Abstract
Background. Annual mass treatment with albendazole and ivermectin is the mainstay of current strategies to interrupt transmission of Wuchereria bancrofti in Africa. More-effective microfilarial suppression could potentially reduce the time necessary to interrupt transmission, easing the economic burden of mass treatment programs in countries with limited resources.
Methods. To determine the effect of increased dose and frequency of albendazole-ivermectin treatment on microfilarial clearance, 51 W. bancrofti microfilaremic residents of an area of W. bancrofti endemicity in Mali were randomized to receive 2 doses of annual, standard-dose albendazole-ivermectin therapy (400 mg and 150 µg/kg; n = 26) or 4 doses of twice-yearly, increased-dose albendazole-ivermectin therapy (800 mg and 400 µg/kg; n = 25).
Results. Although microfilarial levels decreased significantly after therapy in both groups, levels were significantly lower in the high-dose, twice-yearly group at 12, 18, and 24 months. Furthermore, there was complete clearance of detectable microfilariae at 12 months in the 19 patients in the twice-yearly therapy group with data available at 12 months, compared with 9 of 21 patients in the annual therapy group (P<.001 , by Fisher's exact test). This difference between the 2 groups was sustained at 18 and 24 months, with no detectable microfilariae in the patients receiving twice-yearly treatment. Worm nests detectable by ultrasonography and W. bancrofti circulating antigen levels, as measured by enzyme-linked immunosorbent assay, were decreased to the same degree in both groups at 24 months, compared with baseline.
Conclusions. These findings suggest that increasing the dosage and frequency of albendazole-ivermectin treatment enhances suppression of microfilariae but that this effect may not be attributable to improved adulticidal activity.
More than 120 million people in approximately 80 countries are infected with the mosquito-transmitted filarial nematodes, Wuchereria bancrofti or Brugia species. Furthermore, it is estimated that >40 million people have chronic, disabling disease manifestations, including lymphedema, hydrocele, and elephantiasis. Despite successful elimination programs in some countries [1], transmission of lymphatic filariasis remains a problem in many regions of the world. This is particularly true in resource-poor areas of Africa, including Mali, where sustaining the World Health Organization- approved mass treatment regimen of single-dose albendazole (400 mg) and ivermectin (150–200 µg/kg) annually for 4–6 years poses a significant financial burden [2]. Because microfilarial levels in the blood are directly responsible for continued transmission by the mosquito vectors, a more effective suppressive regimen could accelerate the interruption of transmission and shorten the overall duration of the mass treatment program.
Currently available antifilarial drugs with activity against W. bancrofti , the agent of lymphatic filariasis in Africa, include ivermectin, diethylcarbamazine, albendazole, and doxycycline. Of these, ivermectin and diethylcarbamazine have each been shown to be effective in clearing microfilariae from the circulation after single-dose therapy [3], although diethylcarbamazine is contraindicated in most of Africa because of the risk of severe (and possibly life-threatening) posttreatment reactions in patients with concomitant onchocerciasis. The demonstration that the addition of albendazole (400 mg) to annual, singledose therapy with ivermectin (200 µg/kg) prolonged the suppression of microfilaremia without any noticeable increase in adverse effects or posttreatment reactions provided the basis for the current World Health Organization recommendation of annual single-dose ivermectin-albendazole for lymphatic filariasis elimination in Africa [4].
Although early studies in French Polynesia suggested that increased dosage (400 µg/kg) or twice-yearly administration of ivermectin alone was more effective than standard annual therapy in reducing W. bancrofti microfilaremia [5, 6], the effects of these parameters in the setting of combination therapy are unknown. The current study was designed to assess whether twice-yearly administration of albendazole-ivermectin at an increased dosage (800 mg and 400 µg/kg, respectively) is more effective in suppressing microfilarial levels than the current World Health Organization-approved regimen. The effects of this regimen on adult worm burden, as assessed by serum levels of circulating filarial antigen and ultrasonography, were also explored.
Study Population And Methods
Study population. The study was conducted in the villages of Tienekebougou and Bougoudiana, ∼105 km northwest of Bamako, Mali. Prior studies in these villages had demonstrated a high prevalence of W. bancrofti microfilaremia. This region is outside the area of endemicity for onchocerciasis. Patients had received a single dose of albendazole and ivermectin as part of the National Program to Eliminate Lymphatic Filariasis 1 year before the start of the study. The study (NCT00339417) was approved by the ethical review committees of the Faculty of Medicine, Pharmacy, and Dentistry at the University of Bamako (Bamako, Mali) and of the National Institutes of Allergy and Infectious Diseases (Bethesda, Maryland). Community permission for the study was obtained from village elders, and individual oral or written informed consent was obtained from all participants in French or Bambara, the local language.
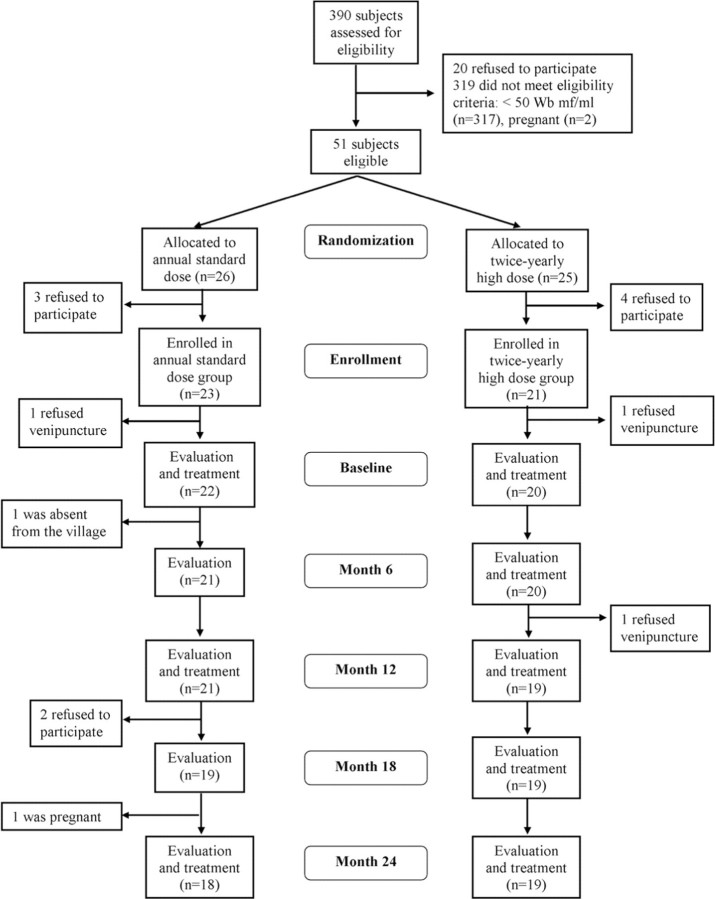
Nonpregnant volunteers (n = 390) of both sexes, 14–65 years of age, were screened with a brief medical history and physical examination and venipuncture between 10 pm and 2 am for detection of W. bancrofti microfilariae by calibrated thick smear of 60 µL of blood, assessment of W. bancrofti circulating antigen levels by enzyme-linked immunosorbent assay (TropBio), and hemoglobin level (Figure 1). Patients were excluded from participating in the drug study for the following reasons: a W. bancrofti microfilarial level of <50 microfiliae/ mL, pregnancy, hemoglobin level <9 g/dL, heavy alcohol use (17 beers or other alcohol-containing drinks per week), temperature >37.5°C, serious medical illness, history of allergy to benzimidazoles or ivermectin, or use of albendazole or ivermectin within the past 6 months.
Figure 1.
Flowchart of study participants.
Study design. Eligible participants were randomly assigned to receive annual treatment with standard-dose albendazole (400 mg) and ivermectin (150 µg/kg) or twice-yearly treatment with high-dose albendazole (800 mg) and ivermectin (400 µg/kg) for 24 months (Figure 1). A brief clinical assessment, including vital signs and pregnancy testing (in women of childbearing age), was performed at each study time point. Study medication was administered under the direct observation of a physician who remained in the study village for 1 week after treatment to assess adverse events. Venipuncture was performed between 10 PM and 2 AM at baseline and every 6 months before treatment for quantification of W. bancrofti microfilarial levels (by Nuclepore filtration of 1 mL of blood) and circulating antigen levels, as well as complete blood cell count. All laboratory assessments were performed by trained personnel unaware of the group assignments. Ultrasonography was performed for male and female volunteers at baseline to identify adult worms in the scrotal and breast lymphatics (filarial dance sign) [7]. Additional ultrasonography was performed at 12 and 24 months. An experienced radiologist, blinded to the study group assignments, performed all ultrasound studies.
Statistical analysis. The primary end point of the study was the difference in W. bancrofti levels between the 2 groups at 12 months. This was evaluated by examining the W. bancrofti clearance rates at 12 months using 2-sided Fisher's exact test and matching confidence intervals [8] and by assessing the difference in the percentage of baseline W. bancrofti microfilarial levels at 12 months by Wilcoxon-Mann-Whitney U test. The Fisher's exact test was performed for all patients with complete data at 12, 18, and 24 months and, in addition, for all randomized patients at 12 months using a conservative pooled imputation sensitivity analysis, where the missing patients in each group are imputed with proportions closest to the total proportion of responders, regardless of treatment group [9]. On the basis of the published data comparing single and multidose regimens for the treatment of lymphatic filariasis [10] and a predicted 10% attrition rate, a sample size of 25 per group was estimated to have 90% power to detect a difference in the 2 groups by Fisher's exact test with a 2-sided α level of .05. One patient in the annual group and 2 patients in the twice-yearly group had no detectable W. bancrofti microfilariae at the baseline visit despite a microfilarial level of >50 microfilariae/mL detected at screening. Those 3 patients have been excluded from the difference in the percentage of baseline W. bancrofti analysis. There were similar dropout rates for both treatment groups (Figure 1), and patients missing responses were assumed to be missing at random and have been excluded from all analyses except the sensitivity analysis, in which conservative imputations were performed. Changes over time in binary variables (eg, presence or absence of eosinophilia) were analyzed using the exact McNemar's test. Calculations were performed in either Prism software, version 5.0c (GraphPad), or R, version 2.10.1 (R Foundation for Statistical Computing), using the exact 2×2 software package, version 1.0.0.
Results
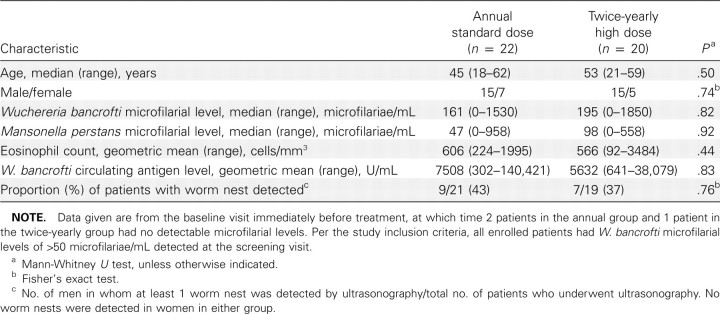
Study population. Patients were recruited and screened in April 2007 and began treatment in July 2007. On the basis of the screening results, 51 eligible patients were identified and randomized to receive standard, annual therapy (n = 26) or high-dose, twice-yearly therapy (n = 25). Seven patients (3 in the annual group and 4 in the twice-yearly group) declined to participate before enrollment, and 2 (1 in each group) signed consent but refused to undergo venipuncture at the baseline visit, leaving 42 patients (22 in the annual group and 20 in the twice-yearly group) in the treatment portion of the study. There were no significant differences between the 2 groups at baseline with respect to median age, sex distribution, eosinophil count, W. bancrofti microfilarial or circulating antigen levels, or the prevalence of worm nests detected by ultrasonography (Table 1). Clinical disease associated with lymphatic filariasis was uncommon in both groups, with hydrocele found in only 3 patients (2 in the annual group and 1 in the twice-yearly group) and a history of lymphedema in only 1 subject (in the annual group). Sixteen patients (8 in each group) reported a history of lymphangitis occurring >1 month before the study. As has been reported in previous studies in this region of Mali, most W. bancrofti-infected patients (38 [90%] of 42) were coinfected with Mansonella perstans.
Table 1.
Baseline Characteristics of the Study Population
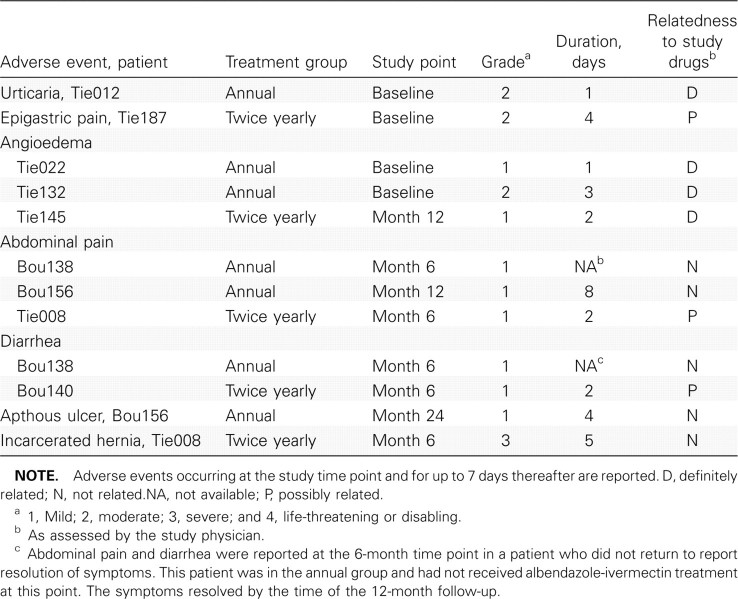
Adverse events. A total of 12 adverse events were reported by 9 study patients (17%). Mild-to-moderate adverse events were reported in both treatment groups and included gastrointestinal symptoms, urticaria, and localized angioedema, all of which are consistent with known effects of anthelmintic (albendazole-ivermectin) therapy (Table 2). None of the patients with angioedema demonstrated respiratory or laryngeal symptoms. The only serious adverse event, an incarcerated hernia requiring surgical intervention, was categorized as unrelated to the study procedures.
Table 2.
Adverse Events in the 2 Study Groups
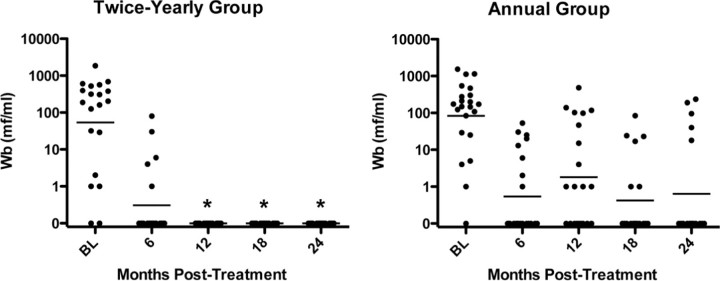
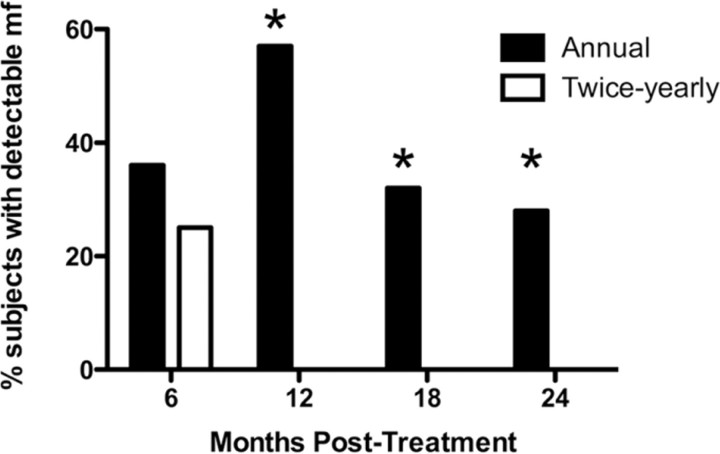
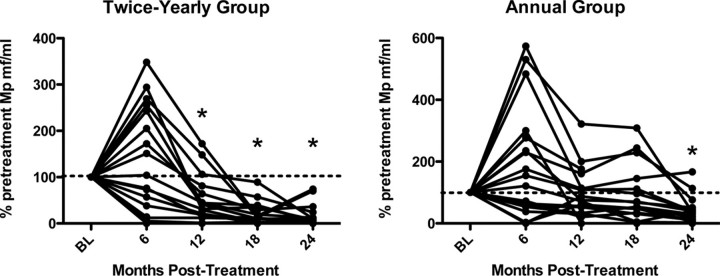
Efficacy. As expected, microfilarial levels decreased significantly in both groups after albendazole-ivermectin treatment (Figure 2). Nevertheless, the percentages of pretreatment W. bancrofti microfilarial levels were significantly decreased in the high-dose, semiannual group at 12, 18, and 24 months compared with the group receiving annual, standard-dose treatment (P<.001 , P<.019 , and P<.044, respectively, by Wilcoxon-Mann-Whitney U test). More importantly, complete W. bancrofti microfilarial clearance was significantly more common in the high-dose biannual group, with no patients having results positive for circulating microfilariae at 12, 18, or 24 months, compared with 12 of 21, 6 of 19, and 5 of 18 patients with positive results in the annual treatment group (P<.001 , P = .020, and P = .020, respectively, by 2-sided Fisher's exact test) (Figure 3). Because all patients in the twice-yearly group cleared at the 3 time points, the odds ratio estimates and upper confidence limits are infinite, whereas the lower 95% confidence limits are 5.05 (12 months), 1.52 (18 months), and 1.28 (24 months). Even the conservative sensitivity analysis at 12 months showed highly significant results (P = .002, by 2-sided Fisher's exact after imputing missing responses in each group in proportion to the overall response rate).
Figure 2.
Reduction of microfilaremia after high-dose, twice-yearly albendazole and ivermectin (A), compared with standard-dose annual treatment (B). Each symbol represents the value for an individual patient. The horizontal line indicates the geometric mean for the group. *P<.05, annual versus twice-yearly treatment (Wilcoxon-Mann-Whitney U test).
Figure 3.
Clearance of microfilaremia after high-dose, twice-yearly albendazole and ivermectin, compared with standard-dose annual treatment. The bars represent the percentage of patients with detectable Wuchereria bancrofti microfilariae at baseline and 6, 12, 18, and 24 months after treatment. Annual, standard-dose treatment is indicated by the black bars and high-dose, twice-yearly treatment is indicated by the white bars. *P<.05, annual versus twice-yearly treatment (Fisher's exact test).
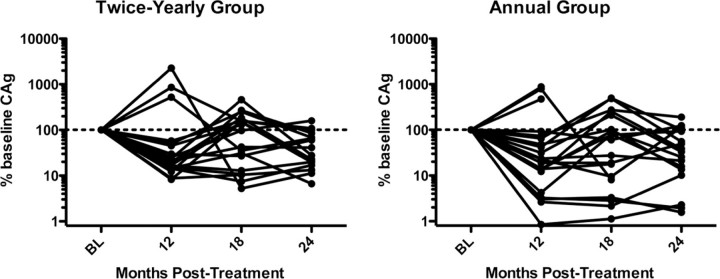
Although no patients had test results negative for W. bancrofti circulating antigen by 24 months, circulating antigen levels decreased in both groups over time (Figure 4). Median percentages of pretreatment levels at 12, 18, and 24 months were 21%, 97%, and 28% in the twice-yearly treatment group, compared with 23%, 60%, and 31% in the annual treatment group (P values were nonsignificant for differences between the groups all time points). The number of patients with worm nests detectable by ultrasonography also decreased significantly from baseline to 24 months in both groups (P = .016 for the annual therapy group and P = .031 for the twice-yearly therapy group, by exact McNemar's test) over time, with only 1 patient with positive results in each group by 24 months.
Figure 4.
Reduction of circulating antigen levels (CAg) after high-dose, twice-yearly albendazole and ivermectin (A), compared with standard-dose, annual treatment (B). Each solid line represents the percentage of pretreatment microfilarial level for an individual patient over time. The dashed line indicates the 100% pretreatment level. *P<.05, annual versus twice-yearly treatment (Wilcoxon-Mann-Whitney U test). BL, baseline.
Effect of treatment on clinical parameters. Progression of clinical disease was comparable in the 2 groups, with 2 previously asymptomatic patients (1 in each group) reporting an episode of lymphangitis and lymphedema at the 6-month study time point and no new cases of hydrocele, chyluria, or elephantiasis. Eosinophilia (absolute eosinophil count >500 cells/mm3) was present at baseline in 25 (60%) of 42 patients with a median baseline eosinophil count of 584 cells/mm3 (range, 92–3484 cells/mm3). Both the number of patients with eosinophilia and the degree of eosinophilia decreased after treatment, with the most significant change occurring at 6 months after baseline, at which time 11 (29%) of 38 patients had peripheral eosinophilia (P = .027 for both groups combined, by exact McNemar's test) with a median eosinophil count of 286 cells/mm3 (range, 0–1133 cells/mm3) (P<.001 , by Wilcoxon signed rank test). Hemoglobin levels did not change in either group over time (data not shown).
Discussion
Although mass treatment with albendazole and ivermectin has proven to be a successful strategy for the suppression of W. bancrofti microfilarial counts in a variety of settings, elimination of transmission has been reported exclusively in countries where diethylcarbamazine has been administered for many years [11–13]. Early estimates of the length of time required to eliminate transmission of W. bancrofti infection were based on the assumption that single-dose antifilarial therapy administered annually for the 4-to 6-year mean reproductive life span of the parasite would be sufficient to permanently interrupt transmission [14]. More recently, computer simulations have demonstrated that the number of years needed to eliminate transmission depends not only on the life span of the parasite but also on the vector species, treatment coverage, drug efficacy, and baseline endemicity [15, 16]. The influence of at least 1 of these parameters, baseline endemicity, on the reduction of transmission has been validated in the field [17]. Drug efficacy is likely to play at least as important a role.
Although a number of studies have suggested that diethylcarbamazine-containing regimens are more effective than ivermectin and albendazole, the use of diethylcarbamazine to enhance efficacy of mass treatment is contraindicated in most of Africa, including Mali, because of the presence of onchocerciasis. Thus, other methods of enhancing drug efficacy are required in these regions. In the present study, we examined the effects of increased dose and duration of albendazole and ivermectin treatment on the suppression of microfilaremia in an area of lymphatic filariasis endemicity in Mali. Not only did the higher, more frequent dosing lead to reduced levels of microfilaremia at 12, 18, and 24 months, but also microfilariae were undetectable in the peripheral blood specimens of all patients in the high-dose, twice-yearly group at these time points. At the community level, enhanced clearance of blood microfilariae could accelerate the interruption of transmission and potentially shorten the duration of mass treatment. Interestingly, no differences in circulating antigen levels or the number of worm nests were seen between the 2 groups, suggesting that the increased efficacy was not due to killing of adult worms.
Ivermectin and albendazole are broad-spectrum anthelmintics that have been shown to decrease the prevalence and intensity of intestinal helminth infections in the setting of mass distribution programs for lymphatic filariasis, providing additional benefit to treated communities [18]. In contrast, the effect of standard-dose annual ivermectin and albendazole on
M. perstans infection has been unimpressive [19]. In the present study, median M. perstans microfilarial levels were unchanged from baseline in the standard annual dose group but decreased significantly in the high-dose, twice-yearly group at 12 and 18 months (Figure 5). Both groups demonstrated a significant decrease in median M. perstans microfilarial levels at 24 months, although the change was less remarkable in the standard-dose annual therapy group. Clearance of M. perstans microfilariae was observed at 24 months in only 1 of 16 M. perstans-positive patients in the standard-dose annual therapy group, compared with 4 of 17 in the high-dose, twice-yearly therapy group (P values were nonsignificant by Fisher's exact test).
Figure 5.
Reduction of Mansonella perstans microfilarial levels after high-dose, twice-yearly albendazole and ivermectin (A), compared with standard-dose, annual treatment (B). Each solid line represents the percentage of pretreatment microfilarial levels for an individual patient over time. The dashed line indicates the 100% pretreatment level. *P<.05, compared with baseline (Wilcoxon-Mann-Whitney U test).
The major limitation of the present study was the lack of sufficient patients to enroll in the study groups receiving increased-dose annual treatment or standard-dose, twice-yearly treatment, precluding independent analysis of the effects of dosage and frequency of dosing on reduction of microfilaremia. This distinction is particularly important from an economic standpoint, because albendazole and ivermectin are donated by Merck and GlaxoSmithKline, respectively, whereas the cost of drug distribution is borne by the lymphatic filariasis elimination programs themselves. Although the similarity between the microfilarial levels in the standard-dose annual therapy group and the high-dose, twice-yearly therapy group at 6 months suggests that frequency of treatment may be the more important factor, additional studies are needed to resolve this question.
In summary, increased-dose, twice-yearly therapy with albendazole and ivermectin was more effective in reducing W. bancrofti microfilarial levels than was standard-dose annual therapy in a region of high endemicity in Mali, West Africa, suggesting that more frequent and/or higher-dose therapy might accelerate the interruption of transmission. Such a strategy could be particularly useful in regions where implementation of the control program has been delayed by economic or political factors. Additional studies are clearly necessary to confirm the findings in areas of differing endemicity and to determine the relative contributions of increased dosage and increased frequency to the observed effect.
Acknowledgments
We thank the Mectizan Donation Program, for providing the albendazole and ivermectin used in the study, and Dr. Massitan, manager of the Malian National Program for the Elimination of Lymphatic Filariasis, and the local health care staff of Kolokani, for their cooperation and facilitation of the field studies.
Financial support. Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and the Task Force for Global Health (to A.D.K.).
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Meeting of the International Task Force for Disease Eradication-29 October 2008. Wkly Epidemiol Rec. 2009;84:89–94. [PubMed] [Google Scholar]
- 2.Goldman AS, Guisinger VH, Aikins M, et al. National mass drug administration costs for lymphatic filariasis elimination. PLoS Negl Trop Dis. 2007;1(1):e67. doi: 10.1371/journal.pntd.0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Addiss DG, Beach MJ, Streit TG, et al. Randomised placebo-controlled trial of ivermectin and albendazole alone and in combination for Wuchereria bacrofti microfilaraemia in Haitian children. Lancet. 1997;350:480–484. doi: 10.1016/S0140-6736(97)02231-9. [DOI] [PubMed] [Google Scholar]
- 4.Horton J, Witt C, Ottesen EA, et al. An analysis of the safety of the single dose, two drug regimens used in programmes to eliminate lymphatic filariasis. Parasitology. 2000;121((Suppl)):S147–S160. doi: 10.1017/s0031182000007423. [DOI] [PubMed] [Google Scholar]
- 5.Cartel JL, Spiegel A, Nguyen Ngnoc L, et al. Compared efficacy or repeated annual and semi-annual doses of ivermectin and diethylcarbamazine for prevention of Wuchereria bancroftifilariasis in French Polynesia. FInal evaluation. Trop Med Parasitol. 1992;43:91–94. [PubMed] [Google Scholar]
- 6.Moulia-Pelat JP, Glaziou P, Nguyen LN, et al. Single doses of ivermectin 400 micrograms/kg: the most effective dosage in bancroftian filariasis. Southeast Asian J Trop Med Public Health. 1995;26:124–127. [PubMed] [Google Scholar]
- 7.Mand S, Marfo-Debrekyei Y, Dittrich M, et al. Animated documentation of the filaria dance sign (FDS) in bancroftian filariasis. Filaria J. 2003;2(1):3. doi: 10.1186/1475-2883-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fay MP. Confidence intervals that match Fisher's exact or Blaker's exact tests. Biostatistics. 2010;11:373–374. doi: 10.1093/biostatistics/kxp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proschan MA, McMahon RP, Shih JH, et al. Sensitivity analysis using an imputation method for missing binary data in clinical trials. J Stat Plan Inference. 2001;96:155–165. [Google Scholar]
- 10.El-Setouhy M, Ramzy RM, Ahmed ES, et al. A randomized clinical trial comparing single and multi-dose combination therapy with diethylcarbamazine and albendazole for treatment of bancroftian filariasis. Am J Trop Med Hyg. 2004;70:191–196. [PubMed] [Google Scholar]
- 11.Cheun H, Lee J, Cho S, et al. Elimination of lymphatic filariasis in the Republic of Korea: an epidemiological survey of formerly endemic areas, 2002–2006. Trop Med Int Health. 2009;14:445–449. doi: 10.1111/j.1365-3156.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- 12.Itoh M, Wu W, Sun D, et al. Confirmation of elimination of lymphatic filariasis by an IgG4 enzyme-linked immunosorbent assay with urine samples in Yongjia, Zhejiang Province and Gaoan, Jiangxi Province, People's Republic of China. Am J Trop Med Hyg. 2007;77:330–333. [PubMed] [Google Scholar]
- 13.Ramzy RM, El Setouhy M, Helmy H, et al. Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet. 2006;367:992–999. doi: 10.1016/S0140-6736(06)68426-2. [DOI] [PubMed] [Google Scholar]
- 14.Ottesen EA, Duke BO, Karam M, et al. Strategies and tools for the control/elimination of lymphatic filariasis. Bull World Health Organ. 1997;75:491–503. [PMC free article] [PubMed] [Google Scholar]
- 15.Stolk WA, De Vlas SJ, Borsboom GJJM, et al. LYMFASIM, a simulation model for predicting the impact of lymphatic filariasis control: quantification for African villages. Parasitology. 2008;135:1583–1598. doi: 10.1017/S0031182008000437. [DOI] [PubMed] [Google Scholar]
- 16.Michael E, Malecela-Lazaro MN, Simonsen PE, et al. Mathematic modelling and the control of lymphatic filariasis. Lancet Infect Dis. 2004;4:223–234. doi: 10.1016/S1473-3099(04)00973-9. [DOI] [PubMed] [Google Scholar]
- 17.Bockarie MJ, Tisch DJ, Kastens W, et al. Mass treatment to eliminate filariasis in Papua New Guinea. N Engl J Med. 2002;347:1841–1848. doi: 10.1056/NEJMoa021309. [DOI] [PubMed] [Google Scholar]
- 18.Ottesen EA, Hooper PJ, Bradley M, et al. The global programme to eliminate lymphatic filariasis: health impact after 8 years. PLoS Negl Trop Dis. 2008;2:e317. doi: 10.1371/journal.pntd.0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asio SM, Simonsen PE, Onapa AW. Mansonella perstans: safety and efficacy of ivermectin alone, albendazole alone and the two drugs in combination. Ann Trop Med Parasitol. 2009;103:31–37. doi: 10.1179/136485909X384929. [DOI] [PubMed] [Google Scholar]