Penicilliosis made up 4.4% of all AIDS admissions and increased approximately 30% in rainy months. In-hospital mortality was 20% and associated with injection drug use, shorter illness, absence of fever or skin lesions, respiratory difficulty, and degree of thrombocytopenia.
Abstract
Background. Penicillium marneffei is an important human immunodeficiency virus (HIV)–associated opportunistic pathogen in Southeast Asia. The epidemiology and the predictors of penicilliosis outcome are poorly understood.
Methods. We performed a retrospective study of culture-confirmed incident penicilliosis admissions during 1996–2009 at the Hospital for Tropical Diseases in Ho Chi Minh City, Viet Nam. Seasonality of penicilliosis was assessed using cosinor models. Logistic regression was used to assess predictors of death or worsening disease based on 10 predefined covariates, and Cox regression was performed to model time-to-antifungal initiation.
Results. A total of 795 patients were identified; hospital charts were obtainable for 513 patients (65%). Cases increased exponentially and peaked in 2007 (156 cases), mirroring the trends in AIDS admissions during the study period. A highly significant seasonality for penicilliosis (P < .001) but not for cryptococcosis (P = .63) or AIDS admissions (P = .83) was observed, with a 27% (95% confidence interval, 14%–41%) increase in incidence during rainy months. All patients were HIV infected; the median CD4 cell count (62 patients) was 7 cells/μL (interquartile range, 4–24 cells/μL). Hospital outcome was an improvement in 347 (68%), death in 101 (20%), worsening in 42 (8%), and nonassessable in 23 (5%) cases. Injection drug use, shorter history, absence of fever or skin lesions, elevated respiratory rates, higher lymphocyte count, and lower platelet count independently predicted poor outcome in both complete-case and multiple-imputation analyses. Time-to-treatment initiation was shorter for patients with skin lesions (hazard ratio, 3.78; 95% confidence interval, 2.96–4.84; P < .001).
Conclusions. Penicilliosis incidence correlates with the HIV/AIDS epidemic in Viet nam. The number of cases increases during rainy months. Injection drug use, shorter history, absence of fever or skin lesions, respiratory difficulty, higher lymphocyte count, and lower platelet count predict poor in-hospital outcome.
The systemic mycosis caused by Penicillium marneffei is endemic in South and Southeast Asia and is a common human immunodeficiency virus (HIV)–associated opportunistic infection, ranking third after tuberculosis and cryptococcal meningitis in Thailand and after Pneumocystis jiroveci pneumonia (PCP) and tuberculosis in Hong Kong [1–3]. It is increasingly diagnosed in immunocompromised individuals from regions where it is not endemic who have traveled to Southeast Asia [3, 4]. HIV-infected individuals with a CD4 cell count <100 cells/μL are particularly at risk and make up the majority of infections in regions of endemicity [1, 3]. Critical features of the epidemiology of P. marneffei, including the natural reservoir and modes of transmission, remain poorly understood. Exposure to soil during the rainy season has been associated with infection, whereas exposure to or consumption of bamboo rats, the only other known natural host of P. marneffei, is not [5]. Considerable efforts to search for the environmental reservoir have been unsuccessful [6, 7]. Untreated disseminated infection is fatal. Treatment with amphotericin B, followed by itraconazole, is effective [8]. Itraconazole is recommended in mild to moderate disease [9, 10]. However, these treatment strategies have never been studied in randomized trials. Despite appropriate therapy, in-hospital fatality rates remain between 10% and 28% [1, 9, 11, 12], and the determinants of poor outcome have not been identified.
Other than Segretain's accidental auto-inoculation injury in 1959 [13], the first reported human infection in Viet Nam occurred in a 29-year-old HIV-infected man who died before the identification of P. marneffei by blood culture at the Hospital for Tropical Disease (HTD) in Ho Chi Minh City (HCMC) in July 1996 [14]. P. marneffei has since become the second most common pathogen isolated from all routine blood cultures after Cryptococcus neoformans at HTD. The epidemiology of penicilliosis in Viet Nam is unknown, and apart from relatively small case series in Southeast Asia [1–3, 9–12], there has not been a large case review of penicilliosis that spans the combination antiretroviral therapy (cART) era. In the context of a growing HIV epidemic in Viet Nam [15], we conducted a large retrospective chart review to determine the clinical features, seasonality, and outcome predictors in penicilliosis.
METHODS
Study Setting and Population
HTD is the largest primary, secondary, and tertiary referral hospital for infectious diseases in Viet Nam, with >5000 HIV-infected patients seen yearly. The present study included all patients admitted with penicilliosis from July 1996 through December 2009. Penicilliosis was defined as any illness in which P. marneffei was isolated from blood, skin scrapings, bone marrow, lymph node, and/or other body fluid samples. Isolation of P. marneffei was done according to standard culture techniques described elsewhere [16]. Cases were identified from the microbiology records, which had all culture data (positive or negative results) recorded consistently since 1992. To ensure that patients with P. marneffei isolated from referral hospitals were included, we additionally searched for hospital charts with penicilliosis as a primary discharge diagnosis. All patients were cross-checked for duplication in the database by name, date of birth, and address. Multiple culture-positive specimens and/or multiple admissions for 1 patient were merged into one case. Only data from the first penicilliosis admission were extracted. The study was approved by the Scientific and Ethical Committee of HTD.
Data Collection and Definition of Hospital Outcome
Clinical and laboratory information were entered into individual case record forms. Hospital outcome was evaluated by a physician investigator (in consultation with a second physician when there was a doubt) and was categorized as follows: (1) death, (2) worsening disease at discharge (ie, patients who were moribund or deteriorated clinically and were taken from hospital to die at home as per local custom), (3) an improvement (negative repeat culture result and resolution of fever, skin lesions, and/or symptoms), or (4) nonassessable (unchanged or left hospital against medical advice). For analysis, poor outcome was defined as death or worsening disease at discharge, good outcome was an improvement, and nonassessable outcome was treated as missing data.
Data from case record forms were independently double-entered into a Microsoft Access 2003 database to ensure data integrity.
Assessment of Seasonality and Statistical Analysis
Seasonality of penicilliosis, cryptococcosis, and AIDS admissions was assessed using Poisson regression models, which modeled monthly admissions depending on a global trend (modeled with a natural cubic spline with 5 degrees of freedom) and a sinusoidal shape for seasonality effect (modeled with a sine and a cosine term [ie, a cosinor model]) [17]. The log number of days per month was used as an offset to correct for unequal lengths of months. To detect seasonality, we tested whether the sine and cosine term could jointly be omitted from the model with use of a likelihood ratio test. In a second step, we modeled the proportion of penicilliosis among all AIDS admissions with use of the same strategy, based on a logistic regression model. Nonduplicate cryptococcosis cases were simultaneously identified from blood and cerebrospinal fluid (CSF) cultures from the microbiology records. AIDS admission numbers were identified from the hospital records, based on International Classification of Disease, Tenth Revision, code B20.
We performed univariate and multiple logistic regressions based on 10 predefined admission covariates: days of illness, history of fever, cough, injection drug use (IDU), presence of skin lesions, dyspnea, respiratory rate, absolute lymphocyte count, platelet count, and hemoglobin level. These covariates were chosen because they either are established HIV/AIDS prognostic factors (IDU and absolute lymphocyte as a CD4 cell surrogate), have been associated with death in AIDS-associated cryptococcosis or histoplasmosis (longer illness, respiratory difficulty, skin lesions, and hemoglobin level) [18–20], or are known outcome predictors in other infectious diseases (fever and platelet count) [21–23]. Multiple imputation was used for missing covariates and nonassessable outcome. We performed a sensitivity analysis, which excluded patients with missing covariates and nonassessable outcome. In a second sensitivity analysis, we additionally excluded patients who left the hospital with worsening disease (ie, only patients with documented death remained in the poor outcome category). We described time to treatment initiation with use of Kaplan-Meier estimates and modeled it with a Cox regression analysis, depending on history of fever or presence of skin lesions. Patients who did not initiate treatment were treated as censored on the day of discharge or death. A multivariate analysis based on multiple imputed data and including all 10 admission covariates was also conducted.
All analyses were performed with the statistical software R, version 2.9.1 [24], and the companion R package MICE for multiple imputation [25].
RESULTS
Penicilliosis Incidence
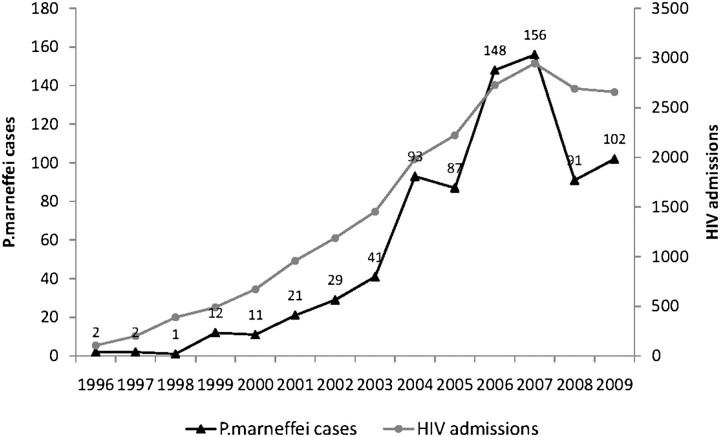
During 1996–2009, the search by microbiology records resulted in 728 cases, and the search by primary discharge-diagnosis resulted in an additional 67 cases, giving a total of 795 nonduplicate cases. The number of penicilliosis cases increased exponentially, from 2 in 1996 to a peak of 156 in 2007, then decreased subsequently to 90 cases per year (Figure 1). Penicilliosis incidence largely mirrored the trends in AIDS admissions at HTD during the same study period (Figure 1). During 2004–2009, penicilliosis accounted for a mean of 4.4% (range, 3.4%–5.4%) of yearly AIDS admissions and for a mean of 6.3% (range, 5%–8.3%) of AIDS-related in-hospital deaths at HTD.
Figure 1.
P. marneffei cases (black line) and AIDS admissions (gray line) at HTD during 1996–2009.
Seasonal Association
Absolute Number of Admissions.
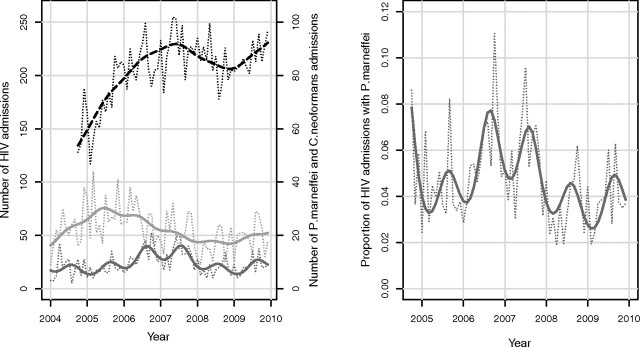
Monthly admission numbers of penicilliosis, cryptococcosis, and AIDS during 2004– 2009 are shown in Figure 2. The Poisson regression model revealed a highly significant seasonality for penicilliosis (P < .001) but not for cryptococcosis (P = .63) or AIDS admissions (P = .83). The seasonality term for penicilliosis has an estimated peak in August, which is in the middle of rainy months, lasting from May to November in HCMC, and an amplitude corresponding to an estimated increase in penicilliosis cases by 27% (95% CI, 14%–41%) during the peak month, compared with the mean. Regression diagnostics did not indicate any problems with the model assumptions or evidence of any unexplained seasonality in the residuals.
Figure 2.
Seasonality of penicilliosis at HTD during 2004–2009. Left panel, Monthly admission numbers of patients with AIDS (black, dashed), P. marneffei infection (dark gray, solid), and C. neoformans infection (light gray, solid) during 2004–2009. Thin dotted lines correspond to actual counts, and thick lines correspond to smoothed values based on a Poisson regression model with terms for a global trend and seasonality. Right panel, Monthly admission numbers of P. marneffei as a proportion of all AIDS admissions. The dotted line corresponds to observed proportions, and the solid line corresponds to smoothed values based on a logistic regression model with terms for a global trend and seasonality.
Proportion of Penicilliosis Cases Among All AIDS Admissions.
Monthly proportion of penicilliosis cases among all AIDS admissions are shown in Figure 2. The logistic regression model revealed a highly significant seasonality for the proportion of penicilliosis (P < .001). Again, the peak month was estimated to be August, and the amplitude corresponded to a 32% (95% CI, 17%–48%) higher odds for a penicilliosis admission during the peak month, compared with the mean.
Clinical and Laboratory Features
Hospital charts were obtainable for 513 (65%) of 795 patients who were admitted to HTD during 2004–2009. All patients were HIV infected; median CD4 count for 62 patients was 7 cells/μL (interquartile range [IQR], 4–24 cells/μL). Penicilliosis was the HIV-presenting illness in 126 (25%) of 513 patients. One hundred three (20%) of 513 patients had been receiving cART for a mean duration of 30 days (IQR:15–120 days). The median age was 28 years (IQR, 25–32 years). Four hundred twenty-one (82%) of 513 patients were men. History of injection drug use was present in 332 (68%) of 485 patients. Four hundred thirty-one (84%) of 513 patients were from HCMC; the remaining 16% were from 21 of 32 southern provinces in Viet Nam.
The clinical and laboratory characteristics of the 513 patients are summarized in Table 1. Symptoms were nonspecific and included, fever, anorexia, fatigue, cough, and gastrointestinal complains. Common signs included cachexia, paleness, skin lesions, hepatomegaly, and/or splenomegaly. The most common laboratory abnormalities were anemia, thrombocytopenia, and elevated transaminase levels. Isolation of P. marneffei from clinical specimens obtained for diagnostic evaluation is reported in Table 2, with highest sensitivities for skin scraping, blood, and lymph node. The cultural yields for peritoneal fluid, CSF, bronchoalveolar lavage fluid, and bone marrow samples were high, but the denominators were small. The median time to identify P. marneffei from skin and blood cultures was 4 days (IQR, 3–5 days) and 5 days (IQR, 4–6 days), respectively. Skin microscopy by Giemsa stain revealed intra- and extracellular yeasts in 123 (80%) of 153 specimens examined; however, detailed description of the yeasts (ie, whether a diagnostic midline septum was observed) was lacking.
Table 1.
Clinical Features of 513 Patients with Penicilliosis in Ho Chi Minh City, Viet Nam
| Patient Characteristic | |
| Demographic characteristic | Value |
| Male sex | 421 (82) |
| Age, years | 28 (25–32) |
| IVDU | 332/485 (68) |
| Receiving ARV | 103 (20) |
| New HIV diagnosis | 126 (25) |
| Symptoms | |
| Days of illness | 15 (7–30) |
| Fever | 419 (82) |
| Anorexia | 318 (62) |
| Fatigue/malaise | 239 (47) |
| Cough | 205 (40) |
| Abdominal pain | 160 (32) |
| Diarrhea | 150 (30) |
| Weigh loss | 92 (18) |
| Signs | |
| Admission temperature, °C | 38 (37–38.5) |
| Highest temperature, °C | 39·5 (38.5–40) |
| Respiratory rate, breaths/min | 24 (22–28) |
| Emanciated/cachexia | 369/467 (79) |
| Pale | 286/397 (72) |
| Thrush | 139/496 (28) |
| Skin lesions | 346/487 (71) |
| Hepatosplenomegaly | 286 (56) |
| Lymphadenopathy | 131 (26) |
| Dyspnoea | 117/468 (25) |
| Decreased mentation | 24 (4.7) |
| Meningismus | 2 (.4) |
| Laboratory Findings | |
| Absolute lymphocyte count, cells/μL | 395 (232–696) |
| Hemoglobin level, g/dL | 8 (6–9.8) |
| Hematocrit, % | 24.9 (19.3–30.3) |
| Platelet count, ×103 cells/μL | 82 (42–150) |
| AST level, U/La(n = 410) | 122 (63–230) |
| ALT level, U/Lb(n = 410) | 60 (34–119) |
| AST:ALT ratio | 2 (1.85–1.93) |
| CD4 cell count, cells/μL (n = 62) | 7 (4–24) |
| Imaging Findings | |
| Abnormal chest radiograph | 357 (70) |
| Reticulonodular | 177/357 (50) |
| Interstitial | 144/357 (39) |
| Alveolar consolidation | 71/357 (20) |
| Pleural effusion | 25/357 (7) |
| Abnormal ultrasound | 312 (60) |
| Hepatomegaly | 197/312 (63) |
| Size, cm | 15.8 (14.3–17.6) |
| Splenomegaly | 176/312 (56) |
| Size, cm | 14.5 (13.2–16) |
| Hepatosplenomegaly | 159/312 (51) |
| Abdominal lymphadenopathy | 159/312 (33) |
| Ascites | 73/312 (23) |
| Spleen abscess | 8/312 (2.6) |
| Concurrent Diagnoses | |
| Clinical pneumonia | 148 (29) |
| M. tuberculosis | 36/148 (24) |
| PCP | 13/148 (8.8) |
| P. marneffei | 12/148 (8.1) |
| Undiagnosed | 87/148 (59) |
| Clinical CNS infection | 59 (11.5) |
| P. marneffei | 20/59 (34) |
| C. neoformans | 7/59 (11.9) |
| P. marneffei and C. neoformans co-infection | 1 |
| M. tuberculosis | 1 |
| No pathogen | 30/59 (50.8) |
| Concurrent OIs | 285 (56) |
| Oral candidiasis | 139/285 (49) |
| Tuberculosis | 62 (22) |
| PCP | 13/285 (4.6) |
| Herpes | 13/285 (4.6) |
| C. neoformans | 7/285 (2.5) |
| Salmonella spp. | 10/285 (3.5) |
| Other bacteria | 9/285 (3.2) |
NOTE. Data are in absolute count (%) for categorical variables and median (interquartile range [IQR]) for continuous data. The denominator is 513 unless otherwise indicated. ARV, antiretrovirals; CNS, central nervous system; IVDU, intravenous drug user; OIs, opportunistic infections; PCP, Pneumocystis jiroveci pneumonia.
Normal range ≤37 U/L.
Normal range ≤40U/L.
Table 2.
P. marneffei Identification from Clinical Specimens of 513 Patients with Penicilliosis
| Clinical Specimens | Number (%) |
| Blood | 395/472 (84) |
| Skin biopsy/scrapings | 186/195 (95) |
| Lymph node | 17/20 (85) |
| Peritoneal fluid | 9/23 (39) |
| CSF | 21/59 (36) |
| Bronchial alveolar lavage | 7/9 |
| Bone marrow | 2/2 |
| Pleural fluid | 0/2 |
| Oral pharyngeal lesions | 8/13 |
| Stool | 6/10 |
Data refer to the number of P. marneffei-positive specimens.
(%) amongst all specimens sampled for diagnostic evaluation within 48 hours of admission.
Clinical pneumonia was diagnosed in 148 (29%) of 513 patients. Of those, a pathogen was identified in 74 (50%) of 148 patients: sputum and/or BAL smear-positive M. tuberculosis in 36, PCP in 13 (not all specimens were tested for PCP), and P. marneffei in 12. Central nervous system (CNS) infection was suspected in 59 (12%) of 513 patients and was definitively diagnosed by CSF culture and/or microscopy in 29 (50%) of 59 patients: P. marneffei was isolated in 20, C. neoformans was microscopically observed and isolated in 7, M. tuberculosis was seen on Zeihl-Neelsen stain in 1, and concurrent P. marneffei and C. neoformans was isolated in one. The clinical features of the patients with CNS symptoms and P. marneffei isolated from the CSF were reported elsewhere [26]. Concurrent opportunistic infections were diagnosed in 285 (56%) of 513 patients, including oral candidiasis (49%), microscopically confirmed tuberculosis (22%), PCP (4.6%), cutaneous herpes (4.6%), cryptococcal meningitis (2.5%), salmonellosis (3.5%), and other bacterial infections (3.2%).
Treatment and Outcome
Appropriate antifungal treatment was initiated in 410 (80%) of 513 patients, with an estimated median time to treatment initiation of 2 days (IQR, 0–5 days). Two hundred fifty-four (62%) of 410 patients received itraconazole (400 mg/day), 147 (36%) of 410 received amphotericin B (0.6–1 mg/kg/day), 4 (1%) of 410 received fluconazole (400 mg/day), and the remaining 5 (1.2%) of 410 received fluconazole followed by itraconazole. Blood transfusion was given for 144 (28%) of 513 patients with symptomatic anemia.
Outcome at discharge was an improvement in 347 (68%) of 513 patients, death in 101 (20%) of 513, worsening in 42 (8%) of 513, and nonassessable in 23 (5%) of 513 (4 left against medical advice, 19 unchanged). The median duration of hospitalization was 9 days (IQR, 4–17 days) and was strongly dependent on the outcome group: patients with documented death died a median of 2 days (IQR, 1–5 days) after admission, and those with worsening disease stayed in the hospital for 2.5 days (IQR,1–4 days). In contrast, patients who showed an improvement at discharge had a median hospitalization duration of 14 days (IQR, 8–19 days). Among 254 patients receiving itraconazole, 208 (82%) had an improvement; 36 (14%) died or had worsening disease. By comparison, 111 (76%) of 147 patients receiving amphotericin B had an improvement; 33 (22%) of 147 died or had worsening disease. The fatality rate among patients receiving amphotericin tended to be higher, but the difference did not reach statistical significance (P = .054, Fisher's exact test).
Admission Risk Factors for Poor Outcome.
The 10 admission covariates used to assess the prediction of outcome are listed in Table 3. The most frequently missing covariates were IDU and presence of skin lesions (both missing in 5%); other covariates were missing in <2%. All covariates were jointly nonmissing and outcome assessable in 411 patients (80%). Results of the logistic regression are displayed in Table 3. All predefined covariates except for cough, dyspnea (highly significant in univariate analysis, borderline significant in multiple logistic regression), and hemoglobin level were significant predictors of outcome in both univariate and multiple logistic regression analysis. Multiple logistic regression was based on multiple imputation of missing data, but a complete-case analysis gave highly consistent results; the only difference was that dyspnea also reached statistical significance (P = .043) in the multiple regression analysis. If patients with worsening disease were also regarded as nonassessable (ie, only patients with death remained in the poor outcome group), results were also consistent, except that the presence of skin lesions was no longer statistically significant (odds ratio, 0.70; 95% CI, 0.33–1.48; P = .35) in the adjusted analysis.
Table 3.
Covariates at Hospital Admission and Association with Poor Outcome in 513 Patients with Penicilliosis
| Characteristics | All patients(N = 513) | Good outcomea (N = 347) | Poor outcomeb (N = 143) | Univariate effectc: OR (95% CI) | Adjusted effect from multiple logistic regressiond: OR (95% CI) |
| IVDU | 332/485 (68%) | 208/331 (63%) | 108/134 (81%) | 2.46 (1.52–3.98), P < .001 | 2.97 (1.67–5.29), P < .001 |
| Days of illnesse | n = 502 15 (7,30) | n = 341 15 (10,30) | n = 138 14 (7,30) | 0.80 (.69–.92), P = .003 | 0.77 (.65–.92),P = .004 |
| History of fever | 436/502 (87%) | 315/342 (92%) | 106/138 (77%) | 0.28 (.16–.50),P < 0·001 | 0.28 (.14–.55), P < .001 |
| Respiratory ratef (breaths/minute) | n = 504 24 (22,28) | n = 338 24 (22,28) | n = 143 28 (24,38) | 3.47 (2.52–4.76), P < .001 | 3.02 (2.08–4.40),P < .001 |
| Cough | 205/513 (40%) | 143/347 (41%) | 51/143 (36%) | 0.79 (.53–1.18), P = .25 | 0.66 (.40–1.10), P = .11 |
| Dyspnoea | 61/513 (12%) | 22/347 (6%) | 36/143 (25%) | 4.97 (2.80–8.82), P < .001 | 2.07 (.99–4.35), P = .05 |
| Skin lesions | 346/487 (71%) | 252/337 (75%) | 81/128 (63%) | 0.58 (.38–.90),P = .01 | 0.54 (.31–.93), P = .03 |
| Absolute Lymphocyte counte (cells/μL) | n = 513 399.0 (232.3,699.6) | n = 347 343.1 (221.6,595.4) | n = 143 601.8 (313.7,1231.3) | 1.36 (1.18–1.58), P < .001 | 1.19 (1.01–1.40), P = .04 |
| Platelet counte (x103 cells/μL) | n = 509 82 (42,150) | n = 344 93·1 (46.9,165.3) | n = 142 62.5 (33.8,102.3) | 0.73 (.63–.85), P < .001 | 0.72 (.61–.86), P < .001 |
| Hemoglobing (g/dL) | n = 508 8·0 (6·0,9·8) | n = 345 8.0 (6.0,9.7) | n = 140 8.0 (5.9,10.3) | 1.03 (.97–1.08), P = .31 | 1.02 (.95–1.09), P = .59 |
Summary statistic is absolute count (%) for categorical variables and median (IQR) for continuous data·
IVDU, intravenous drug user; OR, odds ratios.
Defined as an improvement at hospital discharge with negative repeat culture, resolution of fever, skin lesions, and/or symptoms.
Defined as death or worsening disease at discharge.
Based on patients with non-missing outcome and covariate.
Based on multiple imputation.
OR correspond to the effect of a 2-fold higher value.
OR correspond to the effect of a +10 breaths/minute higher respiratory rate.
OR correspond to the effect of a +1 g/dL higher hemoglobin value.
Time to Treatment Initiation.
The time to treatment initiation was significantly shorter for patients with skin lesions than for those without (hazard ratio [HR], 3.78; 95% CI, 2.96–4.84; P < .001), and the estimated median time to treatment initiation was 1 day (IQR, 0–2 days) after admission for patients with skin lesions, compared with 5 days (IQR, 3–8 days) for those without. There was no evidence that fever affected time to treatment initiation (HR, .91; 95% CI, 0.66–1.24; P = .54). When all covariates were jointly included in a multiple Cox regression model, only presence of skin lesions was significantly associated with a more rapid treatment initiation (HR, 3.27; 95% CI, 2.51–4.27; P < .001).
DISCUSSION
Our study is a large penicilliosis case review that spans the cART era in Viet Nam. We report new epidemiological and clinical data on an emerging opportunistic mycosis from a region of endemicity that faces one of the fastest growing HIV epidemics in the world. We confirm the previous report of the seasonality of penicilliosis from Thailand [27], and we report for the first time, to our knowledge, important and easily measurable predictors of outcome in penicilliosis. Although penicilliosis cases in this study do not represent true incidence of disease, they provide estimates of penicilliosis burden (4.4% among hospitalized patients with AIDS) and mirrored the trends in AIDS admissions at HTD over >1 decade. Both penicilliosis and AIDS trends reflect the HIV/AIDS epidemic in Viet Nam, with an estimated number of people living with HIV increased from 120,000 to 310,000 since 2000 [15]. The decrease in penicilliosis and AIDS incidence at HTD after 2007 may be a reflection of the successful roll-out of cART programs in Viet Nam. In partnership with international agencies, Viet Nam has made considerable progress in the national response to HIV infection, including launching of the National Strategy on HIV/AIDS Prevention and Control in 2005 and decentralizing its HIV management approach by expanding provincial AIDS centers to 58 of 64 provinces in Viet Nam [28]. This may have reduced the numbers of patients referred to HTD. In addition to this, with increased awareness and experience amongst physicians, penicilliosis is often being treated in the outpatient setting, further reducing the numbers of admissions.
Penicilliosis has been associated with exposure to soil in the rainy season [7]. The cases doubled during the wet months in Chiang Mai [27], suggesting that precipitation facilitates an expansion of environmental reservoirs of P. marneffei. In this study, we found an ∼30% increase in penicilliosis cases during the rainy months of May to November in HCMC, whereas cryptococcosis and AIDS admissions showed no seasonal pattern. This study demonstrated the seasonality of penicilliosis and should reenergize research interest in the ecological niche of P. marneffei.
The in-hospital case fatality rate of penicilliosis at HTD was 20%, which increased to 28% if patients with worsening disease at discharge were included. This fatality rate was observed despite initiation of appropriate antifungal therapy in 50% of patients who subsequently died. More patients treated with amphotericin B than those treated with itraconazole had a poor outcome; however, the difference did not reach statistical significance, and amphotericin B may have been chosen for patients with more severe disease. In both complete-case and multiple-imputation analyses, IDU, shorter history, absence of fever or skin lesions, higher respiratory rates, higher absolute lymphocyte count, and lower platelet count independently predicted poor outcome. IDU is an established independent risk factor for death and/or development of AIDS. The association of shorter illness and poor outcome suggests that differences in disease courses and host pathogen factors, rather than delay in seeking care, are contributing to outcome. Absence of fever in patients with systemic mycosis may be a sign of profound immunosuppression, which is a poor prognosis. However, the presence of fever may be advantageous, because it stimulates investigation and admission and, subsequently, earlier diagnosis and treatment. Similarly, presence of skin lesions may be protective, because it results in more rapid initiation of empirical treatment. For this reason, we performed a post-hoc analysis of time to antifungal initiation in association with all admission covariates and found that only presence of skin lesions (not fever) was significantly associated with a more rapid time to antifungal initiation. Respiratory rate, an easily measureable and objective physical sign, was an independent predictor of outcome, unlike cough and dyspnea. The finding that higher rather than lower lymphocyte counts predicted poor outcome is unexpected and should be further investigated. It is possible that lymphocyte count is not a good outcome predictor at such low numbers and over a relatively narrow range. As in other infectious diseases, we found thrombocytopenia to be a predictor of poor outcome [22, 23]; however, admission hemoglobin level did not, perhaps because patients with symptomatic anaemia received blood transfusions. We were unable to ascertain follow-up information after hospital discharge for these patients because of a limitation in our current medical record system, which generates a different hospital number for every inpatient visit from the same patient and does not link with the outpatient care.
Notwithstanding the inherent limitations of a retrospective study design, including confounding and selection bias, this study sheds new light on the epidemiology and clinical prognosis of penicilliosis. The previously reported seasonality of penicilliosis has been confirmed in this large dataset. Better understanding of the ecological niche of P. marneffei may shed light on the modes of disease transmission and may bring about new strategies for disease prevention. The in-hospital mortality remains high and is associated with IDU, shorter illness duration, absence of fever or skin lesions, respiratory difficulty, and the degree of thrombocytopenia. These and other prognostic factors should be further evaluated in prospective studies to guide treatment strategies for this emerging opportunistic infection, particularly in resource-poor settings where disease is endemic.
Acknowledgments
We thank the administration and staff at the Hospital for Tropical Diseases, for giving us access to the hospital admission records, microbiology records, and the hospital charts, and the staff at the Clinical Trial Unit and the Data Management Department at the Oxford University Clinical Research Unit, for assistance in obtaining the hospital charts and data management.
Financial Support. This work was supported by Fogarty International Clinical Research Fellowship (to T.L.); Hawaii Center for AIDS (to T.L. and C.M.S.) and Wellcome Trust Major Overseas Programme (to T.L., M.W., J.N.D., and J.F.). None of the funding sources had any role in the design or execution of the study. The corresponding author had full access to the data and in consultation with other co-authors had the final decision to submit this work for publication.
Potential conflicts of interest. M.K. has served on the scientific advisory board and has stock options for Quanta Life; has received institutional grant support from Merck, BMS, Abbott, Pfizer, and Gilead; has received royalties from Stanford University; and has patents with Yale University, Stanford University, and U.S. Veterans Administration. All other authors: no conflicts.
Contributors. T.L., M.J.K., C.M.S. and J.F. conceived and designed the study. N.H.C., V.M.Q., N.T.C., and N.P.H.L. obtained the penicilliosis, cryptococcosis and AIDS admission numbers from the hospital records. T.L. and P.S.L. obtained the hospital charts and extracted the data. M.W. and T.L. performed the statistical analyses. T.L., M.W., J.N.D. and J.F. wrote the paper. All authors participated in data gathering and in the critical appraisal of the paper
References
- 1.Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated P. marneffei infection in southeast Asia. Lancet. 1994;344:110–3. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 2.Wong KH, Lee SS. Comparing the first and second hundred AIDS cases in Hong Kong. Singapore Med J. 1998;39:236–40. [PubMed] [Google Scholar]
- 3.Duong TA. Infection due to P. marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. 1996;23:125–30. doi: 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 4.Cristofaro P, Mileno MD. P. marneffei infection in HIV-infected travelers. Aids Alert. 2006;21:140–2. [PubMed] [Google Scholar]
- 5.Chariyalertsak S, Sirisanthana T, Supparatpinyo K, Praparattanapan J, Nelson KE. Case-control study of risk factors for P. marneffei infection in human immunodeficiency virus-infected patients in northern Thailand. Clin Infect Dis. 1997;24:1080–6. doi: 10.1086/513649. [DOI] [PubMed] [Google Scholar]
- 6.Chariyalertsak S, Vanittanakom P, Nelson KE, Sirisanthana T, Vanittanakom N. Rhizomys sumatrensis and Cannomys badius, new natural animal hosts of P. marneffei. J Med Vet Mycol. 1996;34:105–10. [PubMed] [Google Scholar]
- 7.Gugnani HC, Paliwal-Joshi A, Rahman H, et al. Occurrence of pathogenic fungi in soil of burrows of rats and of other sites in bamboo plantations in India and Nepal. Mycoses. 2007;50:507–11. doi: 10.1111/j.1439-0507.2007.01402.x. [DOI] [PubMed] [Google Scholar]
- 8.Sirisanthana T, Supparatpinyo K, Perriens J, Nelson KE. Amphotericin B and itraconazole for treatment of disseminated P. marneffei infection in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;26:1107–10. doi: 10.1086/520280. [DOI] [PubMed] [Google Scholar]
- 9.Supparatpinyo K, Nelson KE, Merz WG, et al. Response to antifungal therapy by human immunodeficiency virus-infected patients with disseminated P. marneffei infections and in vitro susceptibilities of isolates from clinical specimens. Antimicrob Agents Chemother. 1993;37:2407–11. doi: 10.1128/aac.37.11.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranjana KH, Priyokumar K, Singh TJ, et al. Disseminated P. marneffei infection among HIV-infected patients in Manipur state, India. J Infect. 2002;45:268–71. doi: 10.1053/jinf.2002.1062. [DOI] [PubMed] [Google Scholar]
- 11.Wu TC, Chan JW, Ng CK, Tsang DN, Lee MP, Li PC. Clinical presentations and outcomes of P. marneffei infections: a series from 1994 to 2004. Hong Kong Med J. 2008;14:103–9. [PubMed] [Google Scholar]
- 12.Supparatpinyo K, Chiewchanvit S, Hirunsri P, Uthammachai C, Nelson KE, Sirisanthana T. P. marneffei infection in patients infected with human immunodeficiency virus. Clin Infect Dis. 1992;14:871–74. doi: 10.1093/clinids/14.4.871. [DOI] [PubMed] [Google Scholar]
- 13.Segretain G. Penicillium marneffei n sp., agent of a mycosis of the reticuloendothelial system. Mycopathologia. 1959;11:327–53. doi: 10.1007/BF02089507. [DOI] [PubMed] [Google Scholar]
- 14.Hien TV, Loc PP, Hoa NTT, et al. First cases of disseminated penicilliosis marneffei infection among patients with acquired immunodeficiency syndrome in Viet nam. Clin Infect Dis. 2001;32:e78–80. doi: 10.1086/318703. [DOI] [PubMed] [Google Scholar]
- 15.Viet nam Ministry of Health. Viet nam HIV/AIDS estimates and projections 2007–2012. Ha Noi: Ministry of Health in Viet Nam; 2009. pp. 43–91. [Google Scholar]
- 16.Viviani MA, Tortorano AM. P marneffei. In: Ajello L, Hay RJ, editors. Medical mycology Topley and Wilson's microbiology and microbial infections. 9th ed. Vol 4. London: Edward Arnold; 1998. pp. 409–19. [Google Scholar]
- 17.Barnett AG, Dobson AJ. Analysing seasonal Health data (Statistics for Biology and Health) Berlin Heidelberg: Springer; 2010. [Google Scholar]
- 18.de Francesco Daher E, de Sousa Barros FA, da Silva Júnior GB, et al. Risk factors for death in acquired immunodeficiency syndrome-associated disseminated histoplasmosis. Am J Trop Med Hyg. 2006;74:600–3. [PubMed] [Google Scholar]
- 19.Visnegarwala F, Graviss EA, Lacke CE, et al. Acute respiratory failure associated with cryptococcosis in patients with AIDS: analysis of predictive factors. Clin Infect Dis. 1998;27:1231–7. doi: 10.1086/514984. [DOI] [PubMed] [Google Scholar]
- 20.Daher EF, Silva GB, Jr., Barros FA, et al. Clinical and laboratory features of disseminated histoplasmosis in HIV patients from Brazil. Trop Med Int Health. 2007;12:1108–15. doi: 10.1111/j.1365-3156.2007.01894.x. [DOI] [PubMed] [Google Scholar]
- 21.Lim WS, Macfarlane JT. Defining prognostic factors in the elderly with community acquired pneumonia, 2001 community acquired pneumonia: a case controlled study of patients aged > or = 75 yrs. Eur Respir J. 2001;17:200–5. doi: 10.1183/09031936.01.17202000. [DOI] [PubMed] [Google Scholar]
- 22.Sy RW, Chawantanpipat C, Richmond DR, Kritharides L. Thrombocytopenia and mortality in infective endocarditis. J Am Coll Cardiol. 2008;51:1824–5. doi: 10.1016/j.jacc.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 23.Srikiatkhachorn A, Green S. Markers of dengue disease severity. Curr Top Microbiol Immunol. 2010;338:67–82. doi: 10.1007/978-3-642-02215-9_6. [DOI] [PubMed] [Google Scholar]
- 24.R Development Core Team. R: a language and environment for statistical computing (version 2.9.1) 2010. Available at: http://www.R-project.org. Accessed 24 July 2010. [Google Scholar]
- 25.Van Buuren S, Groothuis-Oudshoorn K. MICE: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software, forthcoming. 2011. Available at: http://www.stefvanbuuren.nl/publications.html. Accessed 24 July 2010. [Google Scholar]
- 26.Le T, Chi NH, Cuc NTK, et al. AIDS-Associated Penicillium marneffei infection of the central nervous system. Clin Infect Dis Dec. 2010;51:1458–62. doi: 10.1086/657400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chariyalertsak S, Sirisanthana T, Supparatpinyo K, Nelson KE. Seasonal variation of disseminated penicillium marneffei infections in northern Thailand: a clue to the reservoir? J Infect Dis. 1996;173:1490–3. doi: 10.1093/infdis/173.6.1490. [DOI] [PubMed] [Google Scholar]
- 28.Ministry of Health Viet nam. The third country report on following up the implementation to the declaration of commitment on HIV AIDS. 2008. [Google Scholar]