Abstract
In the United States, the human immunodeficiency virus (HIV) epidemic among heterosexual men disproportionately affects individuals involved with the criminal justice system, injection drug and other substance users, and racial and ethnic minorities. These overlapping populations confront similar social and structural disparities that contribute to HIV risk and limit access to HIV testing, treatment, and care. In this review, we discuss barriers to linkage to comprehensive HIV care for specific subpopulations of heterosexual men and examine approaches for enhancing linkage to care for this diverse population.
In 1997, 78% of all AIDS cases in the United States were among men [1]. A decade later, the human immunodeficiency virus (HIV)/AIDS epidemic remains disproportionately concentrated among men, who represent nearly three-fourths of all HIV/AIDS cases and new HIV infections among adults and adolescents [2]. HIV-infected men are also more likely to receive a diagnosis late in the course of infection [3] and have lower CD4 cell counts when care is initiated [4]. In 2007, 46% of men infected through heterosexual contact progressed to AIDS within 12 months, compared with 36% of the total HIV-infected population [2]. Significant racial and ethnic disparities in HIV infection persist. In 2007, black and Hispanic men comprised 57% of all HIV/AIDS diagnoses among men, and black men experienced the highest rate of new HIV infections of any demographic group (115.7/100,000 population) [2]. Racial and ethnic minorities are also disproportionately represented among late diagnoses and are significantly more likely to experience delayed linkage to care [5–11].
Table 1.
Linkage to Care among Heterosexual Men with Human Immunodeficiency Virus Infection: Barriers and Facilitators
| Barriers or Challenges | Successful Strategies |
| Incarceration | Communication between correctional and community providers; comprehensive discharge planning and case management; availability of substance use treatment within and outside correctional facilities |
| Substance dependence | Directly administered antiretroviral therapy; integrated opiate replacement and antiretroviral therapy; case management; integration or colocation of medical care and supportive services |
| Stigma and distrust | Peer engagement and outreach; sustained engagement with target population |
| Structural or environmental barriers | Case management and colocation of services; linkage to health insurance; access to stable housing; job training and placement programs |
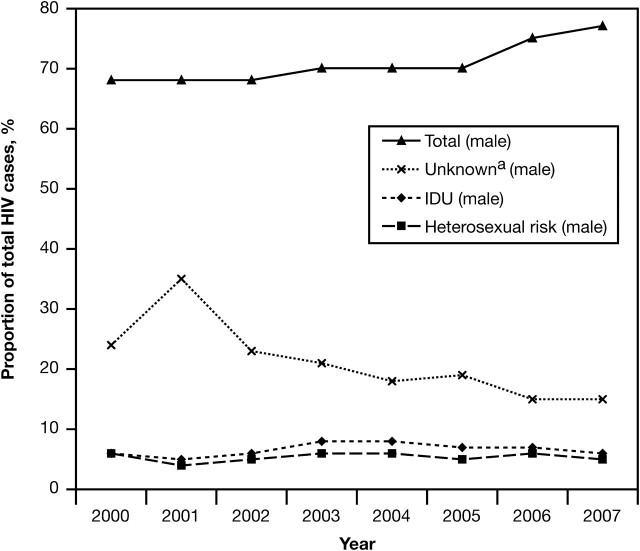
Modes of HIV transmission among men have changed during the last decade [1, 2]. Male-to-male sexual contact remains the primary mode of transmission among men in the United States; however, 16% of HIV-positive men were infected through heterosexual sex and 12% through injection drug use (IDU) in 2007 [2]. Whereas the number of new HIV/AIDS cases among men resulting from IDU has declined during the past 2 decades and stabilized since 2004, infections attributable to heterosexual sex have increased [2]. Increasing rates of heterosexual HIV transmission underscore the potential for a more generalized heterosexual HIV epidemic, and studies in Washington, DC, and Baltimore, Maryland, have identified this trajectory in marginalized urban communities [12, 13]. Figure 1 depicts the proportion of heterosexual men among the total number of persons with HIV infection in the United States between 2000 and 2007 [2, 14–20].
Figure 1.
Estimated proportion of human immunodeficiency virus (HIV) infection among males in the United States, by transmission category, 2000–2007. Source: Centers for Disease Control and Prevention annual HIV/AIDS surveillance reports [2,14–20]. aUnknown indicates other or risk factor not reported or identified.
In the United States, the HIV epidemic among heterosexual men disproportionately affects individuals involved with the criminal justice system, injection drug users (IDUs), other substance users, and racial and ethnic minorities. These overlapping populations confront similar social and structural disparities that contribute to HIV risk and limit access to HIV testing, treatment, and care. In describing these disparities and risks, clinicians and researchers need to be particularly cautious about protecting sensitive health information, such as drug use and sexual risk-taking behaviors. Some researchers have used peer-based interventions and employed research staff of the same race and/or cultural background as the study participants to enhance participants’ comfort with the research and to bolster the quality of data collected [21, 22]. Other studies have used technology such as audio computer-assisted self-interviews to improve rates of reporting of sensitive behaviors and to reduce socially desirable responding [23–26]. In this review, we discuss barriers to linking specific subpopulations of heterosexual men to comprehensive HIV care and examine approaches for enhancing the linkage to care for this diverse population.
EMERGING SUBPOPULATIONS AT RISK
IDU Populations
During the past 2 decades, there has been a significant decline in IDU-related HIV infections [27–29], probably in part because of increases in HIV prevention programs targeted to IDUs, including syringe exchange programs [30, 31]. Despite these declines, IDU-related HIV transmission continues to affect racial and ethnic minorities at disproportionate rates, particularly African American men [32]. Recent data from the Centers for Disease Control and Prevention indicate that between 2004 and 2007, 62% of incident IDU-associated HIV infections were among men and 58% of those infected through IDU were black [32]. In addition, 40% of HIV-infected IDUs received late HIV diagnoses, defined as receiving an AIDS diagnosis within 12 months of HIV diagnosis [32]. Among African Americans in high-risk communities in Houston, Texas, Risser et al found that individuals reporting both IDU and heterosexual anal intercourse had 6.2 times the odds of being HIV infected [33]. In a sample of 3555 drug users and neighborhood controls, McCoy et al found that IDUs and those reporting both IDU and crack cocaine smoking were 9.8 and 5.27 times, respectively, more likely to be HIV infected [34]. These findings demonstrate the need for coordinated efforts between researchers, policymakers, and outreach and community-based organizations to address late HIV diagnoses among IDUs and to target interventions to the needs of specific IDU subpopulations.
Nonparenteral Substance Users
Despite the overall decline in IDU-related HIV infections, the association between nonparenteral substance use and HIV infection has been increasingly demonstrated. In some areas of the United States, HIV prevalence among crack cocaine smokers may be comparable to or greater than among IDUs [35]. Booth et al found that crack cocaine smokers and crack cocaine–smoking IDUs were more likely to report having multiple sexual partners and exchanging sex for drugs or money than those who only injected [36]. McCoy et al found that, compared with neighborhood controls, crack cocaine smokers were 2.2 times more likely to be infected with HIV [34]. Adimora et al also found a statistically significant association between sexual concurrency and crack cocaine smoking in a sample of rural African Americans with recent heterosexually acquired HIV infection [37]. Alcohol use has also been shown to be an important mediator of high-risk sexual behavior among men [38, 39], with additional studies finding strong associations between alcohol use and HIV incidence [40, 41]. Methamphetamine use is yet another emerging risk factor for HIV infection among heterosexual men [42, 43].
Men Who Have Sex With Men and Women
Understanding risk factors among men who have sex with men and women (MSMW) and adapting effective prevention interventions should be priorities, given the potential of MSMW to bridge the epidemics between sexes. Lichtenstein found that bisexual activity is often unprotected among black MSMW [44], and Williams et al identified high rates of IDU and crack use among MSMW [45]. In a sample of mostly low-income, unemployed, minority MSMW, Gorbach et al found that sexual and drug use networks were highly interconnected [46].
Foreign-born Populations
Another characteristic of the changing HIV epidemic among heterosexual males in the United States is the increasing number of HIV-infected persons who are foreign born [47]. This includes legal and illegal immigrants as well as refugees and asylum seekers. The regulatory change in 2009 that removed HIV infection from the list of communicable diseases of public health significance among foreign immigrants may affect the proportion of foreign-born HIV-infected persons in the United States in the coming years [48]. Before this change, HIV-infected immigrants were inadmissible to the country without a government waiver. Heterosexual risk is the predominant mode of HIV transmission among many foreign-born populations [49, 50]; however, relatively little is known about the epidemiology of HIV infection in these populations and the extent to which these individuals engage in HIV care after arrival in the United States.
LINKAGE TO CARE
Correctional Populations
Large numbers of HIV-infected individuals pass through correctional facilities each year. In 2006, 1 in 7 HIV-positive individuals in the United States were incarcerated [51]. Access and adherence to antiretroviral treatment can often be most difficult in the period immediately after release from incarceration. Recently released individuals are at elevated risk for relapse to drug use and sexual and drug-related risk behaviors [52–58] and have difficulty securing stable housing and employment [59–61]. These stressors during community reentry may disrupt engagement in care and lead to worsened virologic outcomes as well as increase the risk of secondary HIV transmission [62–64]. Newly released African American and Latino inmates in particular have difficulty accessing antiretroviral treatment (ART) in the community [65].
The majority of correctional facilities provide some type of discharge planning for HIV-positive inmates (T. M. Hammett, S. Kennedy, S. Kuck, unpublished data, 2007), and studies have found that inmates who receive such assistance are more likely to engage in HIV treatment and care in the community [61, 66]. However, Grinstead et al found that staff responsible for discharge planning may not be informed of inmates’ HIV status or have knowledge of HIV-related services in the community [67], indicating that education of discharge planning staff and coordination with community providers could probably be improved.
Because recently released HIV-infected inmates confront a multitude of challenges during community reentry, initiating and remaining engaged in community-based care often requires intensive and sustained assistance that addresses barriers such as substance dependence, mental illness, unstable housing, unemployment, and lack of health insurance. Intensive case management can be successful in engaging recently released HIV-infected prisoners into medical care and providing linkage to social services [63]. Newly released HIV-infected individuals are also more likely to fill a prescription for ART within 10, 30, or 60 days of release if they receive assistance from a community caseworker in completing the AIDS Drug Assistance Program application [65]. However, fewer than half of state and federal correctional facilities and only 39% of city and county systems provide referrals to case management services for HIV-infected inmates during discharge planning (T. M. Hammett, S. Kennedy, S. Kuck, unpublished data, 2007). Organized discharge planning and intensive case management are critical to facilitating successful linkage to and retention in care within this population and should be implemented on a wide scale.
Substance-Using Populations
Substance use frequently undermines the medical management of HIV among HIV-infected substance users [68], who are also more likely to experience high levels of socioeconomic instability and have limited health care access and utilization [68–70]. In a systematic review of 41 studies examining the relationship between substance use and adherence to ART, Malta et al found that active substance use was widely associated with poor ART adherence [71]. In turn, these associations may create reluctance among physicians to initiate combination ART in active substance users [72].
Involvement with the criminal justice system further complicates the provision of HIV care for substance users. Kerr et al found that incarceration was the strongest predictor for discontinuation of ART among HIV-infected IDUs, with individuals reporting recent incarceration having 5-fold higher odds of discontinuing highly active ART (95% confidence interval [CI], 1.2–18.7) [73]. Furthermore, because of the limited provision of substance-dependence treatment such as opiate replacement therapy (ORT) in correctional facilities [74], substance-dependent individuals undergoing treatment with buprenorphine or methadone in the community may not be able to continue treatment while incarcerated [75]. As a result, they may undergo withdrawal and be less inclined to reinitiate treatment after release [75], which may increase their risk of relapse to drug use and significantly affect their ability to engage in HIV treatment and care. Recently, studies in several cities have demonstrated the feasibility and effectiveness of linking prisoners to ORT during incarceration and after release [76–84].
Despite the challenges to engaging and retaining this population in care, a number of different treatment interventions targeted to HIV-infected substance users have achieved favorable clinical outcomes. Smith-Rohrberg et al conducted a randomized, controlled trial of directly administered ART for IDUs and found improved virologic and immunologic outcomes as well as improved adherence [85]. Integrating substance dependence and HIV treatment is an approach to engaging substance users in care that directly addresses substance use and its associated complications. The efficacy of integrating ORT and HIV treatment has been increasingly examined and models that integrate treatment with buprenorphine-naloxone into HIV primary care have recently been successfully piloted [68, 86–89]. Medication-assisted treatment is also available for individuals dependent on cocaine, methamphetamine, or alcohol, although more work is needed to explore the potential for integrating these therapies with ART and HIV care [90].
Case management and colocation of services can also enhance linkage to care for substance users [91], although interventions using case management alone may be less effective than direct linkage to substance-dependence treatment in this population [92]. In their study, Smith-Rohrberg et al assessed the impact of colocated medical, case management, and referral to substance abuse services among drug users undergoing directly administered ART and found that greater utilization of onsite medical and case management services was independently associated with improved virologic outcomes [85]. The impact of case management on engagement and retention in care has also been demonstrated among substance-using homeless populations [93, 94]. Broadhead et al confirmed the feasibility of using peer health advocates to engage HIV-infected drug users in care and described this social support structure as a more accessible alternative in the context of limited access to integrated substance-dependence treatment and HIV care. The intervention involved weekly provision of peer support and counseling and the provision of nominal monetary rewards to health advocates for successfully promoting their peers’ engagement in care [95].
African-American and Latino Populations
HIV-infected African American and Latino persons are significantly more likely than HIV-infected white persons to be diagnosed and initiated on treatment late in the course of HIV infection. In a modeling analysis using data from the national HIV Research Network to describe HIV survival disparities among specific racial and ethnic groups, Losina et al found that late initiation and early discontinuation of ART were most pronounced among Hispanic subjects, with an additional 3.9 years of life lost from late initiation and early discontinuation of ART compared with 3.5 years of life lost for the entire study population [96]. In a retrospective cohort study, Ulett et al found 2.45 higher odds (95% CI, 1.60–3.74) of delayed linkage to HIV care among African American patients at an HIV/AIDS clinic [97]. Racial and ethnic minorities experience greater marginalization from the health care system and are more likely than their white counterparts to receive lower quality medical care [7, 9, 10, 98–104]. Distrust of the health care system can pose an additional barrier to engaging HIV-infected African American and Latino persons in treatment and care [105–107].
The complex interplay between social, cultural, and economic barriers to care among African American and Latino populations is not fully understood. However, socially and culturally sensitive linkage interventions have been developed in a manner consistent with the adaptation of culturally sensitive and client-centered HIV prevention interventions [108, 109]. Peer and outreach-based interventions that address structural barriers to care have demonstrated effectiveness in linking marginalized racial and ethnic minorities to treatment. The California Bridge Project used peer-based staff in outreach to locate out-of-treatment HIV-infected individuals [110]. Nearly a third of the 325 predominantly African American and Latino clients who reported no history of HIV treatment were linked to care. African American and Latino clients had 2.3 and 3.7 greater odds, respectively, of being linked to care than did white clients; the authors hypothesized that this difference was probably due to the use of outreach staff who reflected the client population demographically. An average of 15.4 contacts were reported among those who were successfully linked compared with 7.1 among those who were not, demonstrating the sustained effort required to engage marginalized individuals in care [110]. Rajabiun et al conducted qualitative interviews with predominantly underserved African American and Latino HIV-positive individuals at 7 sites of the Health Resources and Services Administration–funded Outreach Initiative to identify components of outreach programs that contributed to engagement and retention in HIV care by these populations [111]. Outreach staff improved access to care through locating physicians and clinics, linking clients to health insurance, accompanying them to medical appointments, and facilitating communication with providers. Staff support enhanced clients’ self-efficacy and capacity to cope with the HIV diagnosis, and participants were provided with services such as transportation, food, and housing that addressed structural barriers to care. Forty-five percent of participants achieved undetectable viral loads by 12 months [112]. In another analysis of this multisite study, Cabral et al found that participants reporting ≥9 contacts with outreach staff were half as likely as those with fewer contacts to have substantial gaps in primary care during a 12-month period [113]. Randomized, controlled trials are needed to assess the effect of outreach-based interventions on initiating and retaining disadvantaged minority populations in care [108]. The feasibility of integrating outreach interventions with substance-dependence treatment should also be explored [70, 108, 112].
Interventions that incorporate case management have also been successful in enhancing linkage to care among racial and ethnic minorities. The Antiretroviral Treatment Access Study (ARTAS) was a brief strengths-based case management intervention implemented in health departments and community-based organizations that involved client identification of strengths and abilities and the development of a personalized plan to acquire needed resources. ARTAS successfully linked 79% of recently diagnosed participants (497/626) to a primary HIV care provider within 6 months. Hispanic subjects were more likely to be engaged in HIV care than other racial and ethnic groups (odds ratio, 2.14; 95% CI, 1.03– 4.43) [114]. Gardner et al conducted a randomized controlled trial of ARTAS in 4 states, comparing the efficacy of passive referral to a case management intervention in linking persons recently diagnosed to care. Individuals receiving the strengths-based case management intervention were 41% more likely to see a medical provider in consecutive 6-month intervals than those receiving passive referral to care (relative risk, 1.41; 95% CI, 1.1–1.6). The intervention had a stronger impact on Hispanic participants (relative risk, 2.16; 95% CI, 1.40–3.35) than on participants of other ethnicities [115].
Colocation of medical care and other support services has also been shown to be an important factor in engaging marginalized racial and ethnic minorities in care. Individuals who participated in the ARTAS intervention at a site colocated with HIV medical care providers were more likely to be linked to care [115]. In a program designed to facilitate HIV health care utilization among mostly minority populations in Bronx, New York, through colocation of case management, support groups, mental health, and harm reduction services, Cunningham et al found that case management and HIV support group visits were associated with 1.9 and 2.3 greater odds, respectively, of quarterly medical visits among participants [116].
CONCLUSION
In summary, factors such as substance use, poverty, unemployment, lack of educational opportunities, and marginalization from the health care system constitute multilevel barriers to care for vulnerable subpopulations of HIV-infected heterosexual men. Consequently, interventions that address social and structural barriers to care through case management, colocation of services, and outreach have been shown to enhance linkage to care across these subpopulations. Despite the broad efficacy of these interventions, those involved with the criminal justice system, substance users, and disadvantaged racial and ethnic minorities face distinct challenges to accessing care that also require more targeted strategies. Correctional facilities have the capacity to improve the health of HIV-infected individuals beyond incarceration, where they are arguably most vulnerable, by providing organized and coordinated discharge planning and linkage to intensive case management after release. Although substance-dependent populations are especially challenging to link to and retain in care, the emergence of integrated substance use and HIV treatment offers new possibilities to engage this population. The efficacy of peer- and outreach-based interventions in linking racial and ethnic minorities to care demonstrates the importance of socially and culturally sensitive interventions that foster trust in providers and provide means of overcoming structural barriers to care.
Future work is urgently needed to scale up successful models of linkage to care and to adapt these models to local contexts. This will require additional resources, but, most importantly, it will require collaboration across agencies and institutions and the innovative use of existing resources and capacities. Integration of services is an important example of improving efficiency in delivering comprehensive HIV care. The challenge and complexity of linking HIV-infected heterosexual men to care require renewed efforts to adapt interventions to the needs of diverse subpopulations.
Acknowledgments
Financial support. The authors received no direct financial support for this manuscript, but support for related work is provided by the National Institutes of Health (NIH), Center for AIDS Research (grant number P30-AI-42853); the Center for Drug Abuse and AIDS Research (grant number P30DA013868); and the National Institute on Drug Abuse, NIH (grant number K23DA021095).
Supplement sponsorship. This article was published as part of a supplement entitled “Linkage, Engagement, and Retention in HIV Care,” supported by Bristol-Myers Squibb, Positive Charge Initiative. Editorial support for the supplement was provided by J. Turner at PAREXEL and was funded by Bristol-Myers Squibb, Positive Charge.
Potential conflicts of interest. A.N. has received consulting fees from Mylan. All other authors: no conflicts.
References
- 1.Centers for Disease Control and Prevention. HIV/AIDS surveillance report. 1997;9:1–43. Available at: http://www.cdc.gov/hiv/topics/reports/pdf/hivsur92.pdf. Accessed 7 June 2010. [Google Scholar]
- 2.Centers for Disease Control and Prevention. HIV/AIDS surveillance report. 2007;19:1–63. Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2007report/pdf/2007SurveillanceReport.pdf. Accessed 7 June 2010. [Google Scholar]
- 3.Castilla J, Sobrino P, De La Fuente L, Noguer I, Guerra L, Parras F. Late diagnosis of HIV infection in the era of highly active antiretroviral therapy: consequences for AIDS incidence. AIDS. 2002;16:1945–51. doi: 10.1097/00002030-200209270-00012. [DOI] [PubMed] [Google Scholar]
- 4.Samet JH, Freedberg KA, Savetsky JB, Sullivan LM, Stein MD. Understanding delay to medical care for HIV infection: the long-term non-presenter. AIDS. 2001;15:77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- 5.Wohl DA, Shain L, Adamian M, et al. HIV transmission risk behaviors among HIV-infected individuals released from prison [abstract 36] Program and Abstracts of the 10th Conference on Retroviruses and Opportunistic Infections. 10–14 February 2003. Boston. [Google Scholar]
- 6.Hu DJ, Byers R, Jr., Fleming PL, Ward JW. Characteristics of persons with late AIDS diagnosis in the United States. Am J Prev Med. 1995;11:114–9. [PubMed] [Google Scholar]
- 7.Oramasionwu CU, Brown CM, Lawson KA, Ryan L, Frei CR. Evaluating HIV/AIDS disparities for blacks in the United States: a review of antiretroviral and mortality studies. J Natl Med Assoc. 2009;101:1221–9. doi: 10.1016/s0027-9684(15)31133-0. [DOI] [PubMed] [Google Scholar]
- 8.Oramasionwu CU, Brown CM, Ryan L, Lawson KA, Hunter JM, Frei CR. HIV/AIDS disparities: the mounting epidemic plaguing US blacks. J Natl Med Assoc. 2009;101:1196–204. doi: 10.1016/s0027-9684(15)31130-5. [DOI] [PubMed] [Google Scholar]
- 9.Oramasionwu CU, Skinner J, Ryan L, Frei CR. Disparities in antiretroviral prescribing for blacks and whites in the United States. J Natl Med Assoc. 2009;101:1140–4. doi: 10.1016/s0027-9684(15)31110-x. [DOI] [PubMed] [Google Scholar]
- 10.Moore RD, Stanton D, Gopalan R, Chaisson RE. Racial differences in the use of drug therapy for HIV disease in an urban community. N Engl J Med. 1994;330:763–8. doi: 10.1056/NEJM199403173301107. [DOI] [PubMed] [Google Scholar]
- 11.Wohl AR, Tejero J, Frye DM. Factors associated with late HIV testing for Latinos diagnosed with AIDS in Los Angeles. AIDS Care. 2009;21:1203–10. doi: 10.1080/09540120902729957. [DOI] [PubMed] [Google Scholar]
- 12.Magnus M, Kuo I, Shelley K, et al. Risk factors driving the emergence of a generalized heterosexual HIV epidemic in Washington, District of Columbia networks at risk. AIDS. 2009;23:1277–84. doi: 10.1097/QAD.0b013e32832b51da. [DOI] [PubMed] [Google Scholar]
- 13.Towe VL, Sifakis F, Gindi RM, et al. Prevalence of HIV infection and sexual risk behaviors among individuals having heterosexual sex in low income neighborhoods in Baltimore, MD: the BESURE study. J Acquir Immune Defic Syndr. 2010;53:522–8. doi: 10.1097/QAI.0b013e3181bcde46. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. HIV/AIDS surveillance report. 2000;12:1–44. Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/pdf/hasr1202.pdf. Accessed 7 June 2010. [Google Scholar]
- 15.Centers for Disease Control and Prevention. HIV/AIDS surveillance report. 2001;13:1–44. Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2001report/pdf/2001surveillance-report_year-end.pdf. Accessed 7 June 2010. [Google Scholar]
- 16.Centers for Disease Control and Prevention. HIV/AIDS surveillance report. 2002;14:1–50. Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2002report/pdf/2002SurveillanceReport.pdf. Accessed 7 June 2010. [Google Scholar]
- 17.Centers for Disease Control and Prevention. HIV/AIDS surveillance report. 2003;15:1–46. Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2003report/pdf/2003SurveillanceReport.pdf. Accessed 7 June 2010. [Google Scholar]
- 18.Centers for Disease Control and Prevention. HIV/AIDS surveillance report. 2004;16:1–46. Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2004report/pdf/2004SurveillanceReport.pdf. Accessed 7 June 2010. [Google Scholar]
- 19.Centers for Disease Control and Prevention. HIV/AIDS surveillance report. 2005;17:1–54. Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2005report/pdf/2005SurveillanceReport.pdf. Accessed 7 June 2010. [Google Scholar]
- 20.Centers for Disease Control and Prevention. HIV/AIDS surveillance report. 2006;18:1–55. Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2006report/pdf/2006SurveillanceReport.pdf. Accessed 7 June 2010. [Google Scholar]
- 21.Bartholomew LK, Parcel GS, Kok G, Gottlieb NH. Planning health promotion programs: an intervention mapping approach. San Francisco: Jossey-Bass; 2006. [Google Scholar]
- 22.Purcell DW, McCree DH. Recommendations from a research consultation to address intervention strategies for HIV/AIDS prevention focused on African Americans. Am J Public Health. 2009;99:1937–40. doi: 10.2105/AJPH.2008.152546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metzger DS, Koblin B, Turner C, et al. Randomized controlled trial of audio computer-assisted self-interviewing: utility and acceptability in longitudinal studies. HIVNET Vaccine Preparedness Study Protocol Team. Am J Epidemiol. 2000;152:99–106. doi: 10.1093/aje/152.2.99. [DOI] [PubMed] [Google Scholar]
- 24.Perlis TE, Des Jarlais DC, Friedman SR, Arasteh K, Turner CF. Audio-computerized self-interviewing versus face-to-face interviewing for research data collection at drug abuse treatment programs. Addiction. 2004;99:885–96. doi: 10.1111/j.1360-0443.2004.00740.x. [DOI] [PubMed] [Google Scholar]
- 25.Ghanem KG, Hutton HE, Zenilman JM, Zimba R, Erbelding EJ. Audio computer assisted self interview and face to face interview modes in assessing response bias among STD clinic patients. Sex Transm Infect. 2005;81:421–5. doi: 10.1136/sti.2004.013193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macalino GE, Celentano DD, Latkin C, Strathdee SA, Vlahov D. Risk behaviors by audio computer-assisted self-interviews among HIV-seropositive and HIV-seronegative injection drug users. AIDS Educ Prev. 2002;14:367–78. doi: 10.1521/aeap.14.6.367.24075. [DOI] [PubMed] [Google Scholar]
- 27.Tempalski B, Lieb S, Cleland CM, Cooper H, Brady JE, Friedman SR. HIV prevalence rates among injection drug users in 96 large US metropolitan areas, 1992–2002. J Urban Health. 2009;86:132–54. doi: 10.1007/s11524-008-9328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Des Jarlais DC, Perlis T, Arasteh K, et al. HIV incidence among injection drug users in New York City, 1990 to 2002: use of serologic test algorithm to assess expansion of HIV prevention services. Am J Public Health. 2005;95:1439–44. doi: 10.2105/AJPH.2003.036517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santibanez SS, Garfein RS, Swartzendruber A, Purcell DW, Paxton LA, Greenberg AE. Update and overview of practical epidemiologic aspects of HIV/AIDS among injection drug users in the United States. J Urban Health. 2006;83:86–100. doi: 10.1007/s11524-005-9009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Des Jarlais DC, Semaan S. HIV prevention for injecting drug users: the first 25 years and counting. Psychosom Med. 2008;70:606–11. doi: 10.1097/PSY.0b013e3181772157. [DOI] [PubMed] [Google Scholar]
- 31.Beckwith CG, Moreira CC, Aboshady HM, Zaller N, Rich JD, Flanigan TP. A success story: HIV prevention for injection drug users in Rhode Island. Subst Abuse Treat Prev Policy. 2006;1:34. doi: 10.1186/1747-597X-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. HIV infection among injection-drug users - 34 states, 2004–2007. MMWR Morb Mortal Wkly Rep. 2009;58:1291–5. [PubMed] [Google Scholar]
- 33.Risser JM, Padgett P, Wolverton M, Risser WL. Relationship between heterosexual anal sex, injection drug use and HIV infection among black men and women. Int J STD AIDS. 2009;20:310–4. doi: 10.1258/ijsa.2008.008394. [DOI] [PubMed] [Google Scholar]
- 34.McCoy C, Lai S, Metsch L, Messiah S, Zhao W. Injection drug use and crack cocaine smoking: independent and dual risk behaviors for HIV infection. Ann Epidemiol. 2004;14:535–42. doi: 10.1016/j.annepidem.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Williams ML, Elwood WN, Weatherby NL, et al. An assessment of the risks of syphilis and HIV infection among a sample of not-in-treatment drug users in Houston, Texas. AIDS Care. 1996;8:671–82. doi: 10.1080/09540129650125380. [DOI] [PubMed] [Google Scholar]
- 36.Booth RE, Kwiatkowski CF, Chitwood DD. Sex related HIV risk behaviors: differential risks among injection drug users, crack smokers, and injection drug users who smoke crack. Drug Alcohol Depend. 2000;58:219–26. doi: 10.1016/s0376-8716(99)00094-0. [DOI] [PubMed] [Google Scholar]
- 37.Adimora AA, Schoenbach VJ, Martinson FE, Donaldson KH, Stancil TR, Fullilove RE. Concurrent partnerships among rural African Americans with recently reported heterosexually transmitted HIV infection. J Acquir Immune Defic Syndr. 2003;34:423–9. doi: 10.1097/00126334-200312010-00010. [DOI] [PubMed] [Google Scholar]
- 38.Rees V, Saitz R, Horton NJ, Samet J. Association of alcohol consumption with HIV sex- and drug-risk behaviors among drug users. J Subst Abuse Treat. 2001;21:129–34. doi: 10.1016/s0740-5472(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 39.Kalichman SC, Cain D, Zweben A, Swain G. Sensation seeking, alcohol use and sexual risk behaviors among men receiving services at a clinic for sexually transmitted infections. J Stud Alcohol. 2003;64:564–9. doi: 10.15288/jsa.2003.64.564. [DOI] [PubMed] [Google Scholar]
- 40.Shuper PA, Neuman M, Kanteres F, Baliunas D, Joharchi N, Rehm J. Causal considerations on alcohol and HIV/AIDS—a systematic review. Alcohol Alcohol. 2010;45:159–66. doi: 10.1093/alcalc/agp091. [DOI] [PubMed] [Google Scholar]
- 41.Baliunas D, Rehm J, Irving H, Shuper P. Alcohol consumption and risk of incident human immunodeficiency virus infection: a meta-analysis. Int J Public Health. 2010;55:159–66. doi: 10.1007/s00038-009-0095-x. [DOI] [PubMed] [Google Scholar]
- 42.Rondinelli AJ, Ouellet LJ, Strathdee SA, et al. Young adult injection drug users in the United States continue to practice HIV risk behaviors. Drug Alcohol Depend. 2009;104:167–74. doi: 10.1016/j.drugalcdep.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Cheng WS, Garfein RS, Semple SJ, Strathdee SA, Zians JK, Patterson TL. Binge use and sex and drug use behaviors among HIV(-), heterosexual methamphetamine users in San Diego. Subst Use Misuse. 2010;45:116–33. doi: 10.3109/10826080902869620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lichtenstein B. Secret encounters: black men, bisexuality, and AIDS in Alabama. Med Anthropol Q. 2000;14:374–93. doi: 10.1525/maq.2000.14.3.374. [DOI] [PubMed] [Google Scholar]
- 45.Williams CT, Mackesy-Amiti ME, McKirnan DJ, Ouellet LJ. Differences in sexual identity, risk practices, and sex partners between bisexual men and other men among a low-income drug-using sample. J Urban Health. 2009;86:93–106. doi: 10.1007/s11524-009-9367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorbach PM, Murphy R, Weiss RE, Hucks-Ortiz C, Shoptaw S. Bridging sexual boundaries: men who have sex with men and women in a street-based sample in Los Angeles. J Urban Health. 2009;86:63–76. doi: 10.1007/s11524-009-9370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kent JB. Impact of foreign-born persons on HIV diagnosis rates among blacks in King County, Washington. AIDS Educ Prev. 2005;17:60–7. doi: 10.1521/aeap.2005.17.Supplement_B.60. [DOI] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. Medical examination of aliens: removal of human immunodeficiency virus (HIV) infection from definition of communicable disease of public health significance. Fed Regist. 2009;74:56547–62. [PubMed] [Google Scholar]
- 49.Beckwith CG, DeLong AK, Desjardins SF, et al. HIV infection in refugees: a case-control analysis of refugees in Rhode Island. Int J Infect Dis. 2009;13:186–92. doi: 10.1016/j.ijid.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerani RP, Kent JB, Sides T, et al. HIV among African-born persons in the United States: a hidden epidemic? J Acquir Immune Defic Syndr. 2008;49:102–6. doi: 10.1097/QAI.0b013e3181831806. [DOI] [PubMed] [Google Scholar]
- 51.Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. LoS One. 2009;4:e7558. doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valera P, Epperson M, Daniels J, Ramaswamy M, Freudenberg N. Substance use and HIV-risk behaviors among young men involved in the criminal justice system. Am J Drug Alcohol Abuse. 2009;35:43–7. doi: 10.1080/00952990802342923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grinstead OA, Faigeles B, Comfort M, et al. HIV, STD, and hepatitis risk to primary female partners of men being released from prison. Women Health. 2005;41:63–80. doi: 10.1300/J013v41n02_05. [DOI] [PubMed] [Google Scholar]
- 54.Khan MR, Wohl DA, Weir SS, et al. Incarceration and risky sexual partnerships in a southern US city. J Urban Health. 2008;85:100–13. doi: 10.1007/s11524-007-9237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kidder DP, Wolitski RJ, Pals SL, Campsmith ML. Housing status and HIV risk behaviors among homeless and housed persons with HIV. J Acquir Immune Defic Syndr. 2008;49:451–5. doi: 10.1097/qai.0b013e31818a652c. [DOI] [PubMed] [Google Scholar]
- 56.Morrow KM. HIV, STD, and hepatitis risk behaviors of young men before and after incarceration. AIDS Care. 2009;21:235–43. doi: 10.1080/09540120802017586. [DOI] [PubMed] [Google Scholar]
- 57.Khan MR, Doherty IA, Schoenbach VJ, Taylor EM, Epperson MW, Adimora AA. Incarceration and high-risk sex partnerships among men in the United States. J Urban Health. 2009;86:584–601. doi: 10.1007/s11524-009-9348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Margolis AD, MacGowan RJ, Grinstead O, Sosman J, Kashif I, Flanigan TP. Unprotected sex with multiple partners: implications for HIV prevention among young men with a history of incarceration. Sex Transm Dis. 2006;33:175–80. doi: 10.1097/01.olq.0000187232.49111.48. [DOI] [PubMed] [Google Scholar]
- 59.Rich JD, Holmes L, Salas C, et al. Successful linkage of medical care and community services for HIV-positive offenders being released from prison. J Urban Health. 2001;78:279–89. doi: 10.1093/jurban/78.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harzke AJ, Ross MW, Scott DP. Predictors of post-release primary care utilization among HIV-positive prison inmates: a pilot study. IDS Care. 2006;18:290–301. doi: 10.1080/09540120500161892. [DOI] [PubMed] [Google Scholar]
- 61.Wang EA, White MC, Jamison R, Goldenson J, Estes M, Tulsky JP. Discharge planning and continuity of health care: findings from the San Francisco County Jail. Am J Public Health. 2008;98:2182–4. doi: 10.2105/AJPH.2007.119669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clements-Nolle K, Marx R, Pendo M, Loughran E, Estes M, Katz M. Highly active antiretroviral therapy use and HIV transmission risk behaviors among individuals who are HIV infected and were recently released from jail. Am J Public Health. 2008;98:661–6. doi: 10.2105/AJPH.2007.112656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Springer SA, Pesanti E, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clin Infect Dis. 2004;38:1754–60. doi: 10.1086/421392. [DOI] [PubMed] [Google Scholar]
- 64.White MC, Tulsky JP, Estes M, Jamison R, Long HL. Health and health behaviors in HIV-infected jail inmates, 1999 and 2005. AIDS Patient Care STDS. 2008;22:221–31. doi: 10.1089/apc.2007.0043. [DOI] [PubMed] [Google Scholar]
- 65.Baillargeon J, Borucki MJ, Zepeda S, Jenson HB, Leach CT. Antiretroviral prescribing patterns in the Texas prison system. Clin Infect Dis. 2000;31:1476–81. doi: 10.1086/317478. [DOI] [PubMed] [Google Scholar]
- 66.Baillargeon JG, Giordano TP, Harzke AJ, Baillargeon G, Rich JD, Paar DP. Enrollment in outpatient care among newly released prison inmates with HIV infection. Public Health Rep. 2010;125:64–71. doi: 10.1177/00333549101250S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grinstead O, Seal DW, Wolitski R, et al. HIV and STD testing in prisons: perspectives of in-prison service providers. AIDS Educ Prev. 2003;15:547–60. doi: 10.1521/aeap.15.7.547.24045. [DOI] [PubMed] [Google Scholar]
- 68.Bruce RD, Kresina TF, McCance-Katz EF. Medication-assisted treatment and HIV/AIDS: aspects in treating HIV-infected drug users. AIDS. 2010;24:331–40. doi: 10.1097/QAD.0b013e32833407d3. [DOI] [PubMed] [Google Scholar]
- 69.Celentano DD, Galai N, Sethi AK, et al. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 2001;15:1707–15. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- 70.Rumptz MH, Tobias C, Rajabiun S, et al. Factors associated with engaging socially marginalized HIV-positive persons in primary care. AIDS Patient Care STDS. 2007;21:S30–9. doi: 10.1089/apc.2007.9989. [DOI] [PubMed] [Google Scholar]
- 71.Malta M, Strathdee SA, Magnanini MM, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction. 2008;103:1242–57. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- 72.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45:770–8. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kerr T, Marshall A, Walsh J, et al. Determinants of HAART discontinuation among injection drug users. AIDS Care. 2005;17:539–49. doi: 10.1080/09540120412331319778. [DOI] [PubMed] [Google Scholar]
- 74.Nunn A, Zaller N, Dickman S, Trimbur C, Nijhawan A, Rich JD. Methadone and buprenorphine prescribing and referral practices in US prison systems: results from a nationwide survey. Drug Alcohol Depend. 2009;105:83–8. doi: 10.1016/j.drugalcdep.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitchell SG, Kelly SM, Brown BS, et al. Incarceration and opioid withdrawal: the experiences of methadone patients and out-of-treatment heroin users. J Psychoactive Drugs. 2009;41:145–52. doi: 10.1080/02791072.2009.10399907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O'Grady KE. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. J Subst Abuse Treat. 2009;37:277–85. doi: 10.1016/j.jsat.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kinlock TW, Gordon MS, Schwartz RP, O'Grady K, Fitzgerald TT, Wilson M. A randomized clinical trial of methadone maintenance for prisoners: results at 1-month post-release. Drug Alcohol Depend. 2007;91:220–7. doi: 10.1016/j.drugalcdep.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kinlock TW, Gordon MS, Schwartz RP, O'Grady KE. A study of methadone maintenance for male prisoners: 3-month postrelease outcomes. Crim Justice Behav. 2008;35:34–47. doi: 10.1177/0093854807309111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT. Developing and implementing a new prison-based buprenorphine treatment program. J Offender Rehabil. 2010;49:91–109. doi: 10.1080/10509670903534951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Green T, Zaller ND, Parikh A, et al. Initiation of buprenorphine during incarceration and linkage to treatment upon release [abstract WEPE0187] Program of the XVIII International AIDS Conference. 18–23 July 2010; Vienna. [Google Scholar]
- 81.Zaller ND, Mckenzie M, Green T, et al. Initiation of methadone during incarceration and linkage to treatment upon release: results of a randomized control trial [abstract THPDX103] Program of the XVII International AIDS Conference. 18–23 July 2010; Vienna. [Google Scholar]
- 82.Magura S, Lee JD, Hershberger J, et al. Buprenorphine and methadone maintenance in jail and post-release: a randomized clinical trial. Drug Alcohol Depend. 2009;99:222–30. doi: 10.1016/j.drugalcdep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Springer SA, Chen S, Altice FL. Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: the impact of buprenorphine treatment. J Urban Health. 2010;87:592–602. doi: 10.1007/s11524-010-9438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McKenzie M, Nunn A, Zaller ND, Bazazi AR, Rich JD. Overcoming obstacles to implementing methadone maintenance therapy for prisoners: implications for policy and practice. J Opioid Manag. 2009;5:219–27. doi: 10.5055/jom.2009.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith-Rohrberg D, Mezger J, Walton M, Bruce RD, Altice FL. Impact of enhanced services on virologic outcomes in a directly administered antiretroviral therapy trial for HIV-infected drug users. J Acquir Immune Defic Syndr. 2006;43:S48–53. doi: 10.1097/01.qai.0000248338.74943.85. [DOI] [PubMed] [Google Scholar]
- 86.Basu S, Smith-Rohrberg D, Bruce RD, Altice FL. Models for integrating buprenorphine therapy into the primary HIV care setting. Clin Infect Dis. 2006;42:716–21. doi: 10.1086/500200. [DOI] [PubMed] [Google Scholar]
- 87.Sullivan LE, Bruce RD, Haltiwanger D, et al. Initial strategies for integrating buprenorphine into HIV care settings in the United States. Clin Infect Dis. 2006;43:S191–6. doi: 10.1086/508183. [DOI] [PubMed] [Google Scholar]
- 88.Khalsa J, Vocci F, Altice F, Fiellin D, Miller V. Buprenorphine and HIV primary care: new opportunities for integrated treatment. Clin Infect Dis. 2006;43:S169–72. doi: 10.1086/508179. [DOI] [PubMed] [Google Scholar]
- 89.Lum PJ, Tulsky JP. The medical management of opioid dependence in HIV primary care settings. Curr HIV/AIDS Rep. 2006;3:195–204. doi: 10.1007/s11904-006-0016-z. [DOI] [PubMed] [Google Scholar]
- 90.Bruce R. Medical interventions for addictions in the primary care setting. Top HIV Med. 2010;18:8–12. [PubMed] [Google Scholar]
- 91.Mizuno Y, Wilkinson JD, Santibanez S, et al. Correlates of health care utilization among HIV-seropositive injection drug users. AIDS Care. 2006;18:417–25. doi: 10.1080/09540120500162247. [DOI] [PubMed] [Google Scholar]
- 92.Lucas G, Chaudhry A, Hsu J, et al. Clinic-based treatment of opiod-dependent HIV-infected patients versus referral to an opiod treatment program. Ann Intern Med. 2010;152:704–11. doi: 10.1059/0003-4819-152-11-201006010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kushel MB, Colfax G, Ragland K, Heineman A, Palacio H, Bangsberg DR. Case management is associated with improved antiretroviral adherence and CD4+ cell counts in homeless and marginally housed individuals with HIV infection. Clin Infect Dis. 2006;43:234–42. doi: 10.1086/505212. [DOI] [PubMed] [Google Scholar]
- 94.Bristow DP, Herrick CA. Emergency department case management: the dyad team of nurse case manager and social worker improve discharge planning and patient and staff satisfaction while decreasing inappropriate admissions and costs: a literature review. Lippincotts Case Manag. 2001;7:243–51. doi: 10.1097/00129234-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 95.Broadhead RS, Heckathorn DD, Altice FL, et al. Increasing drug users' adherence to HIV treatment: results of a peer-driven intervention feasibility study. Soc Sci Med. 2002;55:235–46. doi: 10.1016/s0277-9536(01)00167-8. [DOI] [PubMed] [Google Scholar]
- 96.Losina E, Schackman BR, Sadownik SN, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the United States: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009;49:1570–8. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ulett KB, Willig JH, Lin HY, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009;23:41–9. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wells K, Klap R, Koike A, Sherbourne C. Ethnic disparities in unmet need for alcoholism, drug abuse, and mental health care. Am J Psychiatry. 2001;158:2027–32. doi: 10.1176/appi.ajp.158.12.2027. [DOI] [PubMed] [Google Scholar]
- 99.Porter J. The street/treatment barrier: treatment experiences of Puerto Rican injection drug users. Subst Use Misuse. 1999;34:1951–75. doi: 10.3109/10826089909039434. [DOI] [PubMed] [Google Scholar]
- 100.Office of Minority Health, U.S. Department of Health and Human Services. Assessment of state minority health infrastructure and capacity to address issues of health disparity. Washington, DC: US Department of Health and Human Services; 2000. Available at: http://minorityhealth.hhs.gov/Assets/pdf/checked/1/OMHHealthDisparityFRSept00.pdf. Accessed 7 June 2010. [Google Scholar]
- 101.Lundgren LM, Amodeo M, Ferguson F, Davis K. Racial and ethnic differences in drug treatment entry of injection drug users in Massachusetts. J Subst Abuse Treat. 2001;21:145–53. doi: 10.1016/s0740-5472(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 102.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38:96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 103.Smedley BD, Stith AY, Nelson AR. Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Unequal treatmentconfronting racial and ethnic disparities in health care. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 104.Zaller ND, Bazazi AR, Velazquez L, Rich JD. Attitudes toward methadone among out-of-treatment minority injection drug users: implications for health disparities. Int J Environ Res Public Health. 2009;6:787–97. doi: 10.3390/ijerph6020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Musa D, Schulz R, Harris R, Silverman M, Thomas SB. Trust in the health care system and the use of preventive health services by older black and white adults. Am J Public Health. 2009;99:1293–9. doi: 10.2105/AJPH.2007.123927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dovidio JF, Penner LA, Albrecht TL, Norton WE, Gaertner SL, Shelton JN. Disparities and distrust: the implications of psychological processes for understanding racial disparities in health and health care. Soc Sci Med. 2008;67:478–86. doi: 10.1016/j.socscimed.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 107.Armstrong K, Ravenell KL, McMurphy S, Putt M. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283–9. doi: 10.2105/AJPH.2005.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bradford JB. The promise of outreach for engaging and retaining out-of-care persons in HIV medical care. AIDS Patient Care STDS. 2007;21:S85–91. doi: 10.1089/apc.2007.9983. [DOI] [PubMed] [Google Scholar]
- 109.McKleroy VS, Galbraith JS, Cummings B, et al. Adapting evidence-based behavioral interventions for new settings and target populations. AIDS Educ Prev. 2006;18:59–73. doi: 10.1521/aeap.2006.18.supp.59. [DOI] [PubMed] [Google Scholar]
- 110.Molitor F, Waltermeyer J, Mendoza M, et al. Locating and linking to medical care HIV-positive persons without a history of care: findings from the California Bridge Project. AIDS Care. 2006;18:456–9. doi: 10.1080/09540120500217397. [DOI] [PubMed] [Google Scholar]
- 111.Rajabiun S, Mallinson RK, McCoy K, et al. “Getting me back on track”: the role of outreach interventions in engaging and retaining people living with HIV/AIDS in medical care. AIDS Patient Care STDS. 2007;21:S209. doi: 10.1089/apc.2007.9990. [DOI] [PubMed] [Google Scholar]
- 112.Naar-King S, Bradford J, Coleman S, Green-Jones M, Cabral H, Tobias C. Retention in care of persons newly diagnosed with HIV: outcomes of the Outreach Initiative. AIDS Patient Care STDS. 2007;21:S40–8. doi: 10.1089/apc.2007.9988. [DOI] [PubMed] [Google Scholar]
- 113.Cabral HJ, Tobias C, Rajabiun S, et al. Outreach program contacts: do they increase the likelihood of engagement and retention in HIV primary care for hard-to-reach patients? AIDS Patient Care STDS. 2007;21:S59–67. doi: 10.1089/apc.2007.9986. [DOI] [PubMed] [Google Scholar]
- 114.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19:423–31. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 115.Gardner LI, Marks G, Metsch LR, et al. Psychological and behavioral correlates of entering care for HIV infection: the Antiretroviral Treatment Access Study (ARTAS) AIDS Patient Care STDS. 2007;21:418–25. doi: 10.1089/apc.2006.0115. [DOI] [PubMed] [Google Scholar]
- 116.Cunningham CO, Sanchez JP, Li X, Heller D, Sohler NL. Medical and support service utilization in a medical program targeting marginalized HIV-infected individuals. J Health Care Poor Underserved. 2008;19:981–90. doi: 10.1353/hpu.0.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]