Predictors of liver fibrosis were evaluated in women using a noninvasive index (FIB-4). HIV RNA levels were associated with increased FIB-4 in the absence of viral hepatitis, alcohol use, or antiretroviral therapy. These data complement evidence suggesting a potential relationship between HIV infection and hepatic fibrosis.
Abstract
Background. FIB-4 represents a noninvasive, composite index that is a validated measure of hepatic fibrosis, which is an important indicator of liver disease. To date, there are limited data regarding hepatic fibrosis in women.
Methods. FIB-4 was evaluated in a cohort of 1227 women, and associations were evaluated in univariate and multivariate regression models among 4 groups of subjects classified by their human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infection status.
Results. The median FIB-4 scores were 0.60 in HIV-/HCV- women, 0.83 in HIV-/HCV+ women, 0.86 in HIV+/HCV- women, and 1.30 in HIV+/HCV+ women. In the HIV/HCV co-infected group, multivariate analysis showed that CD4+ cell count and albumin level were negatively associated with FIB-4 (P <.0001), whereas antiretroviral therapy (ART) was positively associated with FIB-4 score (P =.0008). For the HIV mono-infected group, multivariate analysis showed that CD4+ cell count (P <.0001) and albumin level (P =.0019) were negatively correlated with FIB-4 score, ART was positively associated with FIB-4 score (P =.0008), and plasma HIV RNA level was marginally associated with FIB-4 score (P =.080). In 72 HIV mono-infected women who were also hepatitis B surface antigen negative, ART naive, and reported no recent alcohol intake, plasma HIV RNA level was associated with increased FIB-4 score (P =.030).
Conclusions. HIV RNA level was associated with increased FIB-4 score in the absence of hepatitis B, hepatitis C, ART, or alcohol use, suggesting a potential relationship between HIV infection and hepatic fibrosis in vivo. A better understanding of the various demographic and virologic variables that contribute to hepatic fibrosis may lead to more effective treatment of HIV infection and its co-morbid conditions.
Liver biopsy is the gold standard for assessing liver disease. However, it is invasive and associated with complications, sampling error, and variability in interpretation, and it is not routinely performed in uninfected, healthy persons or in persons with human immunodeficiency virus (HIV) mono-infection. Moreover, patients may be reluctant to undergo multiple liver biopsies to accurately monitor their disease progression. Consequently, serum biochemical markers have been evaluated as alternative measures of liver damage (reviewed in [1]). For instance, the aspartate aminotransferase (AST)-to-platelet (PLT) ratio index (APRI) was derived and validated in patients with chronic hepatitis C virus (HCV) infection [2]. Sterling et al have described the FIB-4 index, which consists of alanine aminotransferase (ALT) level, AST level, PLT counts, and age, for assessing fibrosis in a large cohort of patients with HIV/HCV co-infection [3]. The FIB-4 index has also been validated as an inexpensive and accurate marker of fibrosis in the context of HCV mono-infection [4]. Using data from the AIDS Clinical Trials Group protocol A5178 (SLAM-C), our group subsequently evaluated 5 such noninvasive indices of liver injury [5]. In that validation study, the FIB-4 index performed best, with 88% specificity for cirrhosis and >86% negative predictive value for severe fibrosis when enhancement algorithms were employed. Thus, these noninvasive markers constitute an inexpensive yet accurate prediction of hepatic fibrosis and may reduce the overall need for liver biopsy in certain high-risk populations. Furthermore, a more comprehensive understanding of the demographic, virologic, and other variables that contribute to hepatic fibrosis may lead to more effective treatment of HIV infection and its associated co-morbid conditions.
To date, there are limited data regarding hepatic fibrosis in women with or without HIV infection and with or without HCV infection; therefore, we examined the factors associated with FIB-4 score in a large natural history study of HIV infection in high-risk women.
METHODS
Study Population and Calculation of FIB-4 Values
From 1993 through 2000, a prospective study of HIV infection—the HIV Epidemiologic Research (HER) Study—was conducted in a cohort of US women [6]. By study design, one-half of the subjects reported injection drug use (IDU), whereas the other half reported only sexual risk behavior. Plasma HIV RNA levels were quantified using either second- or third-generation Quantiplex branched DNA assays (Chiron). Hepatitis B virus (HBV) surface antigen (HBsAg) was evaluated with the Austria II-125 RIA (Abbott Laboratories). Among the 871 HIV-positive women, antiretroviral therapy (ART) status was categorized as none, ART consisting of 1 or 2 drugs, or highly active antiretroviral therapy (HAART). As previously reported for the cohort, 30% of women were receiving ART at baseline, none were receiving HAART, and 70% were ART naive [7]. As described elsewhere, HCV serostatus was determined by Abbott HCV enzyme immunoassay 2.0 or Ortho HCV enzyme-linked immunosorbent assay, version 3.0 [8], performed on the earliest available stored serum sample. Overall, the seroprevalence of HCV infection was 56.5%, and the seroprevalence was 48.0% among HIV-uninfected women and 60.8% among HIV-infected women [9]. For the current cross-sectional analysis, 1227 women—representing 93.6% of the entire HER Study cohort—were selected on the basis of the availability of values for AST level, ALT level, and platelet count at the baseline study visit. As originally described by Sterling et al [3], the FIB-4 value was calculated for each woman at baseline using the following formula: age [years] × AST [IU/L]/(PLT [109/L] × (ALT1/2 [IU/L]). All participants provided informed consent prior to sample and clinical data collection.
Statistical Analyses
Variables selected a priori for associations with increased FIB-4, depending on HIV/HCV infection status, were evaluated in uninfected, HIV–mono-infected, HCV–mono-infected, and HIV/HCV–co-infected women. These variables included race/ethnicity (black/white/other), ART (ever/never at baseline), creatinine level, albumin level, plasma HIV RNA level, ever injected drugs (yes/no), current injection drug use (yes/no), frequency of intercourse with men, and number of male sexual partners in the last 6 months. HBV infection status (HBsAg positive/negative) and alcohol use (yes/no in the past 6 months) were also evaluated when available. CD4+ cell count, plasma HIV RNA level, and ART (yes/no) at baseline were included for HIV–mono-infected and HIV/HCV–co-infected women. However, HCV RNA levels were not routinely determined at baseline and were therefore not further evaluated.
Following graphical inspection, baseline variables were analyzed within each of the 4 groups using univariate regression analyses. All groups and variables were then included simultaneously in a multiple regression analysis to determine (1) which factors were independently associated with FIB-4 and (2) whether these relationships varied across the 4 groups by inclusion of interaction terms between each covariate and HIV and HCV infection status variables. All P values are 2-tailed, and α =.05. All analyses were performed using SAS software (SAS Institute).
RESULTS
Patient Characteristics
The original HER Study population included a total of 1310 women, of which 27 were missing baseline HCV serostatus and were not included in subsequent analyses. Of the remaining 1283 women, age, ALT level, AST level, and platelet counts were available for 1227 women (93.7% of the original cohort) at the baseline study visit. This subset included 213 women with no evidence of HIV or HCV infection, 320 HIV–mono-infected women, 196 HCV–mono-infected women, and 498 HIV/HCV–co-infected women. Among the women evaluated, the 1st and 99th percentiles of the FIB-4 distribution were 0.3 and 5.2 (minimum and maximum values were 0.03 and 22.4). As shown in Table 1, among uninfected control subjects, the median FIB-4 score was 0.60. Using the categorical cutoff values previously established by Sterling et al [3], 206 women (96.7%) in this group had scores associated with no advanced fibrosis (FIB-4<1.45), and only 1 woman (0.5%) had scores associated with advanced fibrosis (FIB-4>3.25). Among HCV–mono-infected women, the median FIB-4 score was 0.82. In this group, 161 women (82.1%) had scores that suggested no advanced fibrosis, whereas 9 women (4.6%) had scores that suggested advanced fibrosis. Among HIV–mono-infected women, the median FIB-4 score was 0.86. A total of 277 women (86.6%) had scores associated with no advanced fibrosis, whereas 4 (1.3%) had scores associated with advanced fibrosis. The median FIB-4 score was 1.30 among HIV/HCV–co-infected women. In this group, 290 (58.2%) had scores that suggested no advanced fibrosis, whereas 43 (8.6%) had scores that suggested advanced fibrosis.
Table 1.
FIB-4 Scores and Categorical Data at Baseline for 1227 Women Enrolled in the HIV Epidemiologic Research Study
| Study group (no. of subjects) | Median FIB-4 score | FIB-4 score <1.45, no. (%) of subjects | FIB-4 score >1.45 but <3.25, no. (%) of subjects | FIB-4 score >3.25, no. (%) of subjects |
| HIV negative/HCV negative (213) | 0.60 | 206 (96.7) | 6 (2.8) | 1 (.5) |
| HIV negative/HCV positive (196) | 0.82 | 161 (82.1) | 26 (13.3) | 9 (4.6) |
| HIV positive/HCV negative (320) | 0.86 | 277 (86.6) | 39 (12.2) | 4 (1.3) |
| HIV positive/HCV positive (498) | 1.30 | 290 (58.2) | 165 (33.1) | 43 (8.6) |
NOTE. As reported by Sterling et al [3], FIB-4 scores <1.45 generally denote no advanced fibrosis, whereas FIB-4 values >3.25 may suggest advanced fibrosis. HCV, hepatitis C virus; HIV, human immunodeficiency virus.
FIB-4 in Women With No Evidence of HIV or HCV Infection
In the uninfected group, creatinine (r =.253; P =.0002) and frequency of sex with men (r =.163; P =.027) were positively associated with increased FIB-4 in univariate analysis (Table 2). Other factors, such as ever having used injection drugs, current IDU, number of male sex partners within the last 6 months, albumin level, CD4+ cell count, HBsAg positivity, and alcohol use were not associated with FIB-4.
Table 2.
Univariate Analysis of Clinical Variables Associated with FIB-4 Score by HIV and HCV Serostatus
| Variable | Uninfected (n = 213) |
HCV mono-infected (n = 196) |
HIV mono-infected (n = 320) |
HIV/HCV co-infected (n = 498) |
||||
| r | P | r | P | r | P value | r | P value | |
| Creatinine | 0.253 | .0002 | 0.076 | .291 | 0.044 | .435 | 0.002 | .970 |
| Frequency of sex with men | 0.163 | .0267 | 0.204 | .010 | 0.113 | .074 | 0.142 | .009 |
| Current IDU | −0.101 | .142 | 0.169 | .018 | 0.045 | .425 | −0.100 | .025 |
| Number of male sex partners in last 6 months | −0.046 | .531 | −0.014 | .863 | −0.132 | .035 | −.034 | .534 |
| Ever injected drugs (1 = yes, 0 = no) | −0.030 | .668 | −0.039 | .587 | 0.215 | .0001 | 0.119 | .008 |
| Albumin | 0.029 | .678 | −0.139 | .053 | −0.188 | .0007** | −0.235 | <.0001** |
| CD4+ cell count | 0.023 | .740 | −0.115 | .111 | −0.401 | <.0001** | −0.332 | <.0001** |
| HBV surface antigen positive | −0.016 | .925 | −0.122 | .155 | −0.183 | .107 | −.018 | .707 |
| Alcohol use in past 6 months | 0.073 | .291 | −0.123 | .086 | 0.115 | .039 | −.005 | .905 |
| Plasma HIV RNA level | 0.207 | .0002 | 0.177 | <.0001 | ||||
| ART | 0.202 | .0008** | 0.166 | .0008** | ||||
NOTE. Values in bold denote positive associations that were statistically significant in univariate analysis, whereas values in italics denote negative associations that were statistically significant in univariate analysis. Asterisks denote associations that were significant in multivariate analysis with P <.10 (*) and P <.05 (**). ART, antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug use.
FIB-4 in HCV Mono-infected Women
In the HCV mono-infection group, frequency of sex with men (r =.204; P =.010) and current IDU (r =.169; P =.018) were positively associated with increased FIB-4 in univariate analysis (Table 2). Number of male partners within the last 6 months, ever having used injection drugs, creatinine level, albumin level, CD4+ cell count, HBsAg positivity, and alcohol use were not associated with FIB-4.
FIB-4 in HIV–Mono-infected Women
In the HIV mono-infection group, plasma HIV RNA level (r =.207; P =.0002), receipt of ART (r =.202; P =.0008), alcohol use in the past six months (r =.115; P =.039), and ever having used injection drugs (r =.215; P =.0001) were positively associated with FIB-4 in univariate analysis (Table 2). CD4+ cell count (r = −.401; P <.0001), albumin level (r = −.188; P =.0007), and number of male partners within the last 6 months (r = −.132; P =.035) were negatively associated with FIB-4. Frequency of sex with men, current IDU, HBsAg positivity, and creatinine level were not associated with FIB-4. The associations between FIB-4 and albumin level, CD4+ cell count, and plasma HIV RNA level were further confirmed among the 152 HIV–mono-infected women who were also ART naive (data not shown). In multivariate analysis, CD4+ cell count (P <.0001) and albumin level (P =.002) remained negatively correlated with FIB-4, whereas plasma HIV RNA level was marginally associated with increased FIB-4 (P =.080).
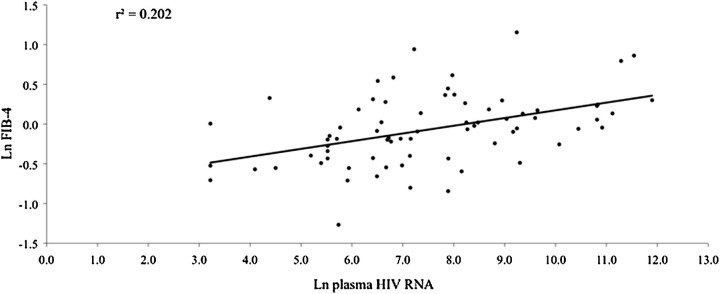
It has been noted previously that 2 of the strongest predictors of liver enzyme elevations—which are key components of the FIB-4 index—are HBV and HCV infections [10]—which suggests that they could mask other underlying factors that lead to increased FIB-4 scores. Therefore, we further examined additional predictors of FIB-4 in a subset of 72 HIV–mono-infected women who were HBsAg negative, ART naive, and reported no recent alcohol intake. The median FIB-4 score was 0.93. Similar to results in the larger HIV–mono-infected and HIV/HCV–co-infected groups, plasma HIV RNA level (r2 =.202; P =.030) was associated with increased FIB-4, as shown in Figure 1. In this subset of HIV–mono-infected women, ever having used injection drugs (r =.345, P =.006) was positively associated with increased FIB-4, whereas CD4+ cell count (r = −.557, P <.0001) was negatively associated with FIB-4.
Figure 1.
Plasma HIV RNA is positively associated with increased FIB-4 among 72 HIV–mono-infected (HBsAg negative, HCV seronegative) ART-naive women who reported no alcohol use during the past 6 months. ART, antiretroviral therapy; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
FIB-4 in HIV/HCV–Co-infected Women
In the HIV/HCV co-infection group, plasma HIV RNA level was once again positively associated (r =.177; P <.001) with FIB-4 in univariate analysis (Table 2). Also positively associated with FIB-4 in this group were the frequency of sex with men (r =.142; P =.0086), receipt of ART (r =.166; P =.0008), and ever having used injection drugs (r =.119; P =.008). Several factors were negatively associated with FIB-4 in this group, including CD4+ cell count (r = −.332; P <.0001), albumin level (r = −.235; P <.0001), and current IDU (r = −.100; P =.025). Number of male sex partners in the last 6 months, HBsAg positivity, alcohol use in the past 6 months, and creatinine level were not associated with FIB-4. The associations between FIB-4 and albumin level, CD4+ cell count, and plasma HIV RNA level were further confirmed among the 264 HIV/HCV–co-infected women who were also ART naive (data not shown). Upon multivariate analysis, CD4+ cell count and albumin level remained negatively associated with FIB-4 (P <.0001) among HIV/HCV–co-infected women, whereas plasma HIV RNA level was no longer significant (P =.245).
There were 43 HIV/HCV–co-infected women with FIB-4 >3.25, indicative of advanced fibrosis. In this group, FIB-4 was negatively associated with albumin level and CD4+ cell count but was positively associated with plasma HIV RNA level (data not shown), in general agreement with the data presented in Table 2.
Effects of Thrombocytopenia
Thrombocytopenia is a common finding among HIV-infected patients, and a relationship among platelet count, HIV RNA levels, and disease progression has been reported [11]. Therefore, we also assessed whether increased FIB-4 scores in persons with HIV infection were due solely to the impact of HIV infection on platelet counts. The median platelet count for the entire HERS cohort was 2.35 × 105 platelets/μL. Median platelet values in each subgroup were as follows: 2.79 × 105 platelets/μL in healthy control subjects, 2.33 × 105 platelets/μL in HIV–mono-infected women, 2.57 × 105 platelets/μL in HCV mono-infected women, and 2.10 × 105 platelets/μL in HIV/HCV–co-infected women. There were 90 (18.1%) HIV/HCV–co-infected women, 35 (10.9%) HIV–mono-infected women, 12 (6.1%) HCV–mono-infected women, and 6 (2.8%) uninfected women with platelet counts <150,000 platelets/μL. To determine the relative contribution of platelet count to the overall FIB-4 score, the actual platelet count for each woman was replaced with the median platelet value of 2.35 × 105 platelets/μL, and the modified FIB-4 (FIB-4-PL) score was re-evaluated. FIB-4-PL values were as follows: 0.73 in healthy control subjects, 0.83 in HIV–mono-infected women, 0.93 in HCV–mono-infected women, and 1.14 in HIV/HCV–co-infected women. The main effects of HIV and HCV infection remained significant (P <.001), with no significant interaction.
DISCUSSION
Liver disease has now surpassed AIDS-defining illnesses as a major cause of morbidity and mortality in several HIV-positive cohorts [12, 13]. Thus, there is a growing need to assess liver disease in this population. Nonetheless, liver biopsies are generally not indicated in patients with HIV/AIDS, because biopsies rarely provide information that influences HIV therapy, and they are not recommended for routine evaluation of abnormal hepatic function test results in the absence of viral hepatitis. Thus, it has been somewhat difficult to directly assess the effects of HIV infection on the liver. HIV infection is associated with a number of hepatic and biliary tract disorders, hepatomegaly, hepatic steatosis, and elevated serum liver enzyme levels [14–17]. Even HIV-positive patients with no evidence of viral hepatitis co-infection exhibit mild-to-moderate increases in liver enzyme levels [18, 19]. These findings are not unexpected, given that several lines of evidence suggest that HIV may directly interact with multiple liver cell types in vivo (reviewed in [20]). For instance, HIV RNA and proviral DNA have been detected in liver biopsy specimens from persons with HIV infection [21–23], and subsequent studies have demonstrated HIV p24 protein and HIV RNA in Kupffer cells, inflammatory mononuclear cells, sinusoidal cells, and hepatocytes [21, 24, 25]. Moreover, expression of HIV entry receptors has been demonstrated in hepatocytes [26–30]. We [31, 32] and others [27, 33, 34] have demonstrated HIV infection of hepatocyte-derived cell lines, as well as primary hepatocytes in vitro. However, the precise mechanisms by which HIV infects and replicates within hepatocytes have not been elucidated. Thus, the effects of soluble HIV proteins and HIV infection on hepatocyte cell function and HCV replication are clearly areas of research that merit additional investigation.
In the current study, plasma HIV RNA level was associated with increased FIB-4 in HIV–mono-infected women in the absence of ART or alcohol use. This finding is supported by additional studies in which HIV load was positively associated with severe fibrosis or increased ALT and/or AST levels [19, 35, 36]. This suggests a possible effect of HIV load on liver enzyme elevations and/or hepatic fibrosis, although additional longitudinal and in vitro studies are necessary to establish causality. Additionally, Kovari et al [37] recently reported that chronic ALT elevations in HIV-infected persons without HBV or HCV co-infection were associated with HIV RNA levels >100,000 copies/mL. There are limited data to suggest that HIV suppression may be associated with decreased rates of hepatic fibrosis, supporting our hypothesis that HIV infection is an independent predictor of liver scarring. For example, Brau et al [38] reported that HIV suppression was associated with less fibrosis in HIV/HCV–co-infected individuals, compared with those individuals without viral suppression. Moreover, use of another noninvasive marker technique, transient elastography, also suggests a strong relationship between the rate of fibrotic progression and ART-associated HIV control [39]. At a cellular level, such findings may be explained, in part, by the interaction of HIV envelope proteins with hepatic stellate cells and stimulation of collagen production [40].
Additional variables associated with FIB-4 in HIV–mono-infected and HIV/HCV–co-infected women included albumin level, CD4+ cell count, and receipt of ART. It is not surprising that albumin levels were negatively associated with FIB-4, given that albumin level is an important indicator of liver dysfunction, and albumin synthesis correlates with the severity of liver disease [41]. Additionally, others have reported that higher CD4+ cell counts and/or HIV suppression were associated with lower ALT/AST levels or noninvasive markers of liver damage [10, 35, 37, 42], although these associations have not been observed in all studies [43]. In HIV treatment–naive patients, a positive correlation between AST (or ALT) levels and HIV load has been reported [19]. Similarly, in HIV positive patients without HBV or HCV infection, chronic elevated ALT levels were associated with high HIV RNA levels [37].
Interestingly, factors such as chronic HBV infection and alcohol use, which are known to promote liver disease, were not associated with increased FIB-4 scores in any subgroup analyzed. For HBV, this finding likely reflects the low prevalence of HBsAg positivity in this cohort, as well as different cut-off values for predicting severe fibrosis and cirrhosis in patients with chronic HBV infection [44], compared with those with HCV infection. For alcohol, it is possible that lifetime alcohol use is a more accurate predictor of liver disease than recent use. Moreover, whereas alcohol and HCV may have a synergistic effect on liver disease, the effect of alcohol may less pronounced in persons with HIV/HCV co-infection, as reported recently [45]. However, a more detailed assessment of alcohol consumption over time in the HER Study may provide additional data on the complex interactions between alcohol, viral hepatitis, HIV infection, and liver disease in women.
Our study design has several distinct advantages over previous analyses. First, the observational nature of the HER Study cohort permits the direct comparison of noninvasive indices of liver fibrosis in HIV–mono-infected, HCV–mono-infected, HIV/HCV–co-infected, and uninfected women from the same cohort with similar risk factors. Moreover, women represent an understudied population with respect to HIV infection, as well as liver disease. Second, at the onset of the HER Study, only 30% of the HIV-positive women were receiving ART, and none were receiving HAART; thus, the contribution of ART to FIB-4 values could be assessed. Third, because of the historical nature of the cohort, prior treatment with interferon-based regimens for HCV infection was quite rare and was therefore highly unlikely to impact liver fibrosis in any appreciable manner. Nonetheless, limitations to our study design should also be noted, including its cross-sectional nature and the use of HCV antibody detection to denote HCV infection, because HCV RNA levels were not assessed at all time points and were therefore not evaluated. It should also be emphasized that the HER Study was conducted at the beginning of the HAART era; thus, the majority of study visits occurred among women who were either ART naive or receiving less than optimally suppressive ART regimens [7]. Newer ART regimens have become simpler, more potent, and better tolerated, and data have emerged suggesting increased survival with the earlier initiation of treatment [46]. Importantly, the findings in the current study may suggest an additional benefit of earlier suppression of HIV replication (ie, prevention of HIV-mediated hepatic fibrosis).
Collectively, these data suggest that advanced HIV disease is associated with more-advanced liver disease. Thus, the FIB-4 index may be widely applicable for the screening of liver fibrosis in HIV-infected populations. Longitudinal studies to determine whether highly effective HIV regimens result in decreased FIB-4 values and reduced hepatic fibrosis in vivo are clearly warranted.
Acknowledgments
We thank the HER Study staff and participants. The HER Study group consists of Robert S. Klein, MD, Ellie Schoenbaum, MD, Julia Arnsten, MD, MPH, Robert D. Burk, MD, Penelope Demas, PhD, and Andrea Howard, MD, MSc, from Montefiore Medical Center and the Albert Einstein College of Medicine; Paula Schuman, MD, Jack Sobel, MD, Suzanne Ohmit, DrPH, William Brown PhD, Michael Long PhD, Wayne Lancaster PhD, and Jose Vazquez, MD, from the Wayne State University School of Medicine; Anne Rompalo, MD, David Vlahov, PhD and David Celentano, PhD, from the Johns Hopkins University School of Medicine; Charles Carpenter, MD, Kenneth Mayer, MD, Susan Cu-Uvin, MD, Timothy Flanigan, MD, Joseph Hogan, ScD, Valerie Stone, MD, Karen Tashima, MD, and Josiah Rich, MD from the Brown University School of Medicine; Ann Duerr, MD, PhD, Lytt I. Gardner, PhD, Chad Heilig, PhD, Scott D. Holmberg, MD, Denise J. Jamieson, MD, MPH, Janet S. Moore, PhD, Ruby M. Phelps, BS, Dawn K. Smith, MD, MPH and Dora Warren, PhD from the Centers for Disease Control and Prevention; and Katherine Davenny, MPH from the National Institute of Drug Abuse.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support The Centers for Disease Control and Prevention and the National Institutes of Health (R21 AI081564 to J.T.B.).
Potential conflicts of interest. K.S. is a board member of Vertex, Bristol-Meyers Squibb, Merck, SciClone, Glaxo SmithKline, Three Rivers, J&J, and Regulus and has received funding through his institution from Roche (Genentech), Schering (Merck), Vertex, Gilead, Bristol-Meyers Squibb, SciClone, Anadys. L.T. has received grant funding through her institution from the National Institute on Drug Abuse [k23DA020383-01] and the National Institutes of Health, Lifespan/Tufts/Brown Center for AIDS Research [P30AI42853]; is a consultant for Vertex; has received payment for lectures/speaker's bureau from Genetech/Roche; and has received grant funding through her institution grant from Roche and Vertex. All other authors: no conflicts.
References
- 1.Poynard T, Morra R, Ingiliz P, et al. Biomarkers of liver fibrosis. Adv Clin Chem. 2008;46:131–160. doi: 10.1016/s0065-2423(08)00404-6. [DOI] [PubMed] [Google Scholar]
- 2.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 3.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 4.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 5.Shire NJ, Rao MB, Succop P, et al. Improving noninvasive methods of assessing liver fibrosis in patients with hepatitis C virus/human immunodeficiency virus co-infection. Clin Gastroenterol Hepatol. 2009;7:471–480. doi: 10.1016/j.cgh.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith D, Warrne D, Vlahov D, et al. Design and baseline participant characteristics of the Human Immunodeficiency Virus Epidemiology Research (HER) Study: a prospective cohort of human immunodeficiency virus infection in US women. Am J Epidemiol. 1997;146:459–469. doi: 10.1093/oxfordjournals.aje.a009299. [DOI] [PubMed] [Google Scholar]
- 7.Mayer K, Hogan J, Smith D, et al. Clinical and immunologic progression in HIV-infected US women before and after the introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;33:614–624. doi: 10.1097/00126334-200308150-00011. [DOI] [PubMed] [Google Scholar]
- 8.Stover C, Smith D, Schmid D, et al. Prevalence of and risk factors for viral infections among human immunodeficiency virus (HIV)-infected and high-risk HIV-uninfected women. J Infect Dis. 2003;187:1388–1396. doi: 10.1086/374649. [DOI] [PubMed] [Google Scholar]
- 9.Thomas D, Rich J, Schuman P, et al. Multicenter evaluation of hepatitis C RNA levels among female injection drug users. J Infect Dis. 2001;183:973–976. doi: 10.1086/319256. [DOI] [PubMed] [Google Scholar]
- 10.Sterling RK, Chiu S, Snider K, Nixon D. The prevalence and risk factors for abnormal liver enzymes in HIV-positive patients without hepatitis B or C coinfections. Dig Dis Sci. 2007;53:1375–1382. doi: 10.1007/s10620-007-9999-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torre D, Pugliese A. Platelets. and HIV-1 infection: old and new aspects. Curr HIV Res. 2008;6:411–418. doi: 10.2174/157016208785861140. [DOI] [PubMed] [Google Scholar]
- 12.Tedaldi E, Baker R, Moorman A, et al. Influence of coinfection with hepatitis C virus on morbidity and mortality due to human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2003;36:363–367. doi: 10.1086/345953. [DOI] [PubMed] [Google Scholar]
- 13.Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 14.Keaveny AP, Karasik MS. Hepatobiliary and pancreatic infections in AIDS: Part one. AIDS Patient Care STDS. 1998;12:347–357. doi: 10.1089/apc.1998.12.347. [DOI] [PubMed] [Google Scholar]
- 15.Kahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. N Engl J Med. 1998;339:33–39. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 16.Bonacini M. Hepatobiliary complications in patients with human immunodeficiency virus infection. Am J Med. 1992;92:404–411. doi: 10.1016/0002-9343(92)90271-c. [DOI] [PubMed] [Google Scholar]
- 17.Ingiliz P, Valantin MA, Duvivier C, et al. Liver damage underlying unexplained transaminase elevation in human immunodeficiency virus-1 mono-infected patients on antiretroviral therapy. Hepatology. 2009;49:436–442. doi: 10.1002/hep.22665. [DOI] [PubMed] [Google Scholar]
- 18.Sterling R, Wilson M, Sanyal A, et al. Impact of highly active antiretroviral therapy on the spectrum of liver disease in HCV-HIV coinfection. Clin Gastroenterol Hepatol. 2004;2:432–439. doi: 10.1016/s1542-3565(04)00129-6. [DOI] [PubMed] [Google Scholar]
- 19.Mata-Marin JA, Gaytan-Martinez JE, Grados-Chavarria BH, Fuentes-Allen JL, Arroyo-Anduiza CI, Alfaro-Mejia A. Correlation between HIV viral and aminotransferases as liver damage markers in HIV infected naive patients: a concordance cross-sectional study. Virol J. 2009;6:181. doi: 10.1186/1743-422X-6-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blackard JT, Sherman K. HCV/ HIV co-infection: time to re-evaluate the role of HIV in the liver? J Viral Hepat. 2008;15:323–330. doi: 10.1111/j.1365-2893.2008.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao Y, Dieterich D, Thomas P, Huang Y, Mirabile M, Ho D. Identification and quantitation of HIV-1 in the liver of patients with AIDS. AIDS. 1992;6:65–70. doi: 10.1097/00002030-199201000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Donaldson YK, Bell JE, Ironside JW, et al. Redistribution of HIV outside the lymphoid system with onset of AIDS. Lancet. 1994;343:383–385. doi: 10.1016/s0140-6736(94)91222-x. [DOI] [PubMed] [Google Scholar]
- 23.Blackard JT, Ma G, Rouster SD, Barrett A, Shata MT, Sherman K. 5th International HIV and Hepatitis Co-Infection Workshop. Lisbon, Portugal: 2009. HIV is frequently detected in liver biopsy tissue. [Google Scholar]
- 24.Housset C, Lamas E, Brechot C. Detection of HIV1 RNA and p24 antigen in HIV-1-infected human liver. Res Virol. 1990;141:153–159. doi: 10.1016/0923-2516(90)90017-d. [DOI] [PubMed] [Google Scholar]
- 25.Housset C, Lamas E, Courgnaud V, et al. Presence of HIV-1 in human parenchymal and non-parenchymal liver cells in vivo. J Hepatol. 1993;19:252–256. doi: 10.1016/s0168-8278(05)80579-3. [DOI] [PubMed] [Google Scholar]
- 26.Cao Y, Friedman-Kein A, Huang Y, et al. CD4-independent, productive human immunodeficiency virus type 1 infection of hepatoma cell lines in vitro. J Virol. 1990;64:2553–2559. doi: 10.1128/jvi.64.6.2553-2559.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee R, Sperber K, Pizzella T, Mayer L. Inhibition of HIV-1 productive infection in hepatoblastoma HepG2 cells by recombinant tumor necrosis factor-a. AIDS. 1992;6:1127–1131. doi: 10.1097/00002030-199210000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Munshi N, Balasubramanian A, Koziel M, Ganju R, Groopman J. Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. J Infect Dis. 2003;188:1192–1204. doi: 10.1086/378643. [DOI] [PubMed] [Google Scholar]
- 29.Balasubramanian A, Ganju R, Groopman J. HCV and HIV envelope proteins collaboratively mediate IL-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J Biol Chem. 2003;278:35755–35766. doi: 10.1074/jbc.M302889200. [DOI] [PubMed] [Google Scholar]
- 30.Vlahakis S, Villasis-Keever A, Gomez T, Bren G, Paya C. Human immunodeficiency virus-induced apoptosis of human hepatocytes via CXCR4. J Infect Dis. 2003;188:1455–1460. doi: 10.1086/379738. [DOI] [PubMed] [Google Scholar]
- 31.Cardona Maya M, Moreno M, Sherman KE, Chougnet C, Nelson JAE, Blackard J. HIV infection of hepatocytes. In: XVII International AIDS Conference. Mexico City: 2008. [Google Scholar]
- 32.Moreno M, Cardona Maya W, et al. 13th International Symposium on Viral Hepatitis and Liver Disease. Washington, DC: 2009. HIV infection of an HCV-producing hepatocyte cell line – a model system for exploring HIV-HCV co-infection in vitro. [Google Scholar]
- 33.Iser DM, Warner N, Revill PA, et al. Coinfection of hepatic cell lines with human immunodeficiency virus and hepatitis B virus leads to an increase in intracellular hepatitis B surface antigen. J Virol. 2010;84:5860–5867. doi: 10.1128/JVI.02594-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao P, Usami O, Suzuki Y, et al. Characterization of a CD4-independent clinical HIV-1 that can efficiently infect human hepatocytes through chemokine (C-X-C motif) receptor 4. AIDS. 2008;22:1749–1757. doi: 10.1097/QAD.0b013e328308937c. [DOI] [PubMed] [Google Scholar]
- 35.Vodkin I, Aouizerat B, Deeks S, Martin J, Peters M. 17th Conference on Retroviruses and Opportunistic Infections. San Francisco, CA: 2010. Factors associated with liver disease severity in a cohort of patients with HIV. [Google Scholar]
- 36.Rhee MS, Sterling RK, McGovern B, Knox TA, Terrin N, Forrester J. American Association for the Study of Liver Diseases. San Francisco, CA: 2008. The effect of HIV RNA on the severity of liver disease in HIV-infected hispanics. [Google Scholar]
- 37.Kovari H, Ledergerber B, Battegay M, et al. Incidence and risk factors for chronic elevation of alanine aminotransferase levels in HIV-infected persons without hepatitis b or c virus co-infection. Clin Infect Dis. 2010;50:502–511. doi: 10.1086/649922. [DOI] [PubMed] [Google Scholar]
- 38.Bräu N, Salvatore M, Ríos-Bedoya CF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Grünhage F, Wasmuth JC, Herkenrath S, et al. Transient elastography discloses identical distribution of liver fibrosis in chronic hepatitis C between HIV-negative and HIV-positive patients on HAART. Eur J Med Res. 2010;15:139–144. doi: 10.1186/2047-783X-15-4-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruno R, Galastri S, Sacchi P, et al. gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Gut. 2010;59:513–520. doi: 10.1136/gut.2008.163287. [DOI] [PubMed] [Google Scholar]
- 41.Leber B, Mayrhauser U, Rybczynski M, Stadlbauer V. Innate immune dysfunction in acute and chronic liver disease. Wiener klinische Wochenschrift. 2009;121:732–744. doi: 10.1007/s00508-009-1288-2. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhry AA, Sulkowski MS, Chander G, Moore R. Hazardous drinking is associated with an elevated aspartate aminotransferase to platelet ratio index in an urban HIV-infected clinical cohort. HIV Med. 2009;10:133–142. doi: 10.1111/j.1468-1293.2008.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Lédinghen V, Barreiro P, Foucher J, et al. Liver fibrosis on account of chronic hepatitis C is more severe in HIV-positive than HIV-negative patients despite antiretroviral therapy. J Viral Hepat. 2008;15:427–433. doi: 10.1111/j.1365-2893.2007.00962.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim BK, Han KH, Park JY, et al. External validation of P2/MS and comparison with other simple noninvasive indices for predicting liver fibrosis in HBV-infected patients. Dig Dis Sci. 2010;55:2636–2643. doi: 10.1007/s10620-009-1070-3. [DOI] [PubMed] [Google Scholar]
- 45.Fuster D, Sanvisens A, Martinez E, et al. 5th International Workshop on HIV & Hepatitis Co-infection. Lisbon, Portugal: 2009. Predictors of increasing FIB-4 values in HCV monoinfected and HCV/HIV coinfected patients. [Google Scholar]
- 46.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]