Abstract
A 32-year-old physician with a history of chickenpox at age 5 and seropositivity to varicella-zoster virus (VZV) at age 30 developed fever and vesicular rash 14 days after examining an immunocompetent patient with localized herpes zoster ophthalmicus. Vesicular viral culture grew VZV, and the physician was diagnosed with VZV reinfection.
The physician developed fever and rash. Over the next 12 hours, the skin eruption became vesicular and spread over her chest and back, and she presented to an infectious diseases specialist for further evaluation. She experienced fever (temperature, 39.2°C), chills, night sweats, lethargy, and myalgias 24 h before developing the skin eruption. The cutaneous lesions started on her abdomen and spread to her chest, back, extremities, face, and oropharynx. The skin lesions were neither painful nor pruritic. Associated symptoms included nausea and vomiting. The patient denied a stiff neck or photophobia. She was otherwise healthy. The patient worked as a pulmonary critical care physician and divided her time between clinical practice and laboratory research. Her occupational exposure history included examination of an immunocompetent adult with localized herpes zoster ophthalmicus 14 days prior to symptom onset. The physician spent approximately 20 min in close contact with the zoster patient and wore gloves during the entire encounter. The lesions were covered with medical gauze, and the physician briefly lifted the gauze for examination. Of note, the zoster patient had received first doses of valacyclovir and oral steroids <12 h earlier. Aside from this patient, the physician had no other known exposures to individuals with varicella infection during the preceding weeks. The physician had contracted chickenpox from her brother at age 5, and she had a positive varicella immunoglobulin G (IgG) antibody enzyme immunoassay result compatible with qualitative immunity at age 30. Varicella serological testing was performed in accordance with hospital credentialing requirements.
On arrival at the infectious diseases clinic, her temperature was 37.3°C, blood pressure 136/78 mmHg, pulse 88/min, and respirations 18/min. She had diffuse well-circumscribed erythematous macules of diameter 1–2 mm, some with central vesicles. Several of the vesicles had umbilication. The macules were located on her trunk, extremities, face, and oropharynx. The lesions spared her palms, soles, and genitalia. There were no petechiae or purpura. Laboratory testing of a serum sample revealed a white blood count of 4900 cells/μL, a hemoglobin level of 13.6 g/dL, and a platelet count of 218,000 cells/μL. The results of a complete metabolic panel were within normal limits. The patient did not have a cough, and a chest radiograph was not performed. Sterile lancing of the vesicle revealed scant clear fluid. A Tzanck smear revealed multinucleated giant cells, and the results of a punch biopsy were consistent with herpesvirus infection. The results of HSV1 and HSV2 stains were unremarkable. VZV was identified by means of immunofluorescence from the viral vesicle culture in MRC-5 and A-549 cells. Acute titers were not obtained. Varicella titers at 1 week revealed IgG ratios of >1:1024 and IgM of 10.16 (positive, >1.10).
VZV reinfection was not suspected at first because of the patient's history of chickenpox in childhood and recent seropositivity. Her rash progressed over several days; there were several hundred lesions in various stages, including erythematous macules, papules, vesicles, and crusted lesions simultaneously (Figure 1). Valacyclovir 1000 mg 3 times a day for 7 days was prescribed on day 3 of the illness. After starting valacyclovir therapy, there was a significant decrease in the number and size of new vesicles. The patient remained febrile for 1 week and experienced significant malaise, necessitating 14 days of sick leave. The vesicular lesions encrusted by 14 days, and there was minimal scarring. There were no other reports of varicella among health care workers exposed to the index herpes zoster opthalmicus patient.
Figure 1.
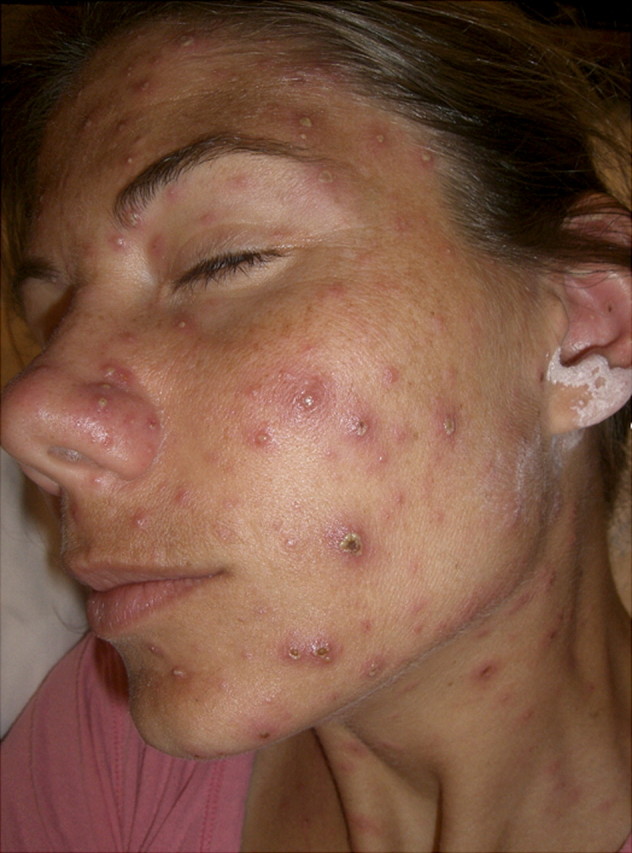
Various stages of varicella-zoster virus lesions present on day 4, including erythematous macules, papules, vesicles, and pustules.
Primary varicella infection confers natural immunity and generally protects against reinfection. Seroprevalence studies indicate that >90% of adults in the United States have antibodies to VZV. Reinfection with VZV is not well understood but may occur more commonly than previously thought. A pediatric varicella active surveillance study revealed 9947 cases of VZV infection; in 4.5%–13.3% of these cases, a previous varicella infection was reported [1]. A similar adult varicella active surveillance study revealed 1,047 cases of VZV infection; in 21% of these cases, the patient reported a history of varicella, and in 3% of cases, the patient had been vaccinated [2]. Importantly, case reports demonstrate that reinfection with VZV can occur even in seropositive individuals [3, 4].
Nosocomial transmission of VZV is well documented in the literature [5–7]. VZV is highly contagious; transmission occurs through direct contact or inhalation of aerosols from vesicular fluid or respiratory tract secretions. Currently, the Centers for Disease Control and Prevention (CDC) recommends that health care providers have evidence of VZV immunity. Evidence of immunity includes documentation of receipt of 2 doses of varicella vaccine, blood test results showing immunity or laboratory confirmation of prior disease, or diagnosis/verification of varicella or zoster infection by a health care provider. However, even health care providers with a documented history and/or positive serum antibody titers have developed VZV reinfection after exposure to contagious patients [5–7].
The CDC recommends airborne and contact isolation for hospitalized patients with varicella or disseminated herpes zoster and for immunocompromised patients with localized herpes zoster [8]. Presumably, these patients have a higher viral burden and more lesions that can aerosolize virus. In contrast, the CDC recommends standard precautions for immunocompetent patients with localized zoster lesions that can be covered, such as the index case in this report [8]. Nosocomial transmission of VZV from immunocompetent patients with herpes zoster has traditionally been thought to occur by means of direct contact with infectious particles. However, nosocomial transmission of VZV from patients with localized herpes zoster has been documented among health care providers who never had direct contact with the index case, which suggests airborne routes of transmission [9–12]. Consequently, some hospitals go beyond the CDC recommendations, placing all patients with varicella and herpes zoster (disseminated or localized) in airborne and contact isolation [13]. Reports suggestive of airborne transmission of VZV from immunocompetent patients with localized herpes zoster, and recovery of DNA samples from the environment of patients with appropriately covered lesions, raise questions about appropriate isolation precautions [9, 10, 14]. The presence of VZV DNA does not necessarily signify infectivity, and more conclusive data on the airborne transmission dynamics of VZV are needed to define optimal isolation strategies.
Our case is unique in that a young, immunocompetent physician with documented childhood disease and recently confirmed serum IgG antibodies became reinfected with VZV after exposure to an immunocompetent patient with localized herpes zoster. This case raises concerns for possible aerosolized transmission of VZV given the lack of direct contact with the vesicular lesions. It also serves as a cautionary tale for health care providers. Seropositivity does not always result in protective immunity against subsequent varicella infection, and health care providers can develop reinfection via occupational exposure.
Acknowledgments
Financial Support. This work was supported by the National Institutes of Health (grant no. T 32HL094296).
Potential conflicts of interest: All authors: no conflicts.
References
- 1.Hall S, Maupin T, Seward J, et al. Second varicella infections: are they more common than previously thought? Pediatrics. 2002;109:1068–73. doi: 10.1542/peds.109.6.1068. [DOI] [PubMed] [Google Scholar]
- 2.Marin M, Watson TL, Chaves SS, et al. Varicella among adults: data from an active surveillance project, 1995–2005. J Infect Dis. 2008;197(suppl 2):S94–100. doi: 10.1086/522155. [DOI] [PubMed] [Google Scholar]
- 3.Gershon AA, Steinberg SP, Gelb L. Clinical reinfection with varicella-zoster virus. J Infect Dis. 1984;149:137–42. doi: 10.1093/infdis/149.2.137. [DOI] [PubMed] [Google Scholar]
- 4.Martin KA, Junker AK, Thomas EE, Van Allen MI, Friedman JM. Occurrence of chickenpox during pregnancy in women seropositive for varicella-zoster virus. J Infect Dis. 1994;170:991–5. doi: 10.1093/infdis/170.4.991. [DOI] [PubMed] [Google Scholar]
- 5.Gurevich I, Jensen L, Kalter R, Cunha BA. Chickenpox in apparently ‘immune’ hospital workers. Infect Control Hosp Epidemiol. 1990;11 510–512. [PubMed] [Google Scholar]
- 6.Ku CH, Liu YT, Christiani DC. Case report: occupationally related recurrent varicella (chickenpox) in a hospital nurse. Environ Health Perspect. 2005;113:1373–5. doi: 10.1289/ehp.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krasinski K, Holzman RS, LaCouture R, Florman A. Hospital experience with varicella-zoster virus. Infect Control. 1986;7:312–6. doi: 10.1017/s019594170006433x. [DOI] [PubMed] [Google Scholar]
- 8.Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(suppl 2):S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josephson A, Gombert ME. Airborne transmission of nosocomial varicella from localized zoster. J Infect Dis. 1988;158:238–41. doi: 10.1093/infdis/158.1.238. [DOI] [PubMed] [Google Scholar]
- 10.Saidel-Odes L, Borer A, Riesenberg K, et al. An outbreak of varicella in staff nurses exposed to a patient with localized herpes zoster. Scand J Infect Dis. 2010;42:620–2. doi: 10.3109/00365541003754436. [DOI] [PubMed] [Google Scholar]
- 11.Leclair JM, Zaia JA, Levin MJ, Congdon RG, Goldmann DA. Airborne transmission of chickenpox in a hospital. N Engl J Med. 1980;302:450–3. doi: 10.1056/NEJM198002213020807. [DOI] [PubMed] [Google Scholar]
- 12.Gustafson TL, Lavely GB, Brawner ER, Jr, Hutcheson RH, Jr, Wright PF, Schaffner W. An outbreak of airborne nosocomial varicella. Pediatrics. 1982;70:550–6. [PubMed] [Google Scholar]
- 13.Weber DJ, Rutala WA, Hamilton H. Prevention and control of varicella-zoster infections in healthcare facilities. Infect Control Hosp Epidemiol. 1996;17:694–705. doi: 10.1086/647206. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Yoshikawa T, Tomitaka A, Matsunaga K, Asano Y. Detection of aerosolized varicella-zoster virus DNA in patients with localized herpes zoster. J Infect Dis. 2004;189:1009–12. doi: 10.1086/382029. [DOI] [PubMed] [Google Scholar]


