Abstract
Antibiotic-refractory Lyme arthritis may result from Borrelia burgdorferi–induced autoimmunity in affected joints. Such patients usually have certain HLA-DRB1 molecules that bind an epitope of B. burgdorferi outer-surface protein A (OspA163–175), and cellular and humoral immune responses to OspA are greater in patients with antibiotic-refractory arthritis than in those with antibiotic-responsive arthritis. Recent work in a mouse model suggests that, during B. burgdorferi infection, OspA in genetically susceptible individuals stimulates a particularly strong TH1 response, which may be one of several factors that can help set the stage for a putative autoimmune response in affected joints. However, vaccination with OspA did not induce arthritis in this mouse model, and case and control comparisons in human vaccine trials did not show an increased frequency of arthritis among OspA-vaccinated individuals. Thus, a vaccine-induced immune response to OspA does not replicate the sequence of events needed in the natural infection to induce antibiotic-refractory Lyme arthritis.
Borrelia burgdorferi, the sole agent of Lyme disease in the United States, is particularly arthritogenic [1]. Months after early infection, if the infection is not recognized and treated with antibiotics, ∼60% of patients experience intermittent or persistent joint swelling and pain, which is a late manifestation of the illness [2]. In Europe, where the infection is usually caused by Borrelia afzelii or Borrelia garinii, arthritis is a less common manifestation of the disease [1].
Lyme arthritis usually resolves after receipt of a 1- or 2-month course of oral doxycycline or a 1-month course of intravenous ceftriaxone therapy [3, 4]. However, in a small percentage of patients in the Northeastern United States, joint inflammation persists for >3 months after >8 weeks of oral antibiotics or >4 weeks of intravenous antibiotic therapy, or both; this condition is called antibiotic-refractory Lyme arthritis [5]. In such patients, polymerase chain reaction (PCR) results for B. burgdorferi DNA in synovial fluid, which are often positive prior to treatment, are usually negative at the conclusion of antibiotic therapy [5, 6], and the results have been uniformly negative in synovial tissue obtained months after antibiotic treatment [5, 7]. This suggests that synovitis in patients with antibiotic-refractory Lyme arthritis may persist after the near or total eradication of spirochetes from the joint with antibiotic therapy.
In 1990, it was reported that patients with chronic Lyme arthritis had increased frequencies of the HLA-DR4 and DR2 alleles [8]. Next, it was noted that patients with antibiotic-refractory arthritis were more likely to have cellular and humoral immune responses to B. burgdorferi outer-surface protein A (OspA) than were patients with antibiotic-responsive arthritis [9, 10], and the severity of joint swelling correlated directly with cellular and humoral immune responses to this spirochetal protein [11, 12]. In July 1998, during the same week that the results of the phase III trials of B. burgdorferi OspA vaccines were reported in the New England Journal of Medicine [13, 14], a seminal report appeared in Science regarding the pathogenesis of antibiotic-refractory Lyme arthritis [15]. In this report, OspA165–173 was identified as the immunodominant T cell epitope of OspA in HLA-DRB1*0401–positive individuals. Moreover, it was noted that this spirochetal epitope had partial sequence homology with a peptide contained in human lymphocyte function–associated antigen-1 (hLFA-1αL332–340), and 6 of 11 patients with antibiotic-refractory arthritis had T cell reactivity with both the OspA and hLFA-1 epitopes, as determined by enzyme-linked immunosorbent spot assay [15]. Thus, it was postulated that antibiotic-refractory arthritis results from molecular mimicry between the OspA and LFA-1 epitopes, leading to autoimmunity within affected synovial tissue.
Although arthritis was not more prevalent in the vaccine groups than it was in placebo groups in the Lyme disease vaccine trials [13, 14], the results of the Science paper raised the question of whether autoimmune arthritis could be a rare complication of OspA vaccination. The purpose of this report is to review the research pertaining to OspA immunity and antibiotic-refractory Lyme arthritis during the subsequent 12 years.
ASSOCIATION OF ANTIBIOTIC-REFRACTORY LYME ARTHRITIS WITH HLA-DR MOLECULES THAT BIND B. BURGDORFERI OSPA165–173
In DRB1*0401-positive individuals, the 9 core amino acids of the immunodominant epitope of OspA are located in positions 165–173 of the protein [15]. To assess patients’ reactivity with this epitope, longer peptides containing amino acids from the peptide-flanking regions were used, because these amino acids influence both HLA-DR binding and T cell receptor recognition. However, for each study, the length of the OspA peptide was slightly different, which is the reason that the subscript numbers for this epitope vary.
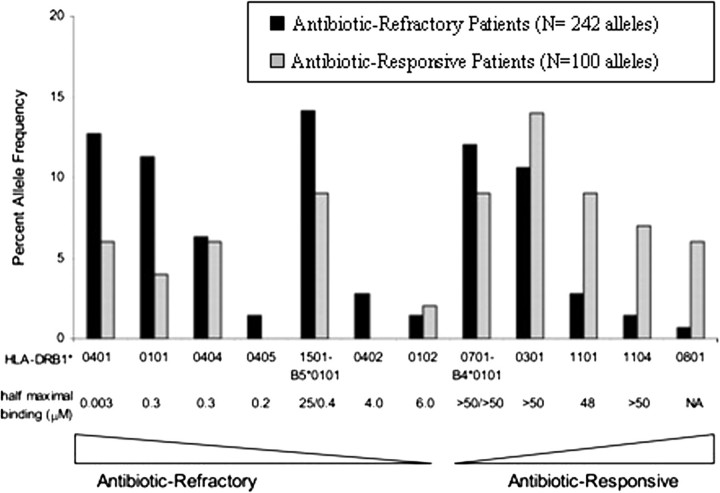
Using molecular techniques, the frequencies of HLA-DRB1 alleles were determined in 121 patients with antibiotic-refractory or antibiotic-responsive Lyme arthritis, and in vitro binding of the OspA163–175 peptide to 14 recombinant DRB molecules was assessed [16]. In general, the DRB molecules that bound OspA163–175 (eg, DRB1*0401, 0101, 0404, and 0405 and DRB5*0101) were more common among patients with antibiotic-refractory arthritis, whereas those that did not bind it (eg, DRB1*0301, 1101 and 1104) were more frequent among patients with antibiotic-responsive arthritis (Figure 1). Altogether, 79% of the patients with antibiotic-refractory arthritis had at least one of the 7 known OspA peptide-binding DR molecules, compared with 46% of the patients with antibiotic-responsive arthritis (odds ratio [OR], 4.4; P < .001). Furthermore, the HLA-DR alleles associated with chronic Lyme arthritis in the previous study [8] were quite consistent with those associated with antibiotic-refractory arthritis in the current study [16].
Figure 1.
Correlation of the relative binding affinity of the Borrelia burgdorferi outer-surface protein A (OspA)163–175 peptide for 14 HLA-DR molecules and percentage allele frequencies of these HLA-DR molecules in patients with antibiotic-refractory Lyme arthritis (antibiotic-refractory patients) or antibiotic-responsive Lyme arthritis (antibiotic-responsive patients). Seven of the 14 DRB molecules, which showed strong-to-weak binding of OspA163–175, were more common among patients with antibiotic-refractory arthritis, whereas the other 7 DRB molecules, which showed negligible or no binding of the peptide, were more common among patients with antibiotic-responsive arthritis. Reproduced with permission from Steere et al [16].
In a search for other Borrelia epitopes with this HLA-DRB1 binding pattern, T cell epitopes were predicted in 17 known immunogenic proteins of B. burgdorferi using the T cell algorithm TEPITOPE, and the HLA-DR binding profiles of 15 candidate peptides were confirmed using in vitro binding assays [17]. In addition, the B. burgdorferi proteome was searched for proteins with sequence homology with OspA165–173. From this work, 1 B. burgdorferi peptide (BB0347392–404) was identified that had a strong refractory-arthritis–associated HLA-DR binding profile, and another peptide (GK297–306) shared sequence homology with OspA165–173. However, patients’ cells did not proliferate in response to either peptide, making it highly unlikely they were involved in refractory arthritis. Thus, recognition of OspA165–173 remains the only currently identified B. burgdorferi epitope associated with this disease course.
OSPA165–173-REACTIVE T CELLS AND OSPA ANTIBODY RESPONSES IN LYME ARTHRITIS
After 1998, a number of reports confirmed and extended observations about the association between OspA-reactive T cells and antibiotic-refractory Lyme arthritis. Using overlapping 20-mer peptides of the OspA protein, it was learned that patients with antibiotic-refractory arthritis often had T cells that proliferated and secreted interferon (IFN) γ in response to 4 or 5 OspA epitopes, including OspA163–175 [12]. Substitution analysis showed that valine (166) and threonine (172) were critical for immunogenicity of the OspA163–175 peptide [18, 19]. Consistent with this result, lymphocytes from 7 patients with antibiotic-refractory arthritis proliferated in response to B. burgdorferi OspA163–175 but not in response to the equivalent B. afzelii or B. garinii peptide [18], which has substitutions in positions 166 and 172, perhaps explaining in part why antibiotic-refractory arthritis has rarely been recognized in Europe.
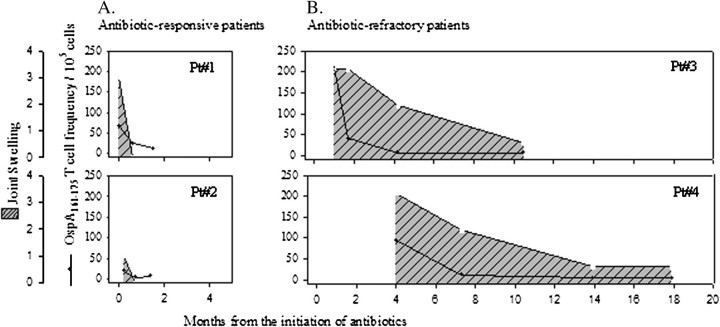
Using newly developed tetramer technology, 50%–60% of DRB1*0401-positive patients with antibiotic-refractory or antibiotic-responsive arthritis had detectable OspA161–175-specific T cells in peripheral blood specimens; these cells were concentrated in synovial fluid, and the frequencies of the cells were greater in patients with antibiotic-refractory arthritis [20]. However, in 2 patients each with antibiotic-refractory or antibiotic-responsive arthritis, the frequencies of these T cells decreased to low or undetectable levels during or soon after antibiotic therapy, which was months before the resolution of synovitis in the patients with antibiotic-refractory arthritis (Figure 2). This suggested that persistent synovitis in these patients was not perpetuated directly by OspA161–175-specfic T cells.
Figure 2.
Correlation of the duration of joint inflammation and the frequency of Borrelia burgdorferi outer-surface protein A (OspA)161–175–specific T cells in peripheral blood in serial samples from 4 patients with antibiotic-responsive Lyme arthritis (antibiotic-responsive patients) or antibiotic-refractory Lyme arthritis (antibiotic-refractory patients). The 2 patients with antibiotic-responsive arthritis were first evaluated prior to antibiotic therapy, and both responded to a 1-month course of oral doxycycline. The 2 patients with antibiotic-refractory arthritis were first seen 1 and 4 months after the start of antibiotic therapy. Patient 3 (Pt#3) was treated with a 2-month course of oral doxycycline and a 1-month course of intravenous ceftriaxone. Patient 4 (Pt#4) received a 1-month course of doxycycline, followed 3 months later by a 1-month course of intravenous ceftriaxone. The cross-hatched areas show the amount of joint swelling, and the lines with closed circles show the frequency of OspA161–175-specific T cells. In both patients with antibiotic-responsive arthritis and those with antibiotic-refractory arthritis, the frequencies of OspA161–175-specific T cells decreased during the period of antibiotic therapy, whereas joint inflammation persisted for months longer in patients with antibiotic-refractory arthritis than in patients with antibiotic-responsive arthritis. Reproduced with permission from Kannian et al [20].
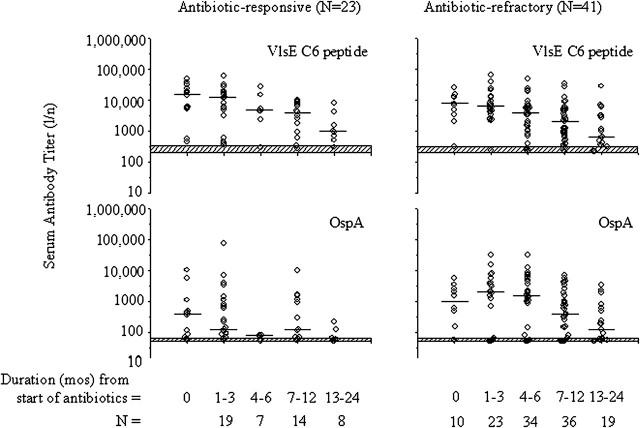
Similarly, in a recent study, ∼70% of patients with either antibiotic-responsive or antibiotic-refractory arthritis had positive antibody responses to OspA, and the values were higher in those with antibiotic-refractory arthritis [21]. However, by the conclusion of antibiotic therapy, which was given for 1–2 months longer in the refractory group, antibody titers to OspA decreased to similar levels in both the patients with antibiotic-responsive arthritis and those with antibiotic-refractory arthritis (Figure 3). Thus, consistent with the negative PCR results for B. burgdorferi DNA in joint fluid after antibiotic treatment [5, 7], the decrease in cellular and humoral responses to OspA after the conclusion of antibiotic therapy in both the antibiotic-refractory and antibiotic-responsive groups suggests that synovitis persisted after the period of infection. Moreover, neither OspA-reactive T cells nor OspA antibody would appear to be directly involved in post-infectious synovial inflammation.
Figure 3.
Serial assessment of immunoglobulin (Ig) G antibody titers to the Borrelia burgdorferi, VlsE C6 peptide and outer-surface protein A (OspA) protein in patients with antibiotic-responsive or antibiotic-refractory Lyme arthritis. The horizontal bars represent the median value in each group. The top of the hatched horizontal bars represents the cutoff value for a positive response; the bottom of the hatched horizontal bars represents the minimal dilution tested. The antibody response to the VlsE C6 peptide and to 2 other outer-surface proteins tested (data not shown) decreased similarly in both patient groups. In contrast, OspA antibody titers tended to be higher in the first 1–6 months after the start of antibiotic therapy in the antibiotic-refractory arthritis group but then decreased to similar levels in both patient groups. Mos, months. Reproduced with permission from Kannian et al [21].
SEARCHING FOR CROSS-REACTIVE T AND B CELL RESPONSES TO OSPA
After the original report of potential cross-reactivity between the OspA and hLFA-1 epitopes [15], OspA163–175-specific T cells were cloned using tetramer reagents [22], and the clones were tested for cross-reactivity with the hLFA-1 peptide [23]. When stimulated with the OspA peptide, nearly all of the clones proliferated and secreted IFN-γ and interleukin13. In contrast, LFA-1αL326–345 stimulated only ∼10% of these clones to proliferate, and they only secreted interleukin13. Thus, the LFA-1 peptide behaved as a partial agonist for only a small percentage of the OspA163-175-reactive clones, and these clones secreted only an anti-inflammatory cytokine. When the binding of these peptides was assessed in vitro using recombinant HLA-DR molecules, the HLA-DRBl*0401 molecule bound both the OspA and LFA-1 peptides, but the DRB1*0101 molecule, which is also associated with antibiotic-refractory arthritis, bound only the OspA peptide and not the LFA-1 peptide [24]. Taken together, these findings cast doubt as to whether the hLFA epitope could serve as a meaningful autoantigen in antibiotic-refractory Lyme arthritis.
In recent years, better characterization of the human proteome has permitted a more thorough search for human proteins with sequence homology with OspA163–175. In a recent search of the published human genome, the 2 peptides with the greatest sequence homology with OspA165–173 were from a protein called MAP kinase activator with WD-repeats (MAWD-BP280–288), which had identity with 8 of the 9 core amino acid residues, and from a protein called tetraspanin-7 (T-span760–68), which had identity with 6 residues [25]. In comparison, LFA-1αL332–340 and 12 other human proteins had epitopes with 5 identical residues to the OspA epitope. MAWD-BP was shown to be expressed in synoviocytes derived from a patient with rheumatoid arthritis, and LFA-1 would presumably be expressed on T cells in joints, but T-span7 was not expressed by either synoviocytes or lymphocytes [25]. Although a minority of patients had T cell–proliferative responses to 1 or more of these 3 self peptides, reactivity was always less than that with the spirochetal peptide (Figure 4). Moreover, in a search for linked T and B cell responses to these putative autoantigens, antibody responses to MAWD-BP [25] or hLFA-1 (unpublished data) were not identified.
Figure 4.
T cell reactivity with 3 self-peptides or the Borrelia burgdorferi outer-surface protein A (OspA)161–175 peptide in each of 11 patients with antibiotic-refractory Lyme arthritis. Among 11 patients, 9 had T cell–proliferative responses to the OspA peptide, 6 had reactivity with the MAP kinase activator with WD-repeats (MAWD-BP) peptide, 3 had responses to the T-span peptide, and 2 had reactivity with the LFA-1 peptide. However, reactivity with the self-peptides was less than that with the spirochetal peptide. Reproduced with permission from Drouin et al [17].
In an attempt to recapitulate relevant antigen-binding specificities in synovial lesions, Ghosh et al [26] generated recombinant antibody probes derived from B cells in patients’ lesions. Using a panel of intra-articular probes, as well as antibody fragments derived from patient peripheral blood samples, cytokeratin 10, which is cross-reactive with OspA, was identified as a target ligand. However, only 3 (20%) of 15 patients with antibiotic-refractory Lyme arthritis had slight antibody responses to this protein.
In summary, several human proteins have been identified that contain cross-reactive T or B cell epitopes with B. burgdorferi OspA. However, cross-reactive immune responses to a given human protein could only be demonstrated in a minority of patients with antibiotic-refractory arthritis, and even in these patients, the cross-reactive responses were usually weak. More importantly, 30%–40% of patients with antibiotic-refractory arthritis lacked OspA immune responses, and even in patients who had such responses, the frequency of OspA165–173-reactive T cells decreased soon after antibiotic treatment. Although it is not possible to look for a structural homologue of the OspA epitope, these findings suggest that molecular mimicry does not explain the association between OspA immunity and antibiotic-refractory Lyme arthritis.
LESSONS FROM A MOUSE MODEL OF ANTIBIOTIC-REFRACTORY LYME ARTHRITIS
Iliopoulou et al [27] recently developed a mouse model of human B. burgdorferi–induced, antibiotic-refractory arthritis. In this model, both the human HLA-DR4 transgene, which is associated with antibiotic-refractory arthritis, and lack of the CD28 co-receptor, which leads to dramatically reduced numbers of T regulatory cells (Treg) [28], were necessary for persistent synovitis after antibiotic treatment of the infection. In contrast, when the DR4-Tg was replaced with the DR11-Tg, which is associated with antibiotic-responsive arthritis, the mice did not develop post-treatment synovitis [29]. Neither the DR4-Tg nor the absence of the CD28 co-receptor, by itself, led to persistent arthritis after treatment [30, 31]. These outcomes in mice are reminiscent of the findings in human patients with antibiotic-refractory Lyme arthritis [16, 32] and are consistent with the infection-induced autoimmunity hypothesis of this illness.
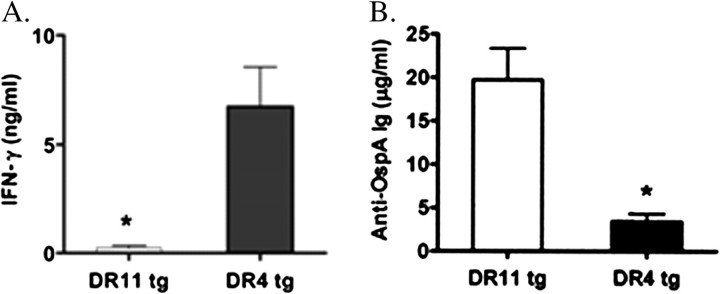
These investigators also evaluated the effects of OspA vaccination alone in this model. Without B. burgdorferi infection of joints, the mice did not develop arthritis [31]. However, T cells from draining lymph nodes from OspA-vaccinated DR4-transgenic mice (DR4-Tg) had significantly greater production of IFN-γ than DR11-Tg mice (Figure 5A). Conversely, DR11-Tg mice had higher antibody titers to OspA than did DR4-Tg mice (Figure 5B). There is a strong correlation between Lyme arthritis severity and IFN-γ in mouse models [33–36], and patients with antibiotic-refractory Lyme arthritis have significantly higher levels of IFN-γ in joint fluid than do patients with antibiotic-responsive arthritis [37].
Figure 5.
Interferon (IFN) γ production from lymph node cells and Borrelia burgdorferi outer-surface protein A (OspA) antibody titers in OspA-vaccinated mice with the DR4 or DR11 transgene lacking the CD28 co-receptor. DR11-tg and DR4-tg mice (15 mice per group) were vaccinated in the footpad (50 μg per mouse) with recombinant OspA (rOspA) in Freund's complete adjuvant. A, production of IFN-γ after in vitro restimulation of popliteal lymph node cells with rOspA (10 μg/mL) was assessed by enzyme-linked immunosorbent assay (ELISA). B, anti-OspA antibody titers were measured by ELISA 2 weeks after OspA vaccination. In both panels, mean values (± standard error of the mean) are given and include the results of 4 or 5 independent experiments. For the comparison in panel A, P = .003. For the comparison in panel B, P = .005. Reproduced with permission from Iliopoulou et al [29].
Although T cell reactivity with OspA epitopes was not examined in mice, this experience provides a possible explanation for the association between OspA165–173 and antibiotic-refractory Lyme arthritis. According to this hypothesis, reactivity with the OspA165–173 peptide, the immunodominant epitope of OspA in DRB1*0401-positive individuals, may lead to a particularly strong TH1 response. This may be one factor that results in high levels of inflammatory cytokines and chemokines in infected joints, which may help set the stage for a putative autoimmune response [38]. However, this marked pro-inflammatory response is probably only one factor in the development of antibiotic-refractory arthritis, and other disadvantageous host factors, such as low numbers of Treg [32], are also likely to be required.
EXPERIENCE WITH VACCINATION WITH OSPA AND ARTHRITIS
Consistent with the lack of joint inflammation from OspA vaccination in the murine model [31], observations in human subjects also suggested that OspA vaccination did not cause antibiotic-refractory Lyme arthritis. During the SmithKline Beecham (now GlaxoSmithKline) phase III Lyme disease trial, which had >10,936 participants, several subjects developed inflammatory arthritis, but these individuals were found in both the vaccine and placebo groups, suggesting that their arthritis was not due to OspA vaccination [13]. In a pediatric safety and immunogenicity study, 1 child had transient ankle swelling after dose 2, which resolved and did not recur after dose 3 [39]. After licensing of the OspA vaccine in December 1998, 2 patients were reported to have transient oligoarticular arthritis after vaccination [40], but this experience seemed to be similar to that noted in the vaccine trials. On the basis of post-marketing surveillance data from the Centers for Disease Control and Prevention and the US Food and Drug Administration Vaccine Adverse Reporting System, the frequency of reports of inflammatory arthritis or rheumatoid arthritis after vaccination was thought to be below the expected level of these diseases in an unvaccinated population [41]. Furthermore, an analysis of HLA-DR profiles and OspA immunity in a subgroup of these patients was not suggestive of a vaccine-induced process [42].
Because OspA vaccination followed soon after by B. burgdorferi infection led to destructive arthritis in a hamster model [43], it was also suggested that vaccination with OspA might be associated with more-severe arthritis if a person subsequently acquired B. burgdorferi infection. In the GlaxoSmithKline trial, 3 of 10,936 participants had Lyme arthritis after vaccination, but they were probably infected prior to vaccination [13]. Similarly, in the Pasteur Méurieux Connaught trial, 3 of 10,305 participants had Lyme arthritis during the follow-up period [14]. Apparently, arthritis in all of these patients responded to antibiotic therapy. In a study of 30 patients with previous Lyme disease, those with a previous history of Lyme arthritis did not have a recurrence of arthritis after vaccination [44]. Although the numbers were small, OspA vaccination in human subjects did not appear to increase the risk or severity of Lyme arthritis if a person subsequently acquired B. burgdorferi infection.
In conclusion, factors known to be associated with the development of antibiotic-refractory Lyme arthritis include more frequent infection of joints with certain inflammatory strains of B. burgdorferi [45], HLA-DR alleles that bind an epitope of OspA (OspA165–173) [16], high joint fluid levels of inflammatory cytokines and chemokines [37], and low numbers of Treg in joint fluid [32]. In addition, we hypothesize that T cell reactivity with OspA165–173 is one of several factors that may lead to a vigorous TH1 inflammatory response in infected joints, which may help to set the stage for a putative autoimmune response. This, rather than molecular mimicry, may be the reason for the association between OspA immunity and antibiotic-refractory arthritis.
Despite this association, it is clear that a vaccine-induced immune response to OspA does not replicate the sequence of events needed in B. burgdorferi–infected joints to induce antibiotic-refractory Lyme arthritis. It remains possible that a vigorous inflammatory response to OspA vaccination may uncover latent autoimmunity in genetically susceptible individuals, as might happen with other types of vaccination or with certain infections. However, if this happened with OspA vaccination, it must have been a rare event that was below the level detectable by case and control comparisons in phase III vaccine trials or in studies of patients who received the vaccine commercially.
Acknowledgments
Financial support. The National Institutes of Health (AR-20358), the English, Bonter, Mitchell Foundation, the Eshe Fund, and the Lyme/Arthritis Research Fund at Massachusetts General Hospital.
Supplement sponsorship. This article was published as part of a supplement entitled “The Need for a New Lyme Disease Vaccine,” sponsored by Baxter Laboratories, the Centers for Disease Control, Fort Collins, CO, and Stanley Plotkin.
Potential conflicts of interest. A.C.S. was the principal investigator of the phase III SmithKline Beecham Lyme disease vaccine trial during the period 1995–1997. All other authors: no conflicts.
References
- 1.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–731. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC, Levin RE, Molloy PJ, et al. Treatment of Lyme arthritis. Arthritis Rheum. 1994;37:878–888. doi: 10.1002/art.1780370616. [DOI] [PubMed] [Google Scholar]
- 4.Dattwyler RJ, Wormser GP, Rush TJ, et al. A comparison of two treatment regimens of ceftriaxone in late Lyme disease. Wien Klin Wochenschr. 2005;117:393–397. doi: 10.1007/s00508-005-0361-8. [DOI] [PubMed] [Google Scholar]
- 5.Steere AC, Angelis SM. Therapy for Lyme arthritis; strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–3085. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 6.Nocton JJ, Dressler F, Rutledge BJ, Rys PN, Persing DH, Steere AC. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid in Lyme arthritis. N Engl J Med. 1994;330:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 7.Carlson D, Hernandez J, Bloom BJ, Coburn J, Aversa JM, Steere AC. Lack of Borrelia burgdorferi DNA in synovial samples in patients with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 1999;42:2705–2709. doi: 10.1002/1529-0131(199912)42:12<2705::AID-ANR29>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 8.Steere AC, Dwyer E, Winchester R. Association of chronic Lyme arthritis with HLA-DR4 and HLA-DR2 alleles. N Engl J Med. 1990;323:219–223. doi: 10.1056/NEJM199007263230402. [DOI] [PubMed] [Google Scholar]
- 9.Kalish RA, Leong JM, Steere AC. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 1993;61:2774–2779. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lengl-Janssen B, Strauss AF, Steere AC, Kamradt T. The T helper cell response in Lyme arthritis: differential recognition of Borrelia burgdorferi outer surface protein A (OspA) in patients with treatment-resistant or treatment-responsive Lyme arthritis. J Exp Med. 1994;180:2069–2078. doi: 10.1084/jem.180.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalish RA, Leong JM, Steere AC. Early and late antibody responses to full-length and truncated constructs of outer-surface protein A of Borrelia burgdorferi in Lyme disease. Infect Immun. 1995;63:2228–2235. doi: 10.1128/iai.63.6.2228-2235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, Field JA, Glickstein L, Molloy PJ, Huber BT, Steere AC. Association of antibiotic treatment-resistant Lyme arthritis with T cell responses to dominant epitopes of outer-surface protein A (OspA) of Borrelia burgdorferi. Arthritis Rheum. 1999;42:1813–1822. doi: 10.1002/1529-0131(199909)42:9<1813::AID-ANR4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 13.Steere AC, Sikand VK, Meurice F, et al. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med. 1998;339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 14.Sigal LH, Zahradnik JM, Lavin P, et al. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. Recombinant outer-surface protein A Lyme disease vaccine study consortium. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [Erratum, N Engl J Med 1998;339:571] [DOI] [PubMed] [Google Scholar]
- 15.Gross DM, Forsthuber T, Tary-Lehman M, et al. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 16.Steere AC, Klitz W, Drouin EE, et al. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med. 2006;203:961–971. doi: 10.1084/jem.20052471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drouin EE, Glickstein L, Kwok WW, Nepom GT, Steere AC. Searching for borrelial T cell epitopes associated with antibiotic-refractory Lyme arthritis. Mol Immunol. 2008;45:2323–2332. doi: 10.1016/j.molimm.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drouin EE, Glickstein LJ, Steere AC. Molecular characterization of the OspA161-175 T cell epitope associated with treatment-resistant Lyme arthritis: differences among the three pathogenic species of Borrelia burgdorferi sensu lato. J Autoimmun. 2004;23:281–292. doi: 10.1016/j.jaut.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Ausubel LJ, O'Connor KC, Baecher-Allen C, et al. Characterization of in vivo expanded OspA-specific human T-cell clones. Clin Immunol. 2005;115:313–322. doi: 10.1016/j.clim.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Kannian P, Drouin EE, Glickstein L, Kwok WW, Nepom GT, Steere AC. Decline in the frequencies of Borrelia burgdorferi OspA161-175-specific T cells after antibiotic therapy in HLA-DRB1*0401-positive patients with antibiotic-responsive or antibiotic-refractory Lyme arthritis. J Immunol. 2007;179:6336–6342. doi: 10.4049/jimmunol.179.9.6336. [DOI] [PubMed] [Google Scholar]
- 21.Kannian P, McHugh G, Johnson BJ, Bacon RM, Glickstein LJ, Steere AC. Antibody responses to Borrelia burgdorferi in patients with antibiotic-refractory, antibiotic-responsive, or non-antibiotic-treated Lyme arthritis. Arthritis Rheum. 2007;56:4216–4225. doi: 10.1002/art.23135. [DOI] [PubMed] [Google Scholar]
- 22.Meyer AL, Trollmo C, Crawford F, et al. Direct enumeration of Borrelia-reactive CD4+ T cells ex vivo by using MHC class II tetramers. Proc Natl Acad Sci U S A. 2000;97:11433–11438. doi: 10.1073/pnas.190335897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trollmo C, Meyer AL, Steere AC, Hafler DA, Huber BT. Molecular mimicry in Lyme arthritis demonstrated at the single cell level: LFA-1α(L) is a partial agonist for outer surface protein A- reactive T cells. J Immunol. 2001;166:5286–5291. doi: 10.4049/jimmunol.166.8.5286. [DOI] [PubMed] [Google Scholar]
- 24.Steere AC, Falk B, Drouin EE, Baxter-Lowe LA, Hammer J, Nepom GT. Binding of outer surface protein A and human lymphocyte function- associated antigen 1 peptides to HLA-DR molecules associated with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 2003;48:534–540. doi: 10.1002/art.10772. [DOI] [PubMed] [Google Scholar]
- 25.Drouin EE, Glickstein L, Kwok WW, Nepom GT, Steere AC. Human homologues of a Borrelia T cell epitope associated with antibiotic-refractory Lyme arthritis. Mol Immunol. 2008;45:180–189. doi: 10.1016/j.molimm.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh S, Seward R, Costello CE, Stollar BD, Huber BT. Autoantibodies from synovial lesions in chronic, antibiotic treatment-resistant Lyme arthritis bind cytokeratin-10. J Immunol. 2006;177:2486–2494. doi: 10.4049/jimmunol.177.4.2486. [DOI] [PubMed] [Google Scholar]
- 27.Iliopoulou BP, Alroy J, Huber BT. Persistent arthritis in Borrelia burgdorferi-infected HLA-DR4-positive CD28-negative mice post-antibiotic treatment. Arthritis Rheum. 2008;58:3892–3901. doi: 10.1002/art.24028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iliopoulou BP, Guerau-De-Arellano M, Huber BT. HLA-DR alleles determine responsiveness to Borrelia burgdorferi antigens in a mouse model of self-perpetuating arthritis. Arthritis Rheum. 2009;60:3831–3840. doi: 10.1002/art.25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng S, Barthold SW, Bockenstedt LK, Zaller DM, Fikrig E. Lyme disease in human DR4Dw4-transgenic mice. J Infect Dis. 1995;172:286–289. doi: 10.1093/infdis/172.1.286. [DOI] [PubMed] [Google Scholar]
- 31.Iliopoulou BP, Alroy J, Huber BT. CD28 deficiency exacerbates joint inflammation upon Borrelia burgdorferi infection, resulting in the development of chronic Lyme arthritis. J Immunol. 2007;179:8076–8082. doi: 10.4049/jimmunol.179.12.8076. [DOI] [PubMed] [Google Scholar]
- 32.Shin S, Shin JJ, Strle K, et al. T regulatory cell numbers and function in patients with antibiotic-refractory or antibiotic-responsive Lyme arthritis. Arthritis Rheum. 2010;62:2127–2137. doi: 10.1002/art.27468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keane-Myers A, Nickell SP. T cell subset-dependent modulation of immunity to Borrelia burgdorferi in mice. J Immunol. 1995;154:1770–1776. [PubMed] [Google Scholar]
- 34.Kang I, Barthold SW, Persing DH, Bockenstedt LK. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–3111. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matyniak JE, Reiner SL. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crandall H, Dunn DM, Ma Y, et al. Gene expression profiling reveals unique pathways associated with differential severity of Lyme arthritis. J Immunol. 2006;177:7930–7942. doi: 10.4049/jimmunol.177.11.7930. [DOI] [PubMed] [Google Scholar]
- 37.Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2007;56:1325–1333. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 38.Steere AC, Glickstein L. Elucidation of Lyme arthritis. Nat Rev Immunol. 2004;4:143–152. doi: 10.1038/nri1267. [DOI] [PubMed] [Google Scholar]
- 39.Sikand VK, Halsey N, Krause PJ, et al. Safety and immunogenicity of a recombinant Borrelia burgdorferi outer surface protein A vaccine against lyme disease in healthy children and adolescents: a randomized controlled trial. Pediatrics. 2001;108:123–128. doi: 10.1542/peds.108.1.123. [DOI] [PubMed] [Google Scholar]
- 40.Rose CD, Fawcett PT, Gibney KM. Arthritis following recombinant outer surface protein A vaccination for Lyme disease. J Rheumatol. 2001;28:2555–2557. [PubMed] [Google Scholar]
- 41.Lathrop SL, Ball R, Haber P, et al. Adverse event reports following vaccination for Lyme disease: December 1998–July 2000. Vaccine. 2002;20:1603–1608. doi: 10.1016/s0264-410x(01)00500-x. [DOI] [PubMed] [Google Scholar]
- 42.Ball R, Shadomy SV, Meyer A, et al. HLA type and immune response to Borrelia burgdorferi outer surface protein a in people in whom arthritis developed after Lyme disease vaccination. Arthritis Rheum. 2009;60:1179–1186. doi: 10.1002/art.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Croke CL, Munson EL, Lovrich SD, et al. Occurrence of severe destructive Lyme arthritis in hamsters vaccinated with outer surface protein A and challenged with Borrelia burgdorferi. Infect Immun. 2000;68:658–663. doi: 10.1128/iai.68.2.658-663.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoen RT, Meurice F, Brunet CM, et al. Safety and immunogenicity of an outer surface protein A vaccine in subjects with previous Lyme disease. J Infect Dis. 1995;172:1324–1329. doi: 10.1093/infdis/172.5.1324. [DOI] [PubMed] [Google Scholar]
- 45.Jones KL, McHugh G, Glickstein L, Steere AC. Analysis of Borrelia burgdorferi genotypes in patients with Lyme arthritis: high frequency of ribosomal RNA intergenic spacer type 1 strains in antibiotic-refractory arthritis. Arthritis Rheum. 2009;60:2174–2178. doi: 10.1002/art.24812. [DOI] [PMC free article] [PubMed] [Google Scholar]