Abstract
Background. Although essential to guide control measures, published estimates of influenza-related seasonal mortality for low- and middle-income countries are few. We aimed to compare influenza-related mortality among individuals aged ⩾65 years in South Africa and the United States.
Methods. We estimated influenza-related excess mortality due to all causes, pneumonia and influenza, and other influenza-associated diagnoses from monthly age-specific mortality data for 1998–2005 using a Serfling regression model. We controlled for between-country differences in population age structure and nondemographic factors (baseline mortality and coding practices) by generating age-standardized estimates and by estimating the percentage excess mortality attributable to influenza.
Results. Age-standardized excess mortality rates were higher in South Africa than in the United States: 545 versus 133 deaths per 100,000 population for all causes (P < .001) and 63 vs 21 deaths per 100,000 population for pneumonia and influenza (P=.03). Standardization for nondemographic factors decreased but did not eliminate between-country differences; for example, the mean percentage of winter deaths attributable to influenza was 16% in South Africa and 6% in the United States (P < .001). For all respiratory causes, cerebrovascular disease, and diabetes, age-standardized excess death rates were 4—8-fold greater in South Africa than in the United States, and the percentage increase in winter deaths attributable to influenza was 2—4-fold higher.
Conclusions. These data suggest that the impact of seasonal influenza on mortality among elderly individuals may be substantially higher in an African setting, compared with in the United States, and highlight the potential for influenza vaccination programs to decrease mortality.
Influenza infection is associated with substantial global mortality each winter season, with the greatest burden experienced in individuals aged ⩾65 years [1]. The incidence of influenza-related mortality in elderly individuals ranges from 80–150 deaths per 100,000 population for all causes and 16–22 deaths per 100,000 population for pneumonia and influenza in the United States and Europe [2–4]. Studies from high-income tropical locations, such as Hong Kong and Singapore, have found rates of excess deaths similar to those in temperate northern hemisphere countries [5, 6]. Mortality is greatest in seasons where the more severe influenza virus subtype A/H3N2 predominates, compared with the milder influenza A/H1N1 or B viruses [2].
Influenza diagnoses are generally not confirmed in the laboratory, and influenza-related deaths may be attributed to other comorbid conditions or secondary complications of infection [7]. Consequently, influenza burden estimates are generated from indirect statistical methods applied to broad disease outcomes and are quantified as the seasonal increase in mortality above a baseline of expected mortality [3, 8–10]. Seasonal increases associated with influenza virus circulation have been demonstrated for a number of mortality outcomes, including all-cause mortality, pneumonia and influenza, diabetes, cerebrovascular disease, and ischemic heart disease [3, 5, 6, 9]. By contrast, cancer deaths show no clear seasonality [9].
It has been suggested that the mortality burden associated with the 1918 influenza pandemic was greatest in low-income countries [11, 12]. Possible contributing factors include high baseline mortality rate, poor nutritional status, high prevalence of comorbid conditions, young age distribution of population, poverty, and poor access to health care [12]. While there are numerous published studies evaluating seasonal influenza-related mortality in more-affluent northern hemisphere and tropical countries [3, 4, 13], only 2 studies provide information on mortality associated with influenza in Africa, and they are geographically and temporally limited [14, 15].
Estimates of seasonal influenza mortality burden are useful to identify groups at elevated risk of mortality and to identify geographical high-risk areas. The perception that influenza does not cause substantial mortality in Africa may contribute to the underutilization of influenza vaccines, antivirals, and other control measures in this setting [16]. In addition, it is important to obtain baseline seasonal estimates of mortality burden, to allow comparison with the impact of unusual events, such as the occurrence of the novel influenza A/H1N1 2009 pandemic.
Here, we aimed to compare the influenza mortality burden between South Africa and the United States, using multiple years of data, 1998–2005, and focusing on individuals aged ⩾65 years.
Methods
Mortality data. Data on underlying causes of death for individuals aged ⩾65 years were obtained from Statistics South Africa [17–19] from 1998-2005 and from the US National Center for Health Statistics from 1997–2005 [20]. Data included 4 age groups (65–69 years, 70–74 years, 75–79 years, and ⩾80 years), month and year of death, and underlying causes of death. For South Africa, it is estimated that, from 1998 to 2005, the completeness of reporting of deaths increased from 73% to 90% for male individuals and from 63% to 88% for female individuals [18]. Causes of death were manually coded and were verified by trained coders, and coding practices were consistent over the study period [17–19]. Data were not adjusted to account for underreporting and thus represent a minimum estimate of mortality.
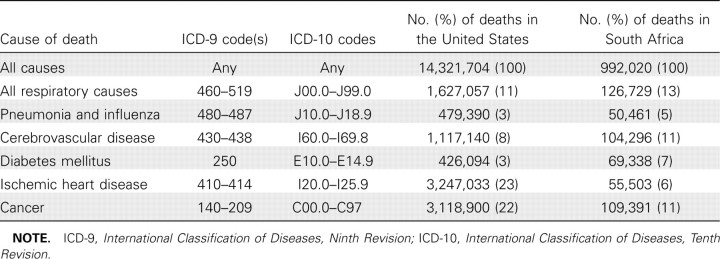
We used the International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, Tenth Revision (ICD-10) to compile monthly mortality time series for pneumonia and influenza, all respiratory diseases, diabetes, ischemic heart disease, cerebrovascular disease, cancer, and all causes (Table 1). For the United States, we adjusted for the conversion from ICD-9 to ICD-10 in 1999, by multiplying the reported number of pneumonia and influenza deaths in 1998 by published comparability ratios [21].
Table 1.
Comparison of Causes of Death in Persons Aged ⩾65 Years in South Africa and the United States, Based on All Deaths Occurring during 1998–2005
Population denominators. We obtained annual population estimates in individuals aged ⩾65 years for South Africa and the United States from Statistics South Africa [22] and the US Census Bureau [23], respectively. For South Africa in 1998–1999, age-specific denominators were unavailable and were estimated by linear interpolation from data available from 2000 onward.
Influenza viral isolation data. The climate in South Africa ranges from temperate to subtropical, and influenza virus circulation is seasonal [24]. Data on the number, timing, and subtype of influenza virus isolates in South Africa were obtained from laboratory-based sentinel surveillance for influenza-like illness [24]. Total annual numbers of influenza virus isolates varied markedly between study years, because of changes in the surveillance methodology used over the study period, and do not represent the true influenza burden. To confirmthe validity of the mortality data as an indicator of influenza activity in South Africa, we compared the timing of peak viral isolation data with the timing of peak influenza mortality, as in past research [9, 25]. We considered an influenza subtype (A/H3N2, A/H1N1, or B) to be dominant during the influenza season when it accounted for at least 50% of circulating viruses.
Estimation of influenza-related excess mortality. Influenza related excess mortality was estimated for each of the 8 influenza seasons and 4 age groups for South Africa (winters 1998–2005) and the United States (winters 1997/1998 through 2004/2005). To model the expected level of mortality in the absence of influenza virus activity, we used a Serfling-type linear regression approach, which includes harmonic terms to model seasonality and terms for time trends [2, 26]. We first identified influenza epidemic periods by applying the model to influenza specific deaths for all ages, excluding winter months (December—March in the United States and May—August in South Africa) and by estimating the expected mortality in the absence of influenza circulation. We defined “epidemic months” as the months for which reported influenza-specific deaths exceeded the upper 95% confidence limit of that predicted by the model (the “epidemic threshold”), separately for each winter. We then applied the seasonal regression model to monthly mortality rates for broader death categories and specific age groups, after standardizing for month length and excluding data from winter months. Rates of monthly excess mortality were calculated as the observed minus the predicted mortality rate for all “epidemic months.” Seasonal excess mortality was estimated as the sum of the monthly excess mortality after adjustment for true month length. All model terms included were statistically significant (P < .001), but additional terms were not (P > .05). Overall, excess mortality regression models fitted the South African data for persons aged ⩾65 years well, especially for pneumonia and influenza (R2=0.82). For other influenzaassociated causes of death (all causes, all respiratory causes, diabetes, ischemic heart disease, and cerebrovascular disease), R2 ranged from 0.68 to 0.77.
To control for differences in the population age structure between the United States and South Africa, we adjusted estimates for each 5-year age band to the US population in 2000. Nondemographic differences between countries, such as access to health care, socioeconomic factors, and coding practices (for cause-specific deaths), may also affect baseline and influenza-related mortality rates and may bias geographical comparisons. To adjust for differences in baseline mortality, we calculated 2 measures of the proportion of deaths attributable to influenza, estimated as the excess deaths divided by the model-predicted baseline of deaths in summer and winter, respectively, as used in past research [26, 27]. We used negative binomial regression models to compare annual excess mortality rates between countries and used logistic regression to compare percentage excess deaths.
Ethics. Ethical approval for the study was obtained from the University of the Witwatersrand Human Research Ethics Committee (Medical).
Results
Description of mortality data in South Africa and the United States. In South Africa, there were 3,831,176 deaths reported during the study period, and age data were available for 3,808,121 (99%). Of deaths, 992,020 (26%) occurred among individuals aged ⩾65 years; the annual number of reported deaths in elderly individuals increased by 18% during the study period. The proportion of deaths coded as pneumonia and influenza, all respiratory causes, cerebrovascular disease, and diabetes was similar between South Africa and the United States, but a larger proportion of deaths among elderly individuals in the United States were due to ischemic heart disease and cancer (Table 1).
Timing of viral activity and mortality in South Africa. In South Africa, laboratory-based sentinel surveillance demonstrates that influenza causes seasonal outbreaks during May—August (Figure 1) [24]. During the study period, timing of peak viral activity coincided with peak mortality for pneumonia and influenza and all causes in 6 of 8 years (0-month difference), and there was a 1-month delay in peak mortality in 2 years (1998 and 2000 for pneumonia and influenza; 2000 and 2004 for all causes). For comparison, influenza A/H3N2 was the predominant subtype in 4 of 8 seasons in South Africa (Figure 1) and 6 of 8 seasons in the United States (1997/1998, 1998/1999, 1999/2000, 2001/2002, 2003/2004, and 2004/2005) [28].
Figure 1.

Observed and predicted (baseline) deaths in persons aged ⩾65 years, 1998–2005, South Africa. A, Reported influenza deaths, predicted deaths, epidemic threshold (upper 95% confidence interval of predicted deaths), and number of influenza viral isolations (all ages) by month. B, Deaths due to pneumonia and influenza (P&I). C, Deaths due to all causes. Note that the number of deaths predicted is under the assumption that influenza viruses were not circulating.
The mean rate of excess deaths due to pneumonia and influenza and all causes in seasons where influenza A/H3N2 predominated was elevated, compared with seasons when influenza A/H1N1 or B predominated, for all diagnoses evaluated; however, this difference was not statistically significant (data not shown), because of the small number of observations available for comparison (Table 2).
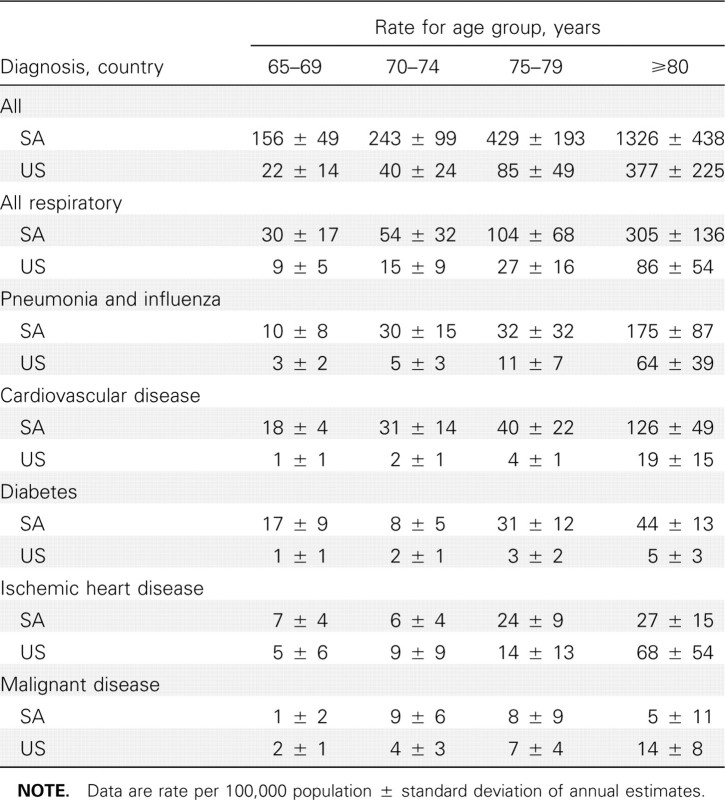
Comparison of influenza-related excess death rates in South Africa and the United States. Rates of excess mortality due to pneumonia and influenza and all causes in elderly individuals were higher in South Africa than in the United States (in South Africa, 42 and 340 deaths per 100,000 population, respectively; in the United States, 22 and 112 deaths per 100,000 population, respectively; P=.08 for pneumonia and influenza; P < .001 for all causes) (Table 3). For all diagnoses evaluated except cancer, the estimated influenza-associated excess mortality increased with age, and the age-specific influenza-related excessmortality rates were higher in South Africa than in the United States (Table 4).
Table 3.
Comparison of Mean Seasonal Estimates of Influenza-Related Excess Mortality in Individuals Aged ⩾65 Years in South Africa (SA) for 1998–2005 and in the United States (US) for the 1997/1998 to 2004/2005 Seasons
Table 4.
Age-Specific Estimates of Influenza-Related Excess Mortality Rates per 100,000 Population Aged ⩾65 Years in South Africa (SA) and the United States (US)
After standardization for population age structure, the differences in the rates of excess pneumonia and influenza and all-cause mortality per 100,000 population increased between the 2 countries (P=.03 for pneumonia and influenza; P= .01 for all-cause mortality; Table 3). After standardization for coding practices and baseline mortality, the differences in influenza-related excess mortality were decreased, but South African mortality estimates remained significantly higher. In addition, the proportion of all winter deaths attributable to influenza was higher in South Africa than in the United States on average (16% vs 6%; P < .001) (Table 3). Similarly, the percentage of all deaths attributable to influenza standardized by the summer baseline was higher in South Africa (19% vs 8%; P < .001) (Table 3).
Rates of excess deaths due to all respiratory causes, cerebrovascular disease, and diabetes were generally greater in South Africa than in the United States, and differences remained when rates were standardized for demographic characteristics, deathcoding practices, and baseline mortality differences (Table 3). Excess mortality rates for ischemic heart disease were not significantly different between the United States and South Africa. In both countries, deaths due to cancer had little seasonal variation that could be attributed to influenza.
Discussion
Our study is the first nationally representative study fromAfrica to estimate seasonal influenza-associated excess mortality. Overall, rates of seasonal influenza-related excess mortality among adults aged ⩾65 years in South Africa were substantially greater than those observed in the United States. These patterns were consistent for a variety of death outcomes that have been used in the past as indicators of influenza disease burden, including all causes, pneumonia and influenza, all respiratory diseases, cerebrovascular disease, and diabetes [3, 5, 9]. Standardization for demographic characteristics, as well as proxies of death-coding practices and baseline health, accounted for some but not all of these differences. These data support increased efforts for control of seasonal influenza in elderly individuals in South Africa and other low- and middle-income countries, where the excess seasonal mortality burden could be greater than previously thought.
Studies from several northern hemisphere, temperate countries, as well as from urbanized tropical areas such as Hong Kong and Singapore, have yielded estimates of influenza-related excess mortality in elderly persons remarkably similar to those from the United States [2–6, 29, 30]. By contrast, unusually severe outbreaks of influenza A/H3N2 occurred in Madagascar and the Democratic Republic of Congo in 2002, suggesting that populations in African countries may experience increased risk of severe outcomes following influenza infection [15, 31]. Casefatality ratios for these outbreaks were high, possibly because of limited access to medical care, high prevalence of underlying illness and malnutrition, and crowded living conditions. Remote areas with less frequent influenza virus activity could experience higher influenza-related mortality [15, 31], although serological studies suggest that rural African populations may be exposed to influenza each season [32, 33]. While there are several published reports describing influenza circulation from various locales in Africa, surveillance remains limited, especially in rural areas; thus, the epidemiology and seasonality of interpandemic influenza virus activity remain unclear in this region [34, 35].
A previous study evaluated influenza-associated excess mortality in elderly individuals occurring at a single South African hospital in 1997–1999 [14] and found lower excess mortality rates than ours (82–221 deaths per 100,000 population aged ⩾65 years). Given that only 50% of deaths occur in the hospital, this study may have substantially underestimated the influenza mortality burden [14, 19]. In addition, the hospital study covered only a short period and was conducted in an urban community with relatively good access to health care, limiting the generalizability of the findings [14].
Our study had several potential limitations. Some deaths in South Africa were not registered [19]; however, reporting improved during the study period, and under-reporting is unlikely to vary with season. Approximately 14% of deaths in South Africa were nonspecifically coded, which is higher than in the United States (0.8%–1.2%). If anything, this difference should lead to underestimation of the excess mortality in South Africa, especially for disease-specific mortality indicators. Standardization for baseline should account for differences in coding practices or baseline mortality between settings and did not eliminate differences in the excess death rate between countries. We also observed a slight increase in the proportion of deaths not specifically coded in winter in South Africa (⩽1%). However, given that the seasonal component explained only 9% of the variance in nonspecifically coded deaths (P=.01), we did not adjust further for this factor. We also note that our analyses of all-cause mortality are not prone to biases in the proportion of nonspecific codes.
All-cause excess mortality is less likely to be subject to differences in coding practices, and the most pronounced and consistent differences between South Africa and the United States were for this mortality outcome. We also found similar between-country differences for other diagnoses, such as pneumonia and influenza, all respiratory deaths, cardiovascular disease, and diabetes. Although not significant, because of small sample size, we found elevated excess mortality in seasons when influenza A/H3N2 viruses predominated in South Africa, as has been seen in previous studies [2]. These patterns support the hypothesis that excess deaths were due to influenza and not to other causes, such as temperature variation. Moreover, the observation of elevated mortality in South Africa is robust because our study period saw fewer influenza A/H3N2-dominated seasons in South Africa (4 of 8 seasons), compared with the United States (6 of 8 seasons) [2]. Overall, our data suggest that South African populations experience risk factors similar to those of other countries, including older age, and that the impact of influenza is worse in seasons dominated by the A/H3N2 subtype.
This study highlights that seasonal influenza-related excess mortality in Africa is likely greater than that observed in wealthier countries. Possible contributing factors may include socioeconomic differences, as well as variability in the contributing role of cocirculating viral and bacterial respiratory pathogens. Studies have suggested that much of the morbidity and mortality associated with influenza infection may be due to secondary bacterial infection, particularly Streptococcus pneumoniae [36, 37]. In addition, the relative contribution of other respiratory viruses, such as respiratory syncytial virus, to excess mortality in elderly individuals has been debated in the literature. The type of modeling we have used in this study is thought to account for the relatively constant year-to-year mortality from respiratory syncytial virus in the baseline [3, 38]. Our approach, however, cannot evaluate the relative contribution of secondary bacterial infections to influenza mortality in the United States and South Africa, and this should be explored in further studies.
The observed differences in excess mortality are not explained by differences in vaccination coverage between US (∼65%) and South African (15% of insured population in one study) elderly individuals, because influenza-associated mortality in US elderly individuals has remained constant since the 1980s, despite increased vaccination coverage [2, 39]. Human immunodeficiency virus (HIV)/AIDS comorbidity is also probably not a contributing factor in persons aged ⩾65 years, unless high HIV prevalence in younger populations is associated with increased intensity of influenza transmission in the community. It has been suggested that pandemic influenza-related mortality may be greater in lower socioeconomic settings [12]. In 2001, it was estimated that >40% of South Africans resided in a rural settings and >50% were living in poverty [40, 41]. Although it is difficult to predict how representative estimates from South Africa are of the situation elsewhere in Africa, it is likely that nonspecific factors related to poverty may have contributed at least in part to the observed increased excess mortality.
The mortality burden of the 2009 influenza A/H1N1 pandemic clearly differs from that of seasonal influenza, with the highest apparent mortality risk in younger adults with underlying health conditions [42, 43]. We have demonstrated elevated influenza-associated mortality in South African elderly individuals. Our study is consistent with the concept that African populations in general may be at higher risk for severemortality from pandemic influenza [12], which may be confirmed as more-robust data become available from the African continent. Future studies are needed to estimate influenza disease burden in younger African populations and to evaluate the impact of underlying host susceptibility, socioeconomic factors, and cocirculating bacterial and viral pathogens. Such data are key to predict the potential impact of influenza pandemics and to provide support for influenza vaccination programs and other control measures in developing countries. The model presented here could be applied to any near-real-time data available from South Africa, to estimate the burden in each wave as the pandemic progresses.
Table 2.
Annual Excess Deaths due to Pneumonia and Influenza (P&I) and All Causes in Individuals Aged ⩾65 Years, South Africa, 1998–2005
Acknowledgments
This research was conducted in the context of the Multinational Influenza Seasonal Mortality Study (MISMS), an ongoing international collaborative effort to understand influenza epidemiological and evolutionary patterns, led by the Fogarty International Center, National Institutes of Health (http://www.origem.info/misms/index.php). Funding for this project comes from the Office of Global Health Affairs' International Influenza Unit in the Office of the Secretary of the Department of Health and Human Services.
Financial support. Research and Policy for Infectious Disease Dynamics (RAPIDD) program of the Science and Technology Directorate, Department of Homeland Security, and Fogarty International Center, National Institutes of Health (to L.S.).
Potential conflicts of interest. L.S. has received funding for a research study from Wyeth-Pfizer and consulting fees from SDI Health, a data warehouse business in Plymouth Meeting, PA. All other authors: no conflicts.
Footnotes
Presented in part: 3rd European Influenza Conference, Villamoura, Portugal, September 2008 (abstract 9–021).
References
- 1.Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis. 1998;178:53–60. doi: 10.1086/515616. [DOI] [PubMed] [Google Scholar]
- 2.Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005;165:265–72. doi: 10.1001/archinte.165.3.265. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Rizzo C, Viboud C, Montomoli E, Simonsen L, Miller MA. Influenzarelated mortality in the Italian elderly: no decline associated with increasing vaccination coverage. Vaccine. 2006;24:6468–75. doi: 10.1016/j.vaccine.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 5.Wong CM, Chan KP, Hedley AJ, Peiris JS. Influenza-associated mortality in Hong Kong. Clin Infect Dis. 2004;39:1611–7. doi: 10.1086/425315. [DOI] [PubMed] [Google Scholar]
- 6.Chow A, Ma S, Ling AE, Chew SK. Influenza-associated deaths in tropical Singapore. Emerg Infect Dis. 2006;12:114–21. doi: 10.3201/eid1201.050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine. 1999;17((Suppl 1)):S3–10. doi: 10.1016/s0264-410x(99)00099-7. [DOI] [PubMed] [Google Scholar]
- 8.Serfling RE. Methods for current statistical analysis of excess pneumonia-influenza deaths. Public Health Rep. 1963;78:494–506. [PMC free article] [PubMed] [Google Scholar]
- 9.Reichert TA, Simonsen L, Sharma A, Pardo SA, Fedson DS, Miller MA. Influenza and the winter increase in mortality in the United States, 1959–1999. Am J Epidemiol. 2004;160:492–502. doi: 10.1093/aje/kwh227. [DOI] [PubMed] [Google Scholar]
- 10.Lui KJ, Kendal AP. Impact of influenza epidemics on mortality in the United States from October 1972 to May 1985. Am J Public Health. 1987;77:712–6. doi: 10.2105/ajph.77.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918–1920 “Spanish” influenza pandemic. Bull Hist Med. 2002;76:105–15. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 12.Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 19181918–20 pandemic: a quantitative analysis. Lancet. 2006;368:2211–8. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- 13.Dushoff J, Plotkin JB, Viboud C, Earn DJ, Simonsen L. Mortality due to influenza in the United States—an annualized regression approach using multiple-cause mortality data. Am J Epidemiol. 2006;163:181–7. doi: 10.1093/aje/kwj024. [DOI] [PubMed] [Google Scholar]
- 14.Karstaedt AS, Hopley M, Wong M, Crewe-Brown H, Tasset-Tisseau A. Influenza- and respiratory syncitial virus-associated adult mortality in Soweto. S Afr Med J. 2009;99:750–4. [PubMed] [Google Scholar]
- 15.Influenza outbreak in the district of Bosobolo, Democratic Republic of the Congo, November-December 2002. Wkly Epidemiol Rec. 2003;78:94–6. [PubMed] [Google Scholar]
- 16.Martin DJ, Schoub BD. Influenza—the forgotten vaccination. S Afr Med J. 1997;87:869–71. [PubMed] [Google Scholar]
- 17.Mortality and causes of death in South Africa, 2005: findings from death notification. Statistics South Africa: Pretoria; 2007. Statistics South Africa. [Google Scholar]
- 18.Mortality and causes of death in South Africa, 2003 and 2004. Statistics South Africa: Pretoria; 2006. Statistics South Africa. [Google Scholar]
- 19.Mortality and causes of death in South Africa, 1997–2003: findings from death notification. Statistics South Africa: Pretoria; 2005. Statistics South Africa. [Google Scholar]
- 20.National Vital Statistics System. http://www.cdc.gov/nchs/nvss.htm. Accessed 30 March 2010.
- 21.Anderson RN, Miniño AM, Hoyert DL, Rosenberg HM. Comparability of cause of death between ICD-9 and ICD-10: preliminary estimates. Natl Vital Stat Rep. 2001;49:1–32. [PubMed] [Google Scholar]
- 22.Statistics South Africa. Population statistics. http://www.statssa.gov.za/publications/populationstats.asp. Accessed 1 June 2009.
- 23.US Census Bureau Population estimates. http://www.census.gov/popest/national/national.html. Accessed 30 March 2010.
- 24.McAnerney JM, Johnson S, Schoub BD. Surveillance of respiratory viruses: a 10-year laboratory-based study. S Afr Med J. 1994;84:473–7. [PubMed] [Google Scholar]
- 25.Viboud C, Bjornstad ON, Smith DL, Simonsen L, Miller MA, Grenfell BT. Synchrony, waves, and spatial hierarchies in the spread of influenza. Science. 2006;312:447–51. doi: 10.1126/science.1125237. [DOI] [PubMed] [Google Scholar]
- 26.Viboud C, Grais RF, Lafont BA, Miller MA, Simonsen L. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. J Infect Dis. 2005;192:233–48. doi: 10.1086/431150. [DOI] [PubMed] [Google Scholar]
- 27.Serfling RE, Sherman IL, Houseworth WJ. Excess pneumonia-influenza mortality by age and sex in three major influenza A2 epidemics, United States, 1957–58, 1960 and 1963. Am J Epidemiol. 1967;86:433–41. doi: 10.1093/oxfordjournals.aje.a120753. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention Seasonal influenza (flu) http://www.cdc.gov/flu. Accessed 30 March 2010.
- 29.Viboud C, Boelle PY, Pakdaman K, Carrat F, Valleron AJ, Flahault A. Influenza epidemics in the United States, France, and Australia, 1972–1997. Emerg Infect Dis. 2004;10:32–9. doi: 10.3201/eid1001.020705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li CK, Choi BC, Wong TW. Influenza-related deaths and hospitalizations in Hong Kong: a subtropical area. Public Health. 2006;120:517–24. doi: 10.1016/j.puhe.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Outbreak of influenza, Madagascar, July-August 2002. Wkly Epidemiol Rec. 2002;77:381–4. [PubMed] [Google Scholar]
- 32.McGregor IA, Schild GC, Billewicz WZ, Williams K. The epidemiology of influenza in a tropical (Gambian) environment. Br Med Bull. 1979;35:15–22. doi: 10.1093/oxfordjournals.bmb.a071535. [DOI] [PubMed] [Google Scholar]
- 33.van Riet E, Adegnika AA, Retra K, et al. Cellular and humoral responses to influenza in Gabonese children living in rural and semi-urban areas. J Infect Dis. 2007;196:1671–8. doi: 10.1086/522010. [DOI] [PubMed] [Google Scholar]
- 34.Dosseh A, Ndiaye K, Spiegel A, Sagna M, Mathiot C. Epidemiological and virological influenza survey in Dakar, Senegal: 1996–1998. Am J Trop Med Hyg. 2000;62:639–43. doi: 10.4269/ajtmh.2000.62.639. [DOI] [PubMed] [Google Scholar]
- 35.Gachara G, Ngeranwa J, Magana JM, et al. Influenza virus strains in Nairobi, Kenya. J Clin Virol. 2006;35:117–8. doi: 10.1016/j.jcv.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–3. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–70. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simonsen L, Viboud C. Respiratory syncytial virus infection in elderly adults. N Engl J Med. 2005;353:422–3. [PubMed] [Google Scholar]
- 39.van Vuuren A, Rheeder P, Hak E. Effectiveness of influenza vaccination in the elderly in South Africa. Epidemiol Infect. 2009;137:994–1002. doi: 10.1017/S0950268808001386. [DOI] [PubMed] [Google Scholar]
- 40.Friedman I, Bengu L. Durban, South Africa: Health Systems Trust; 2008. Fifteen year review of income poverty alleviation programmes in the social and related sectors. [Google Scholar]
- 41.Day C, Gray A. Health and related indicators. In: Barron P, Roma-Reardon J, editors. South African health review. Cape Town: Human Sciences Research Council; 2008. pp. 239–396. [Google Scholar]
- 42.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 43.Human infection with pandemic A (H1N1) 2009 influenza virus: clinical observations in hospitalized patients, Americas, July 2009—update. Wkly Epidemiol Rec. 2009;84:305–8. [PubMed] [Google Scholar]