate |
Rituximab Sensitive Rate (95% CI) |
Rituximab Refractory Rate (95% CI) |
|---|---|---|---|
| CR + PR | 59·4% | 62·5% (47·4-76·1%) | 52·4% (29·8-74·3%) |
| CR | 18·8% | 16·7% (7·5-30·2%) | 23·8% (8·2-47·2%) |
| PR | 40·6% | 45·8% (31·4-60·8%) | 28·6% (11·3-52·2%) |
Figure 1. Distribution of time-to-event endpoints of Mantle Cell Lymphoma Patients treated with Temsirolimus and Rituximab (duration of response, time to progression, and overall survival).
A) Rituximab-sensitive patients (group 1), B) Rituximab-refractory patients (group 2).
Toxicity
All 69 treated patients were evaluable for adverse events. Three patients (4%) died while on study. The cause of death in all 3 was due to disease progression and felt to be unrelated to treatment. Nine patients experienced 13 grade 4 non-hematologic events with 4 events considered at least possibly related to treatment. Seven patients experienced 14 grade 4 hematologic adverse events with 13 events considered at least possibly related to treatment. Thrombocytopenia (5 patients experienced 8 adverse events) and neutropenia (3 patients experienced 5 adverse events) were the only grade 4 hematologic adverse events reported that are related to treatment. Serious adverse events (≥ grade 4) seen in this study were thrombocytopenia in 5 patients (7·2%), neutropenia in 3 (4·3%), dyspnea in 1 (1·4%), hemorrhage in 1 (1·4%), ischemia in 1 (1·4%), sexual dysfunction in 1 (1·4%), and death in 3 patients (4·3%). The most commonly seen toxicities by patient group are shown in Tables 3a and 3b. Grade 3 infection rates (at least possibly related to treatment) were: group 1: 7/48 (14·6%) and group 2: 2/21 (9·5%). There were no grade 4/5 infections. Only one patient experienced grade 3 febrile neutropenia in the rituximab sensitive group. A total of 47 patients (33 in group 1, 14 in group 2) had temsirolimus delayed due to toxicity at some time during treatment. The median number of cycles received per patient was 6 (range=1-12). Twenty-one patients completed the study per protocol and 14 of these had a dose adjustment during therapy. Forty-two patients experienced a total of 89 dose adjustments for either drug, however only one patient had a dose adjustment for rituximab._The median percentage of time that the full dose of therapy was administered on time as per protocol was similar between responding (median 80%; range 15-100%) and non-responding patients (median 75%; range 10-100%). Patients ended treatment due to the following reasons: disease progression (34·8%; 24/69), completed study per protocol (30·4%; 21/69), adverse event (18·8%; 13/69), refused further treatment (7·2%; 5/69), died on study (2·9%; 2/69), other medical problems (2·9%; 2/69), alternate treatment (1·4%; 1/69), and symptomatic deterioration (1·4%; 1/69).
Table 3a.
Adverse Events Related to Therapy Seen in >10% of Patients with Rituximab-Sensitive Mantle Cell Lymphoma (Group 1, n=48)
| ADVERSE EVENT | Grade | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| N | % | N | % | N | % | N | % | |
| Hypertriglyceridemia | 27 | 56·3 | 7 | 14·6 | 3 | 6·3 | ||
| Fatigue | 16 | 33·3 | 12 | 25·0 | 8 | 16·7 | ||
| Thrombocytopenia | 17 | 35·4 | 11 | 22·9 | 4 | 8·3 | 4 | 8·3 |
| Hypercholesterolemia | 24 | 50·0 | 9 | 18·8 | 1 | 2·1 | ||
| Anemia | 14 | 29·2 | 13 | 27·1 | 2 | 4·2 | ||
| Leukopenia | 12 | 25·0 | 10 | 20·8 | 6 | 12·5 | ||
| Rash | 11 | 22·9 | 11 | 22·9 | ||||
| Neutropenia | 3 | 6·3 | 8 | 16·7 | 7 | 14·6 | 3 | 6·3 |
| Hyperglycemia | 12 | 25·0 | 5 | 10·4 | 2 | 4·2 | ||
| Oral ulcers | 6 | 12·5 | 13 | 27·1 | ||||
| Diarrhea | 7 | 14·6 | 8 | 16·7 | 1 | 2·1 | ||
| Edema | 6 | 12·5 | 4 | 8·3 | 4 | 8·3 | ||
| Anorexia | 4 | 8·3 | 8 | 16·7 | ||||
| Dysgeusia | 8 | 16·7 | 4 | 8·3 | ||||
| Weight loss | 9 | 18·8 | 2 | 4·2 | 1 | 2·1 | ||
| Nausea | 8 | 16·7 | 3 | 6·3 | ||||
| Sensory neuropathy | 6 | 12·5 | 4 | 8·3 | 1 | 2·1 | ||
| Nail changes | 7 | 14·6 | 2 | 4·2 | 1 | 2·1 | ||
| Dyspnea | 2 | 4·2 | 4 | 8·3 | 2 | 4·2 | 1 | 2·1 |
| Pneumonitis | 3 | 6·3 | 2 | 4·2 | 4 | 8·3 | ||
| Elevated alkaline phosphatase | 7 | 14·6 | ||||||
| Cough | 7 | 14·6 | ||||||
| Hypocalcemia | 6 | 12·5 | 1 | 2·1 | ||||
| Lymphopenia | 2 | 4·2 | 5 | 10·4 | ||||
| Arthralgia | 5 | 10·4 | 1 | 2·1 | ||||
| Constipation | 4 | 8·3 | 2 | 4·2 | ||||
| Elevated creatinine | 4 | 8·3 | 1 | 2·1 | 1 | 2·1 | ||
| Headache | 5 | 10·4 | 1 | 2·1 | ||||
| Hypoalbuminemia | 4 | 8·3 | 2 | 4·2 | ||||
| Pruritus | 6 | 12·5 | ||||||
| Myalgia | 3 | 6·3 | 2 | 4·2 | ||||
| Pneumonia | 5 | 10·4 | ||||||
| Hemorrhage | 1 | 2·1 | ||||||
| Ischemia/infarction | 1 | 2·1 | ||||||
| Sexual dysfunction | 1 | 2·1 | ||||||
Table 3b.
Adverse Events Related to Therapy Seen in >10% of Patients with Rituximab-Refractory Mantle Cell Lymphoma (Group 2, n=21)
| ADVERSE EVENT | Grade | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| N | % | N | % | N | % | N | % | |
| Anemia | 6 | 28·6 | 10 | 47·6 | ||||
| Fatigue | 4 | 19·0 | 10 | 47·6 | 2 | 9·5 | ||
| Hypertriglyceridemia | 10 | 47·6 | 4 | 19·0 | 2 | 9·5 | ||
| Leukopenia | 4 | 19·0 | 9 | 42·9 | 3 | 14·3 | ||
| Thrombocytopenia | 6 | 28·6 | 2 | 9·5 | 7 | 33·3 | 1 | 4·8 |
| Hypercholesterolemia | 11 | 52·4 | 3 | 14·3 | ||||
| Neutropenia | 4 | 19·0 | 3 | 14·3 | 5 | 23·8 | ||
| Hyperglycemia | 6 | 28·6 | 3 | 14·3 | 1 | 4·8 | ||
| Nausea | 5 | 23·8 | 4 | 19·0 | 1 | 4·8 | ||
| Rash | 5 | 23·8 | 4 | 19·0 | 1 | 4·8 | ||
| Anorexia | 5 | 23·8 | 4 | 19·0 | ||||
| Weight loss | 4 | 19·0 | 2 | 9·5 | 1 | 4·8 | ||
| Diarrhea | 3 | 14·3 | 2 | 9·5 | 1 | 4·8 | ||
| Elevated alkaline phosphatase | 5 | 23·8 | ||||||
| Dyspnea | 3 | 14·3 | 2 | 9·5 | ||||
| Sensory neuropathy | 4 | 19·0 | 1 | 4·8 | ||||
| Constipation | 2 | 9·5 | 2 | 9·5 | ||||
| Edema | 4 | 19·0 | ||||||
| Hypokalemia | 3 | 14·3 | 1 | 4·8 | ||||
| Oral ulcers | 4 | 19·0 | ||||||
| Elevated aspartate aminotransferase | 3 | 14·3 | ||||||
| Cough | 2 | 9·5 | 1 | 4·8 | ||||
| Elevated creatinine | 1 | 4·8 | 2 | 9·5 | ||||
| Lymphopenia | 1 | 4·8 | 2 | 9·5 | ||||
| Muscle weakness | 1 | 4·8 | 1 | 4·8 | 1 | 4·8 | ||
| Nail changes | 1 | 4·8 | 2 | 9·5 | ||||
| Pneumonia | 1 | 4·8 | 2 | 9·5 | ||||
| Pruritus | 2 | 9·5 | 1 | 4·8 | ||||
Correlative Studies
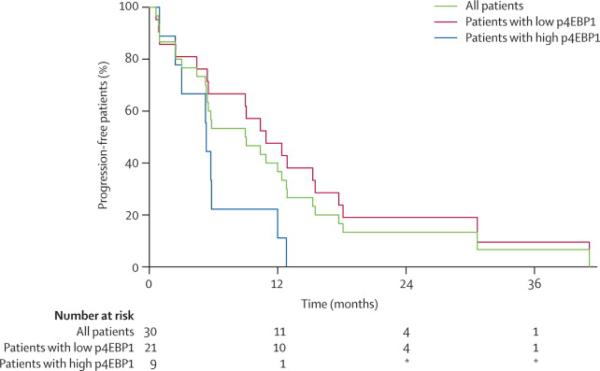
In an exploratory analysis, immunohistochemistry was performed on the pretreatment biopsies of 33 patients who had adequate biopsy specimens available. Specimens were not available on all patients as many patients in this cooperative group study had core needle biopsies performed to confirm recurrent disease resulting insufficient tissue to perform immunohistochemistry. Expression of established markers of T-cell subsets, including TH1, TH2, Treg and cytotoxic T-cells (Tbet, GATA3, FoxP3 and CD8), monocytes and dendritic cells (CD68 and CD11c), as well as the relevant proteins involved in mTOR signaling pathway (raptor, rictor, p4EBP1, pAKT and p70S6K) were tested and correlated with response to therapy and TTP. None of these proteins detected by immunohistochemistry correlated with response to therapy. The pretreatment expression of p4EBP1, however, was significantly associated with TTP (Figure 2). The median TTP for patients with low p4EBP1 expression was 10 months compared to 5.2 months for patients with high p4EBP1 expression (p=0.02). The small sample size, limited power and multiple testing done in this subset analysis require that this finding be replicated in larger independent datasets.
Figure 2. Clinical outcome of Mantle Cell Lymphoma Patients by Immunohistochemical staining for p4EBP1.
Time to progression after treatment with temsirolimus and rituximab in mantle cell lymphoma patients with high or low expression of p4EBP1 (p=0·02).
Discussion
Our findings indicate that mTOR inhibitors in combination with rituximab could have a role in the treatment of patients with relapsed and refractory MCL. Because there is no clear treatment of choice for patients with relapsed MCL and traditional lymphoma salvage therapies commonly provide limited clinical benefit, novel combinations are clearly needed for these patients. mTOR inhibitors have shown activity in patients with relapsed lymphomas including MCL 10,11,19,29,30. In a previous phase II trial of single-agent temsirolimus for patients with relapsed MCL, an ORR of 38% was seen when a dose of 250mg weekly was used confirming that targeting the PI3K pathway at the level of mTOR can produce tumor responses 10. Hematologic toxicity at this dose was significant and a subsequent trial using temsirolimus at a dose of 25mg weekly was performed. A similar response rate of 41% was seen using this lower dose 11. In both studies, however, complete responses were rare (CR rate 3-4%). A subsequent randomized phase III trial comparing temsirolimus at two dose levels to investigator’s choice for relapsed MCL showed a lower ORR for temsirolimus but demonstrated a significantly improved PFS and ORR when compared to the investigator’s choice of therapy 19.
While single agent temsirolimus clearly has activity in relapsed MCL, combination approaches with other agents may significantly improve the response rate. Rituximab has been used as a single agent to treat relapsed and refractory MCL and reported response rates range from 27-37% 20–22. The addition of further doses of rituximab beyond the standard four doses does not seem to improve the response rate 21. In this study of temsirolimus in combination with rituximab, the ORR was 59.4% with 18·8% complete responses and 40.6% partial responses. The median TTP in this study was 9·7 months, with a median TTP in the rituximab-sensitive patients of 10·9 months and 5·4 months in the rituximab-refractory patients. Overall, the response rate for the combination was promising and the complete response rate was higher compared to that seen with either agent alone. The significantly higher ORR seen in this study when compared to the phase III trial of temsirolimus alone may not only be due to the addition of rituximab but may also be due to the fact that patients in the randomized phase III trial had received a greater median number of prior therapies 19. However, in comparison to a TTP of 3·4-7 months seen in previous studies of temsirolimus alone 10,11,19, the TTP of 9·7 months for all patients and 10.9 months for those with rituximab-sensitive disease observed in this study suggests an additive effect when rituximab is added to temsirolimus. Based on the encouraging results seen in this study when standard doses of rituximab were added to temsirolimus, it may be appropriate to explore a dose-dense schedule of rituximab in combination with temsirolimus in future studies.
Consistent with previous studies showing that temsirolimus can cause thrombocytopenia and neutropenia 10,11,19,29, hematologic toxicity was the most common side effect seen in the present trial. The frequency of grade 3 and 4 hematologic toxicity when rituximab was added to temsirolimus did not appear to be different to that seen in the previous studies of temsirolimus alone. Aside from hematologic toxicity, the other more frequently seen grade 3 and 4 toxicities in this study included elevated serum cholesterol and triglycerides, hyperglycemia, fatigue and dyspnea. The frequency of these toxicities in this study also appeared similar to what was observed in previous studies of single agent temsirolimus suggesting that rituximab can be safely added to temsirolimus without a significant increase in toxicity.
To identify prognostic markers of clinical outcome, we performed immunohistochemistry on pretreatment tumor biopsies to measure the expression of proteins associated with mTOR signaling or expressed on intratumoral cells in the tumor microenvironment. Increased expression of p4EBP1, one of the targets of mTOR, was associated with a significantly shorter TTP suggesting that the expression of p4EBP1 may be useful in identifying patients more likely to benefit from temsirolimus-containing therapy. The importance of 4EBP1 as a potential prognostic marker for patients treated with a temsirolimus-containing regimen is supported by the finding in a phase II trial in multiple myeloma that clinical efficacy of the drug was associated with maximal reduction in phosphorylated 4EBP1 in peripheral blood mononuclear cells 18. In addition, in previous in vitro experiments with lymphoma cells, we saw a substantial reduction in pS6 but not p4EBP1 when the cells were treated with rapamycin suggesting that rapamycin and rapalogues such as temsirolimus may not significantly affect 4EBP1 31. This suggests that the high expression of p4EBP1 in the tumor cells prior to treatment may not be adequately or durably suppressed by temsirolimus and therefore predicts a greater likelihood of progression after therapy. This analysis however was done on a small subset of the patients included in this study and therefore needs to be confirmed in other studies.
We conclude that the combination of temsirolimus and rituximab has substantial antitumor activity in patients with relapsed MCL with an ORR, complete response rate and TTP that are superior that that seen in relapsed MCL patients treated with either temsirolimus or rituximab alone. Furthermore, the addition of rituximab to temsirolimus does not result in a significant increase in toxicity. The expression of p4EBP1 in pretreatment biopsy specimens correlated with the TTP and could potentially be used to identify patients who are more likely to benefit from this combination. To clearly determine the role of this effective and well-tolerated combination in the management of patients with relapsed MCL, randomized studies comparing temsirolimus and rituximab to other salvage therapies are planned.
Acknowledgments
Supported in part by grants CA25224 and CA92104 from the National Institutes of Health and the Predolin Foundation. Presented in part at the 51st Annual Meeting of the American Society of Hematology.
Funding: National Institutes of Health (grants CA25224 and CA92104) and the Predolin Foundation.
Footnotes
ClinicalTrials.gov Identifier: NCT00109967
Conflicts of Interest TEW is listed as one of the inventors on a patent application assigned to Mayo Foundation that claims methods for treating mantle cell lymphoma using temsirolimus. Mayo Foundation licensed the patent application and through the Mayo Foundation license revenue sharing policy TEW received a single remuneration from an upfront consideration payment made to Mayo from Wyeth in 2004 and a final milestone payment in 2009. There is no further remuneration. This remuneration was not tied to accrual in the clinical trial reported herein. The other authors declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Argatoff LH, Connors JM, Klasa RJ, et al. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood. 1997;89:2067–2078. [PubMed] [Google Scholar]
- 2.Velders GA, Kluin-Nelemans JC, De Boer CJ, et al. Mantle-cell lymphoma: a population-based clinical study. J Clin Oncol. 1996;14:1269–1274. doi: 10.1200/JCO.1996.14.4.1269. [DOI] [PubMed] [Google Scholar]
- 3.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 4.Zhou Y, Wang H, Fang W, et al. Incidence trends of mantle cell lymphoma in the United States between 1992 and 2004. Cancer. 2008;113:791–798. doi: 10.1002/cncr.23608. [DOI] [PubMed] [Google Scholar]
- 5.Geisler C, Kolstad A, Laurell A, et al. Mantle cell lymphoma - does primary intensive immunochemotherapy improve overall survival for younger patients? Leuk Lymphoma. 2009;50:1249–1256. doi: 10.1080/10428190903040030. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27:511–518. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- 7.Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150:200–208. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich S, Tielesch B, Rieger M, et al. Patterns and outcome of relapse after autologous stem cell transplantation for mantle cell lymphoma. Cancer. 2010 Nov 29; doi: 10.1002/cncr.25756. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Witzig TE, Geyer SM, Ghobrial I, et al. Phase II trial of single-agent temsirolimus (CCI-779) for relapsed mantle cell lymphoma. J Clin Oncol. 2005;23:5347–5356. doi: 10.1200/JCO.2005.13.466. [DOI] [PubMed] [Google Scholar]
- 11.Ansell SM, Inwards DJ, Rowland KM, Jr, et al. Low-dose, single-agent temsirolimus for relapsed mantle cell lymphoma: a phase 2 trial in the North Central Cancer Treatment Group. Cancer. 2008;113:508–514. doi: 10.1002/cncr.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, Bjornsti MA, Houghton PJ. Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther. 2003;2:222–232. doi: 10.4161/cbt.2.3.360. [DOI] [PubMed] [Google Scholar]
- 13.Dudkin L, Dilling MB, Cheshire PJ, et al. Biochemical correlates of mTOR inhibition by the rapamycin ester CCI-779 and tumor growth inhibition. Clin Cancer Res. 2001;7:1758–1764. [PubMed] [Google Scholar]
- 14.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 15.Gera JF, Mellinghoff IK, Shi Y, et al. AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J Biol Chem. 2004;279:2737–2746. doi: 10.1074/jbc.M309999200. [DOI] [PubMed] [Google Scholar]
- 16.Noh WC, Mondesire WH, Peng J, et al. Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res. 2004;10:1013–1023. doi: 10.1158/1078-0432.ccr-03-0043. [DOI] [PubMed] [Google Scholar]
- 17.Hidalgo M, Rowinsky EK. The rapamycin-sensitive signal transduction pathway as a target for cancer therapy. Oncogene. 2000;19:6680–6686. doi: 10.1038/sj.onc.1204091. [DOI] [PubMed] [Google Scholar]
- 18.Farag SS, Zhang S, Jansak BS, et al. Phase II trial of temsirolimus in patients with relapsed or refractory multiple myeloma. Leuk Res. 2009;33:1475–1480. doi: 10.1016/j.leukres.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 20.Foran JM, Rohatiner AZ, Cunningham D, et al. European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. J Clin Oncol. 2000;18:317–324. doi: 10.1200/JCO.2000.18.2.317. [DOI] [PubMed] [Google Scholar]
- 21.Ghielmini M, Schmitz SF, Cogliatti S, et al. Effect of single-agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research (SAKK) J Clin Oncol. 2005;23:705–711. doi: 10.1200/JCO.2005.04.164. [DOI] [PubMed] [Google Scholar]
- 22.Coiffier B, Haioun C, Ketterer N, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 23.Shan D, Ledbetter JA, Press OW. Signaling events involved in anti-CD20-induced apoptosis of malignant human B cells. Cancer Immunol Immunother. 2000;48:673–683. doi: 10.1007/s002620050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golay J, Zaffaroni L, Vaccari T, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95:3900–3908. [PubMed] [Google Scholar]
- 25.Wurflein D, Dechant M, Stockmeyer B, et al. Evaluating antibodies for their capacity to induce cell-mediated lysis of malignant B cells. Cancer Res. 1998;58:3051–3058. [PubMed] [Google Scholar]
- 26.Jazirehi AR, Vega MI, Chatterjee D, et al. Inhibition of the Raf-MEK1/2-ERK1/2 signaling pathway, Bcl-xL down-regulation, and chemosensitization of non-Hodgkin’s lymphoma B cells by Rituximab. Cancer Res. 2004;64:7117–7126. doi: 10.1158/0008-5472.CAN-03-3500. [DOI] [PubMed] [Google Scholar]
- 27.Kanelli S, Ansell SM, Habermann TM, et al. Rituximab toxicity in patients with peripheral blood malignant B-cell lymphocytosis. Leuk Lymphoma. 2001;42:1329–1337. doi: 10.3109/10428190109097760. [DOI] [PubMed] [Google Scholar]
- 28.Cheson B, Horning S, Coiffier B, et al. Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphoma. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM, van Besien K, Karrison T, et al. Temsirolimus has activity in non-mantle cell non-Hodgkin’s lymphoma subtypes: The University of Chicago phase II consortium. J Clin Oncol. 2010;28:4740–4746. doi: 10.1200/JCO.2010.29.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witzig TE, Reeder CB, Laplant BR, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia. 2011;25:341–347. doi: 10.1038/leu.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta M, Ansell SM, Novak AJ, et al. Inhibition of histone deacetylase overcomes rapamycin-mediated resistance in diffuse large B-cell lymphoma by inhibiting Akt signaling through mTORC2. Blood. 2009;114:2926–2935. doi: 10.1182/blood-2009-05-220889. [DOI] [PMC free article] [PubMed] [Google Scholar]