Abstract
Penicillium marneffei is an important human immunodeficiency virus-associated opportunistic infection endemic in Southeast Asia. Central nervous system infection has not been described. We report the first case series of 21 human immunodeficiency virus-infected patients who presented with a syndrome consistent with acute central nervous system infection and who had Penicillium marneffei isolated from cerebrospinal fluid.
Penicillium marneffei is emerging as an important opportunistic pathogen among human immunodeficiency virus (HIV)—infected and immunocompromised residents of (and travelers to) Southeast Asia, Northeastern India, and Southern China [1]. No definitive mode of acquisition has been found, but inhalation has been implicated [2, 3]. The infection has been described in both immunocompromised (>80%) and immunocompetent individuals [4]. Immunocompromised individuals develop disseminated disease involving the reticuloendothelial, skin, respiratory, and gastrointestinal systems. P. marneffei is commonly isolated from skin lesions (90%), blood (76%), bone marrow (100%), and lymph nodes (34%) [5]. There are 2 reports of isolation of P. marneffei from cerebrospinal fluid (CSF) of patients with penicilliosis [5, 6]. However, clinical details were lacking in these cases, and despite the fact that other members of the Penicillium genus are known to cause central nervous system (CNS) disease, penicilliosis presenting as a predominantly CNS infection has not been described [6]. We report the clinical characteristics and outcomes of 21 HIVinfected adult patients who presented with symptoms consistent with a CNS infection and who had P. marneffei isolated from CSF.
Patients. The Hospital for Tropical Diseases in Ho Chi Minh City is the largest referral center for infectious diseases in Vietnam, with >5000 HIV-infected patients seen yearly. From January 2004 through December 2009, 677 incident cases of penicilliosis were retrospectively identified from our hospital records. Diagnosis was confirmed by culture of P. marneffei from skin scrapings, blood, lymph nodes, bone marrow, or other body tissue. Fifty-eight (8.6%) of these patients had lumbar puncture performed for suspected CNS infection. A definitive diagnosis of CNS infection was established by CSF culture and/or microscopy (Gram, India, and Zielh-Neelsen stains) for 29 patients (50%); 20 were due to P. marneffei, 7 to Cryptococcus neoformans, 1 to Mycobacterium tuberculosis, and 1 to both P. marneffei and C. neoformans. The clinical features and outcomes of the 21 patients with P. marneffei cultured from CSF samples were reviewed and are reported in Table 1. The study was approved by the Ethical Committee of the Hospital for Tropical Diseases.
Table 1.
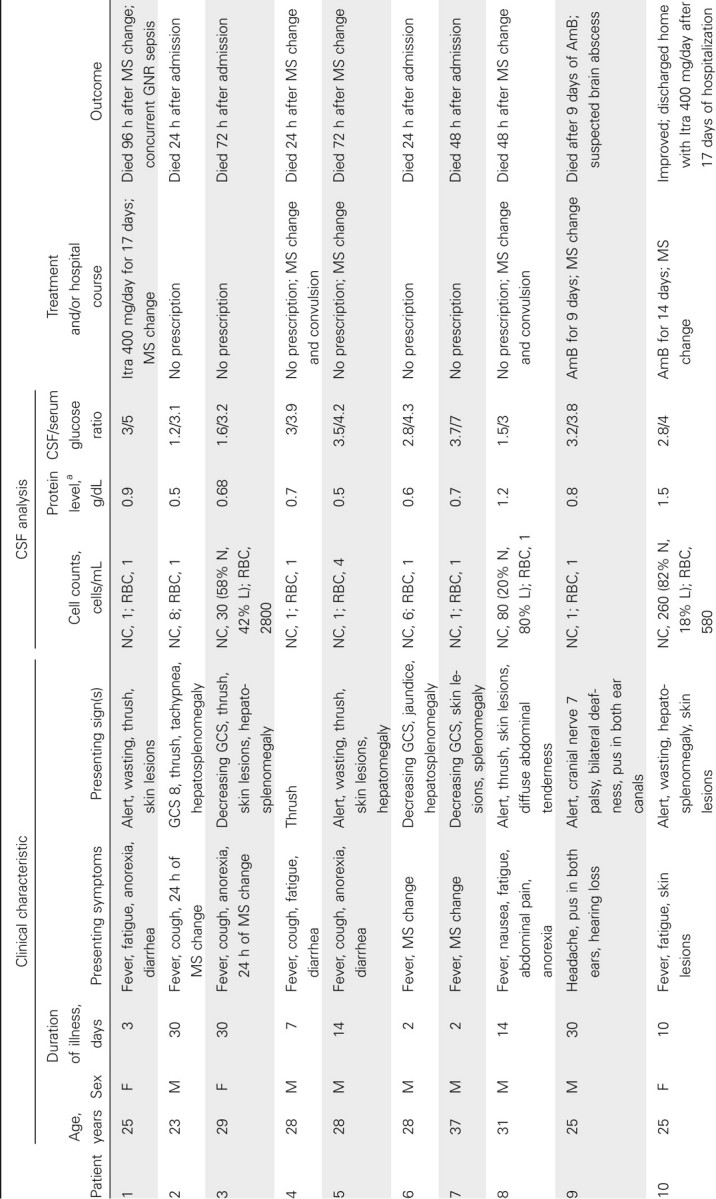
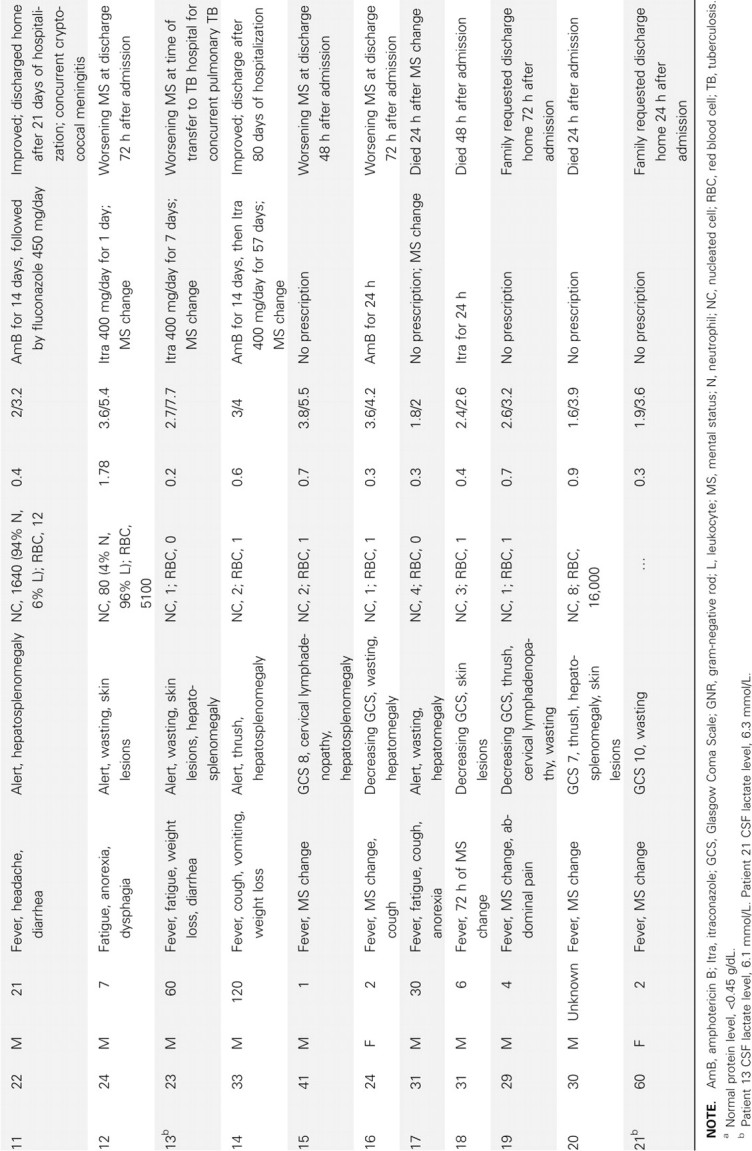
Clinical Features of 21 HIV-Infected Patients with Penicillium marneffei Isolated from Cerebrospinal Fluid (CSF)
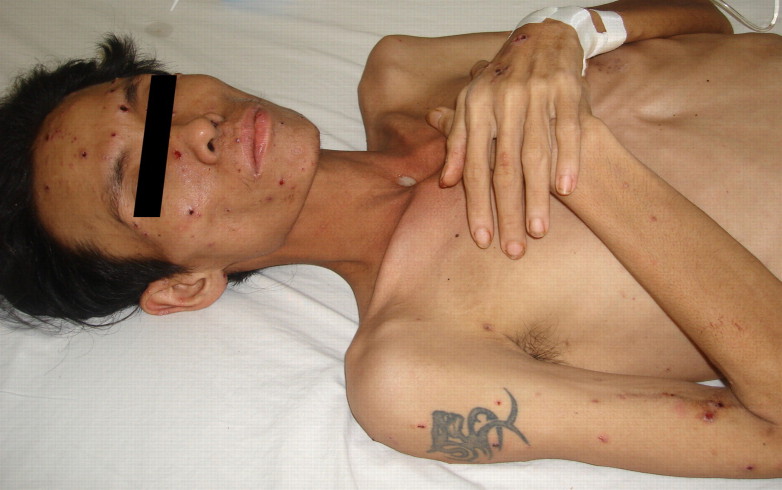
The 21 patients were admitted to Hospital for Tropical Diseases from April 2004 through July 2009. All were HIV infected; the median CD4 count was 11 cells/µL (interquartile range [IQR], 10–12 cells/µL). The median age was 28 years (IQR, 25–31 years); 76% were men. The median duration of illness was 9 days (IQR, 3–30 days). Patients experienced fever (90%), anorexia (57%), fatigue (52%), cough (33%), and diarrhea (29%) prior to the onset of altered mentation that prompted the hospital visits (48%). The median duration of altered mentation was 2 days (IQR, 1.25–2 days). The median temperature was 38.3°C (IQR, 37°C–39°C). On examination, characteristic umbilicated skin lesions were present in 10 patients (photograph of skin lesions in Figure 1), oral thrush in 9, hepatosplenomegaly in 12, and lymphadenopathy in 3 patients. Blood tests showed anemia in 20 patients (median hemoglobin level, 7.2 g/dL; IQR, 5.6–9 g/dL), thrombocytopenia in all patients (median platelet count, 85,000 cells/µL; IQR, 31,000–125,500 cells/µL), and elevated transaminase levels in 18 patients (median alanine transaminase level, 220 units/L; IQR, 109–306 units/L; median aspartate transaminase level, 130 units/L; IQR, 86–181 units/L). Symptoms of altered mentation, including confusion, agitation, or drowsiness, were the presenting features in 10 patients and developed in all remaining patients during hospitalization. Headache was the presenting symptom in 2 patients. Signs of meningeal irritation were absent. Generalized convulsions and facial nerve palsy were observed in 2 and 1 patients, respectively. The CSF analysis is shown in Table 1. The CSF opening pressure was not routinely measured; in 1 patient it measured 180 mm CSF. CSF was clear in all patients and acellular in 14 patients. Among the 7 patients with CSF pleocytosis, the median nucleated cell count was 80 cells/mL (IQR, 19–170 cells/mL), with neutrophil predominance in 3 patients. CSF protein level was elevated (>0.45 g/dL) in 71% of patients, with a median protein level of 0.7 g/dL (IQR, 0.5–0.88 g/dL). The median CSF/serum glucose ratio was 0.65 (IQR, 0.5–0.78); 5 patients had CSF/serum glucose ratios <0.5. Gram stain of CSF was negative in all patients. India ink stain of CSF was positive in 1 of 19 patients tested, and C. neoformans was cultured from blood and CSF samples of this patient (patient 11). Zielh-Neelsen stain of CSF was negative in all 17 patients examined. A diagnosis of tuberculosis was made in 2 patients by positive Zielh-Neelsen stain of sputum and lymph node biopsy. The mean time to identify P. marneffei from CSF culture was 4.4 days (range, 2–8 days) and from blood culture was 4.3 days (range, 3–9 days). Blood culture was performed in 18 patients; 13 grew P. marneffei, 1 grew Escherichia coli, 1 grew Enterococcus species, 1 grew unidentified gram-negative rods, and 1 grew both P. marneffei and C. neoformans.
Figure 1.
Umbilicated skin lesions characteristic of penicilliosis.
Outcomes. Three patients survived and had experienced improvement of symptoms at hospital discharge, 1 was transferred to another hospital for tuberculosis treatment after his condition deteriorated, 12 died within 24–72 h after hospital admission, and 5 were taken from the hospital to die at home, which is common practice in Vietnam. Patients taken home to die were moribund and received no further effective medical care, and all were expected to have died, giving an overall mortality of 81%. Among the 18 patients with a poor outcome, the diagnosis was not established prior to death, and no antifungal drugs were given in 12 patients. Two patients received 2 doses of itraconazole (400 mg/day) on the basis of characteristic skin lesions, 1 received 1 dose of amphotericin B on the basis of preliminary blood culture results indicating growth of yeasts, and the remaining 3 patients received 7–17 days of itraconazole or amphotericin B treatment but had concurrent comorbid conditions. The 3 patients who survived and had improvement of symptoms at hospital discharge started receiving amphotericin B within 24 h after developing CNS symptoms, on the basis of detection of unidentified yeasts fromblood culture (patients 10 and 14) or positive India ink stain of CSF (patient 11), and all received a total of 14 days of amphotericin B therapy before switching to itraconazole or fluconazole.
Discussion. This report describes a new clinical syndrome associated with P. marneffei infection in HIV-infected patients. The syndrome is characterized by an acute onset of altered mental status with confusion, agitation, or depressed consciousness in the setting of a subacute febrile illness with nonspecific constitutional symptoms. Symptoms of increased cranial pressure and signs of meningeal inflammation were notably uncommon or absent. Characteristic umbilicated skin lesions were present in only one-half of the patients. CSF analysis varied from acellular to mild pleocytosis, with normal to mildly elevated protein levels and normal to mildly low glucose levels. CSF microscopy for P. marneffei was negative. CSF culture for P. marneffei took a mean of 4 days for identification. P.marneffei was not always isolated from blood cultures of these patients. The disease course was rapidly progressive, and inpatient mortality was very high. Early initiation of amphotericin B was administered in 5 patients, 3 of whom survived, whereas all 15 patients who did not receive amphotericin B or itraconazole died.
To our knowledge, P. marneffei has never been described as a CNS pathogen. P. marneffei was isolated from the meninges of 1 patient in a review of 155 published penicilliosis cases and from 3 of 20 CSF specimens in a case series of 80 patients with penicilliosis from Thailand [4, 5]. However, the clinical features of those patients were not provided. The patients in this case series had CNS symptoms consistent with a CNS infection, and P. marneffei was the single pathogen isolated from CSF samples from 20 of 21 patients. It is not possible to exclude the involvement of M. tuberculosis by CSF and sputum microscopy and of other opportunistic viral pathogens not tested in this case series; therefore, it remains uncertain whether the CNS syndrome in these patients can be wholly attributed to P. marneffei. Concurrent growth of C. neoformans and P. marneffei has been observed from blood and CSF samples of HIV-infected patients at our hospital [7], as occurred for patient 11 in this case series. P. marneffei grows much slower (at least 24–48 h later) than C. neoformans from clinical specimens, and neither has been observed to have competitive cultural advantages over the other. Unlike M. tuberculosis, cryptococcus is unlikely to have been missed, although cryptococcal antigen tests, if they were clinically available, would have been more informative. Selected members of the other 225 Penicillium species are known to cause CNS disease. Penicillium commune was isolated from multiple brain and lung autopsy specimens from a patient with acute leukemia who was receiving antibiotics and steroids [8]. Penicillium chrysogenum was isolated from CSF and brain biopsy samples of 2 nonimmunocompromised individuals with CNS symptoms [9, 10]. An unidentified Penicillium species was isolated from multiple brain lesions of a patient with chronic liver disease at autopsy [6]. These reports demonstrate the neurotropic potential of invasive Penicillium species, and P. marneffei, the most invasive of Penicillium species, is likely not an exception.
CNS infections are medical emergencies, and early empirical therapy can prevent irreversible neuronal damage and save lives. In HIV-infected patients with CD4 count <100 cells/µL who present with a subacute febrile syndrome, including changes in mental status, and who live or have traveled to endemic regions, disease due to P. marneffei should be considered, along with viral encephalitis, tuberculosis, and cryptococcal meningoencephalitis. Given the long culture incubation time and high disease mortality, heightened clinical suspicion and prompt empirical treatment with a CNS-penetrating antifungal drug, such as amphotericin B, are critical in the management of these patients.
Acknowledgments
Financial support. Fogarty International Clinical Research Fellowship (T.L.) and Wellcome Trust UK Major Overseas Programmes (T.L., J.F., and J.N.D.).
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Vanittanakom N, Cooper CR, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19((1)):95–110. doi: 10.1128/CMR.19.1.95-110.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imwidthaya P. Update of penicilliosis marneffei in Thailand. Mycopathologia. 1994;127:135–137. doi: 10.1007/BF01102912. [DOI] [PubMed] [Google Scholar]
- 3.Hilmarsdottir I, Coutellier A, Elbaz J, et al. A French case of laboratory-acquired disseminated Penicillium marneffei infection in a patient with AIDS. Clin Infect Dis. 1994;19:357–358. doi: 10.1093/clinids/19.2.357. [DOI] [PubMed] [Google Scholar]
- 4.Duong TA. Infection due to P. marneffei, an emerging pathogen: review of 155 reported cases. Clin Infect Dis. 1996;23:125–130. doi: 10.1093/clinids/23.1.125. [DOI] [PubMed] [Google Scholar]
- 5.Supparatpinyo K, Khamwan C, Baosoung V, Nelson KE, Sirisanthana T. Disseminated Penicillium marneffei infection in southeast asia. Lancet. 1994;344((8915)):110–113. doi: 10.1016/s0140-6736(94)91287-4. [DOI] [PubMed] [Google Scholar]
- 6.Noritomi DT, Bud GL, Beer I, Da Silva AS, De Cleva R, Gama-Rodrigues JJ. Multiple brain abscesses due to Penicillium spp infection. Rev Inst Med Trop Sao Paulo. 2005;47((3)):167–170. doi: 10.1590/s0036-46652005000300010. [DOI] [PubMed] [Google Scholar]
- 7.Le T, Hong Chau TT, Kim Cuc NT, et al. AIDS-associated Cryptococcus neoformans and Penicillium marneffei coinfection: a therapeutic dilemma in resource-limited settings. Clin Infect Dis. 2010;51((9)):e65–e68. doi: 10.1086/656685. [DOI] [PubMed] [Google Scholar]
- 8.Huang S, Harris LS. Acute disseminated penicillosis. Am J Clin Pathol. 1963;39:167–174. doi: 10.1093/ajcp/39.2.167. [DOI] [PubMed] [Google Scholar]
- 9.Kantarcioglu AS, Apaydin H, Yucel A, et al. Central nervous system infection due to Penicillium chrysogenum. Mycoses. 2004;47:242–248. doi: 10.1111/j.1439-0507.2004.00974.x. [DOI] [PubMed] [Google Scholar]
- 10.Liratsopulos G, Ellis M, Nerringer R, Denning DW. Invasive infection due to Penicillium species other than P. marneffei. J Infect. 2002;45:184–195. doi: 10.1053/jinf.2002.1056. [DOI] [PubMed] [Google Scholar]