Abstract
Grounded in a socio-ecological framework, we describe salient health care system and policy factors that influence engagement in human immunodeficiency virus (HIV) clinical care. The discussion emphasizes successful programs and models of service delivery and highlights the limitations of current, fragmented health care system components in supporting effective, efficient, and sustained patient engagement across a continuum of care. A fundamental need exists for improved synergies between funding and service agencies that provide HIV testing, prevention, treatment, and supportive services. We propose a feedback loop whereby actionable, patient-level surveillance of HIV testing and engagement in care activities inform educational outreach and resource allocation to support integrated “testing and linkage to care plus” service delivery. Ongoing surveillance of programmatic performance in achieving defined benchmarks for linkage of patients who have newly diagnosed HIV infection and retention of those patients in care is imperative to iteratively inform further educational efforts, resource allocation, and refinement of service delivery.
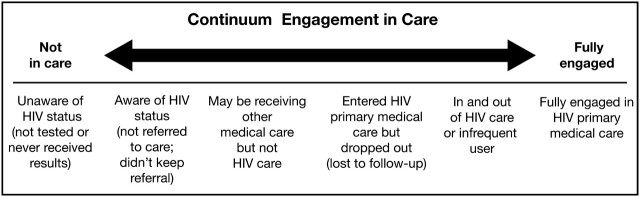
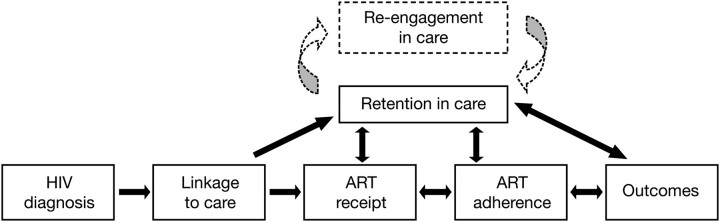
In recent years, the concept of adherence for patients infected with the human immunodeficiency virus (HIV) has expanded beyond antiretroviral therapy (ART) to include adherence to clinical care [1], which is commonly referred to as engagement in care. The Health Resources and Services Administration (HRSA) has operationalized a continuum of engagement [2, 3], ranging from those who are not aware of their HIV status through individuals who are fully engaged in HIV care (Figure 1). Embedded within this continuum of care are the processes of engagement: linkage, retention, and re-engagement in care for those patients who are lost to follow-up (Figure 2)[4].
Figure 1.
Health Resources and Services Administration, Human Immunodeficiency Virus (HIV)/AIDS Bureau, continuum of engagement in HIV care. Reprinted from Cheever [2] with permission from the University of Chicago Press.
Figure 2.
Blueprint for human immunodeficiency virus (HIV) treatment success outlining the requisite steps from HIV testing and diagnosis to achieve optimal clinical outcomes. The processes of engagement in HIV medical care—linkage, retention, and re-engagement—are essential intermediaries for achieving maximal success of a ”test and treat” approach to secondary HIV prevention. ART, antiretroviral therapy. Adapted from Ulett et al [4] with permission from Mary Ann Liebert.
In addition to having well-documented health implications for individual patients [5–7], engagement in HIV care plays a vital role in preventing new HIV infections, which is a critical public health consideration. A “test and treat” (TnT) approach to preventing HIV infections has garnered considerable interest and enthusiasm in a relatively short period of time [8]. Notably, the success of TnT is contingent upon linkage of patients with newly diagnosed infection (the “test” portion of TnT) to clinical care, such that access to ART (the “treat” portion of TnT) is achieved [9].
The vital role of retention in care beyond initial linkage to ensure sustained ART cannot be overstated. Numerous studies have documented high rates of attrition within the first year after enrollment in HIV care, and poor retention has been linked with inconsistent ART receipt and adherence [4, 5, 7, 10]. In an attempt to more explicitly define the process, TnT has recently been referred to as “Testing, Linkage to Care plus” (TLC+), where the “plus” equals ART [11]. To date, however, there are relatively few evidence-based behavioral- or systems-level interventions designed to address each of the essential steps of engagement in care. Moreover, identified efficacious programs have yet to be widely disseminated, implemented, and integrated as standard of care, thereby limiting their potential impact on public health.
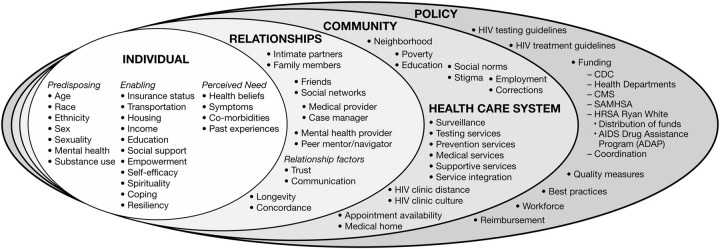
In the current article, our objectives in discussing engagement in HIV care are 3-fold: (1) to review health care system– and policy-level factors that influence engagement in HIV care after HIV testing and diagnosis, (2) to describe successful programs and health care systems, and (3) to provide initial system- and policy-level recommendations to improve the current state-of-the-science and standard of practice. A socio-ecological perspective (Figure 3) provides a framework for conceptualizing the complex interplay of individual-, relationship-, community-, health care system–, and policy-level factors that influence the processes of engagement in HIV care [12].
Figure 3.
A social-ecological perspective, which provides the framework for this article's arguments, serves as a road map outlining the complex interplay of individual, relationship, community, health care system, and policy factors that influence the processes of engagement in care. CDC, Centers for Disease Control and Prevention; CMS, Center for Medicare and Medicaid Services; HIV, human immunodeficiency virus; HRSA, Health Resources and Services Administration; SAMHSA, Substance Abuse and Mental Health Services Administration.
HEALTH CARE SYSTEM
Surveillance
Real-time surveillance of all components of engagement in HIV care is critical to maximize the use of limited resources, guide the deployment of evidence-based interventions, and monitor and respond appropriately to emerging outbreaks, to optimize individual- and population-level HIV health outcomes. Notably, the ability to track and respond effectively to true HIV infection incidence in real time is an important goal and, although extraordinarily difficult to implement, remains a top priority.
Unfortunately, current health care systems have limited patient-level surveillance data regarding engagement in HIV care and very little capability to measure actual incident cases of HIV infection [13]. Estimates of the number of patients in HIV medical care are often based on dated surveillance data or extrapolated by merging utilization and claims data collected from public and private sources to provide relatively crude population estimates [14, 15]. The Centers for Disease Control and Prevention (CDC) Medical Monitoring Project has developed a representative sample to allow for surveillance of clinical outcomes and behaviors among a nationally representative sample of HIV-infected patients that will be used to inform prevention and service delivery at a population level [16]. The shift to patient-level reporting for individuals who receive HIV supportive and/or treatment services funded by the Ryan White HIV/AIDS Program may also allow for improved surveillance for patients supported by this program, who are estimated to account for roughly one-half of HIV-infected individuals in the United States. However, more actionable and innovate systems that capture real-time surveillance data are required to adequately identify engagement in HIV care challenge areas and to ultimately guide health care policy.
HIV Testing
Linkage to care is clearly dependant upon HIV testing and timely receipt of results. However, despite continued emphasis on the importance of linkage to medical services for patients with newly diagnosed infections [17], the activities of testing and linkage are often uncoupled. Moreover, surveillance of the proportion of patients with newly diagnosed infection entering medical care in a timely fashion is often not captured by organizations that provide HIV testing services. Benchmarks of success in this area are not well defined and, accordingly, are not integrated into HIV testing service delivery and funding paradigms. Failure to tightly link HIV testing to HIV care is a key reason for the estimated 300,000 patients who, despite knowing their HIV status, are not in care and thus pose a greater risk for transmitting HIV to uninfected individuals [18].
Supportive Services
The importance of supportive services in fostering engagement by HIV-infected individuals in HIV care has long been recognized. Case management, mental health services, substance abuse treatment, transportation, and housing assistance have proven to be invaluable in linking HIV-infected patients to and retaining them in care [19, 20]. Despite funding through the Ryan White HIV/AIDS Program, limited availability and accessibility of these services are frequently encountered by HIV-infected patients across the United States.
Clinical Care Services
At the clinic level, delays from initial call to initial medical visit have been associated with HIV-infected patients' failure to engage in HIV care [21]. Lack of flexibility of clinic hours to accommodate work schedules and dependant care activities also serves as a barrier to consistent HIV care [6]. Within the clinic, culturally appropriate services, coupled with a prevailing climate of acceptance of all individuals, are essential components for encouraging patients to both seek and remain in care. Similarly, the importance of the patient-provider relationship has been linked to adherence to ART and to HIV care [6, 22], highlighting the need for system-level implementation of educational and training activities for health care providers and clinic staff members who care for HIV-infected individuals.
HEALTH CARE POLICY
HIV Testing and Treatment Guidelines
The revised CDC HIV testing recommendations, which call for voluntary, routine, opt-out HIV testing of persons aged 13–64 years in all health care settings, have resulted in numerous initiatives to expand such services in both medical and nonmedical venues [17, 23]. Notably, however, Walensky et al [24] found that targeting HIV screening resources toward ensuring that individuals who have positive test results actually receive their results and are effectively linked to care was, in fact, more cost effective than offering testing to additional people. Although the importance of linkage to care is emphasized in the CDC guidelines, implementation has often focused on increasing the number of tests performed, with considerably less programmatic emphasis on linking patients to HIV care.
Traditionally, HIV treatment guidelines have focused on recommendations regarding the use of ART and the management of opportunistic infections [25–27], with limited attention to the importance of engagement in care. In contrast to prevailing recommendations, the guidelines for primary care of HIV infection issued by the HIV Medicine Association (HIVMA) in 2009 included a new recommendation: “…emphasis should be placed on the importance of adherence to care rather than focusing solely on adherence to medications” [1]. Language that underscores the clinical importance of the expanded spectrum of adherence to include engagement in HIV care should be an essential element of all future HIV treatment guidelines.
Inadequate Funding of HIV Treatment Services
The Ryan White HIV/AIDS Program administered by HRSA has been a life-saving program, serving as a “payer of last resort” for HIV-infected individuals. However, the program is inadequately funded to effectively address the increasing number of patients who rely on these services and who face increasingly complex medical (eg, comorbidities unrelated to AIDS) and supportive care (eg, housing and substance abuse treatment) needs [28]. For example, despite ∼40% increase in the volume of patients, funding for Ryan White Part C increased by only 2.4% from 2002 through 2008 [29]. Furthermore, within the past year, 13 states witnessed the return of waiting lists for their AIDS Drug Assistance Programs (ADAP) [30]. The implications of ADAP waiting lists for HIV testing and care-seeking behaviors at the individual level are largely unknown, but the inability to access ART—whether real or perceived—may reduce a patient's motivation to seek out and/or sustain engagement in HIV care. Beyond the individual-level, ADAP waiting lists may negatively influence community-level attitudes and acceptance of HIV testing and subsequent care-seeking behaviors, particularly among impoverished and underserved communities, if there is a prevailing perception that treatment for HIV infection is not readily available.
Workforce Considerations
In recent years, concerns have been raised regarding the capacity of the workforce and medical infrastructure serving HIV-infected patients to absorb the influx of patients with newly diagnosed infection through expanded HIV testing initiatives [31]. HIVMA and the Forum for Collaborative HIV Research recently conducted a survey of clinic capacity and workforce challenges faced by Ryan White Part C providers, who provide care to >25% of HIV-infected patients in the United States [32]. Workforce shortages were reported by a majority of survey respondents. Moreover, a lack of qualified clinicians specializing in HIV care, which has resulted from poor reimbursement and inadequate funding support, was identified as a considerable challenge in recruiting and retaining providers in primary HIV care in the future. Regional variation was also observed among respondents, with more-pronounced workforce limitations in the South, relative to other regions of the United States, which translated to longer average waiting times for patient appointments in this underserved region.
Provider Reimbursement
Current reimbursement for HIV medical service delivery is inadequate to support the complex, multidisciplinary care needs of a growing and aging HIV-infected population. Indeed, HIV clinical care increasingly focuses on the provision of primary medical care because of increasing rates of diabetes, hypertension, and other medical and psychosocial comorbidities (eg, depression), as well as the medical complexities associated with aging populations, in the HIV-infected population [28]. Despite increased demands on health care providers to adequately respond to the expansive, comprehensive treatment needs of contemporary HIV care, provider reimbursement accounts for <2% of overall health care expenditures for HIV-infected patients, which raises questions about the long-term sustainability of comprehensive HIV medical care [33, 34]. In addition to serving as a deterrent toward recruiting and retaining quality health care providers in the workforce, inadequate reimbursement serves as a disincentive for clinics to provide expansive services, thereby exacerbating challenges in fostering engagement in care.
FRAGMENTATION OF FUNDING AND HIV SERVICE DELIVERY
Despite numerous successes in the delivery of services to HIV-infected patients, service provision remains largely fragmented [35]. HIV testing and prevention activities are largely under the purview of the CDC and local and state health departments. In contrast, HIV treatment and supportive services are funded predominantly through the HRSA, the Center for Medicare and Medicaid Services, and private insurers, with public, private, and nonprofit organizations delivering services at the local level. Although varying levels of integration exist within and between service delivery organizations and funding agencies that provide testing and/or prevention and medical and/or supportive services, the vast majority of activities are uncoordinated. Fragmentation and disparate approaches to these critical activities and surveillance methods undoubtedly hinder effective engagement in HIV care efforts and lead to suboptimal use of limited funds and resources. Individuals with HIV infection rely on testing, prevention, treatment, and supportive services across their lifespan, which they should receive across a continuum, rather than in discrete, fragmented pieces.
The creation of “medical homes” for the provision of comprehensive, co-located HIV services is an incredible success largely afforded by the Ryan White HIV/AIDS Program [36]. However, such homes are inadequate to fully address the complex micro- and macro-level factors pertaining to engagement in HIV care when operating in isolation (Figure 3). A fundamental need exists for improved synergies among the public, private, and nonprofit funding and service agencies responsible for the provision of HIV testing, prevention, treatment, and supportive services across the continuum of engagement [37].
A WAY FORWARD: MODEL PROGRAMS AND SYSTEMS FOR ENGAGEMENT IN CARE
To date, relatively few studies have developed and evaluated patient- and/or systems-level interventions aimed at improving patient engagement in HIV care. As a result, limited empirical data exist to support “best practices” for improving engagement in HIV care, albeit with some notable exceptions. Below, we highlight a few promising patient-level interventions and integrated systems-level approaches that, together, provide a starting point for the development, refinement, and expansion of future efforts to build evidence-based strategies to improve engagement in HIV care.
Case Management
One of the most compelling behavioral interventions to improve HIV-infected patient linkage to care is the CDC's Antiretroviral Treatment Access Study (ARTAS). This randomized, controlled trial evaluated a 90-day strengths-based case management intervention in which individuals with newly diagnosed HIV infection received up to 5 case manager contacts to facilitate linkage to medical care [38]. Indeed, a greater proportion of patients who received the case management–based intervention were enrolled in care within 6 months, compared with patients in the control group (78% vs 60%; P < .001). The absolute effect size of nearly 20% reflects the fact that, for every 5 patients with newly diagnosed infection who received the brief case management intervention, 1 additional person was linked to HIV care, representing a highly favorable number needed to treat. Importantly, similar results were found in an effectiveness trial conducted with 10 community-based organizations and health departments (79% of patients were linked to care after 6 months) [39], providing compelling evidence to support widespread dissemination and implementation of case management intervention in real-world settings.
Navigation Models
In recent years, health system navigation has emerged as a promising approach to improving engagement in HIV care [40]. Patient navigators are often HIV-infected peers or near-peers who share similar cultural and socioeconomic backgrounds as the patient, often playing a distinct and complementary role to case managers and other supportive service providers. Navigators may assist patients in their awareness and utilization of medical and supportive service resources available in a complex, fragmented health care system, and they often also work with patients to develop behavioral skills to improve self-care and enhance patient-provider communication. Patient-level health systems navigation has demonstrated promising results in a series of HRSA Special Projects of National Significance activities [40].
Client-Oriented New Patient Navigation to Encourage Connection to Treatment (Project CONNECT), a system-level, new-patient navigation program, was launched at the University of Alabama at Birmingham 1917 HIV Clinic in January 2007 in response to a 31% new patient “no show” rate [21]. CONNECT employs a patient-focused orientation visit, during which a trained staff member interacts with patients who are establishing care to help identify and troubleshoot anticipated barriers to sustained engagement in HIV care. Orientation visits are conducted within 5 days after the initial patient call, in contrast to the historical average 28-day wait until an initial provider visit. Project CONNECT has proven to be successful; the proportion of patients who failed to enroll in HIV care decreased from 31% to 18% after implementation (odds ratio, 0.54; 95% confidence interval, 0.38–0.76) [41, 42].
Integrated Health Care Systems
Integrated health care systems, such as Kaiser Permanente and the US Department of Veterans Affairs (VA), provide insight into systems-level approaches aimed at improving patient engagement in HIV care. Although such approaches may not be readily transferable to the general health care system, which is more fragmented, they nonetheless provide important examples and lessons learned for future systems-level approaches.
For example, Kaiser Permanente has adopted quality metrics for linkage and retention in HIV care and employs a shared electronic medical record for patient surveillance and to provide feedback to regional networks regarding performance (M. Horberg, personal communication). Eighty-nine percent of patients with newly diagnosed HIV infection in the Kaiser Permanente system are linked to HIV medical care within 90 days, whereas 77% and 86% meet quality measures for retention in care based on medical visits and completion of HIV-specific laboratory tests, respectively [43]. Elements of case management and systems navigation are employed to foster linkage to HIV medical care, and best practices are shared across the Kaiser Permanente network.
The VA provides a wide array of integrated medical and supportive services to a diverse population of eligible beneficiaries across a range of socioeconomic and cultural backgrounds. A multi-component systems intervention capitalizing on a shared electronic medical record across VA centers and incorporating provider education, feedback, electronic reminders, and social marketing has proven to be successful in increasing HIV testing and case identification rates [44]. Currently, practices for linkage to care include identification of an HIV care coordinator at each VA medical center who is alerted to a positive HIV test result and charged with facilitating connection to medical care (A. Gifford, personal communication). Studies are ongoing to develop and test optimal system-level approaches to facilitate engagement in HIV care that will inform best practices and subsequent dissemination and implementation efforts throughout the VA system.
RECOMMENDATIONS
Despite the promise of health care reform and a national HIV/AIDS strategy, a substantial increase in domestic funds for HIV programs is unlikely to occur [45]. To maximize the use of current funds and resources to optimize patient care and health status, a critical appraisal of local and national HIV testing and engagement in HIV care funds for service provision is urgently needed. Decisive actions to improve linkage to and retention in HIV care activities are imperative, such that maximal health benefits of the significant advances in HIV treatment are achieved at the individual and population levels.
We propose a feedback loop, as advocated by others [46], as a systematic approach to address the challenges and improve patient engagement in care within the fragmented American health care system (Figure 4). We suggest that this approach is applicable at both a local and national level, with the urgent need for substantive integration of efforts across agencies to develop and support sustainable implementation and dissemination of evidence-based strategies. It is essential that all key stakeholders at the local and national level, including funders; providers of testing, prevention, clinical and supportive services; and the priority populations that we aim to serve are invested partners in each step of this process [37, 47]. Efforts to bolster the domestic workforce caring for HIV-infected patients, ensure antidiscrimination laws are adequately monitored and enforced, and definitively address ADAP funding to eliminate and avoid future waiting lists are also imminently needed to improve engagement in HIV care.
Figure 4.
Schematic of a proposed feedback loop identifying target areas to improve the processes of engagement in care by patients infected with human immunodeficiency virus (HIV). Enhanced, real-time patient-level surveillance with appropriate attention to patient protections should inform educational initiatives and resource allocation for integrated testing, linkage to care plus (TLC+) service delivery. Ongoing evaluation of programmatic performance in achieving defined benchmarks for linkage of patients with newly diagnosed infection and retention of those in care are imperative to inform feedback and further educational efforts and refinement of service delivery and resource allocation. Although health information technology may facilitate implementation of the steps encompassed in this feedback loop, there is an essential human element integral to the successful performance of these activities.
Moving Toward an Integrated and Coordinated System of HIV Care
Improved, actionable, patient-level surveillance of HIV testing and engagement in HIV care activities is required, with full attention to ensuring individual protections (Figure 4 and Table 1). Real-time surveillance at the local and national levels should inform resource allocation and educational efforts to areas of greatest need. Deliberate educational and social marketing activities addressing the importance of engagement in HIV care are essential for all stakeholder groups, including health professionals; agencies providing HIV testing, prevention, medical, and/or supportive services; and the community at large. Furthermore, HIV testing and treatment guidelines need to place greater emphasis on linkage to and retention in care activities, because these intermediary steps are vital to the success of the TLC+ paradigm. HIV testing and linkage to care must be explicitly coupled, with the expectation that agencies that offer HIV testing implement strategies and monitor performance to effectively link patients with newly diagnosed infection to HIV care.
Table 1.
Recommendations for Advancing the Science and Practice of Engagement in care by Patients Infected with Human Immunodeficiency Virus (HIV)
| Domain | Recommendation(s) |
| Surveillance/evaluation | Develop integrated health informatics systems to collect real-time, actionable, patient-level surveillance of HIV testing and engagement in care activities at both local and national levels. |
| Standardize national quality benchmarks for linkage and retention in care and hold agencies that provide HIV testing, prevention, treatment, and supportive service accountable for meeting minimum standards. | |
| Information/education | Deploy educational and social marketing campaigns aimed at emphasizing the importance of engagement in HIV care. |
| Incorporate engagement in care information in all HIV treatment guidelines as a component of an expanded spectrum of adherence beyond antiretroviral medications and in relation to testing, linkage to care plus (TLC+) initiatives. | |
| Resource allocation | Critical assessment of current funding paradigm and appropriate reallocation of funds to optimize HIV testing, prevention, treatment, and care services to accurately reflect the needs of current HIV patient population. |
| Allocation of federal HIV funds for dissemination and implementation of cost-effective, integrated TLC+ programs. | |
| TLC+ service delivery | Coordinate activities from funding agencies and service delivery organizations that provide HIV testing, prevention, medical and supportive services to facilitate integrated TLC+ programs. |
| Develop additional evidence-based individual- and systems-level interventions to improve linkage and retention in HIV care. Cost-effectiveness, dissemination, and implementation studies are notably lacking and are imperative to inform policy and practice decisions. |
Standardized national quality benchmarks for linkage (eg, 80% within 3–6 months) and retention in care are needed, with transparent reporting required among organizations receiving federal support for service provision. There is an urgent need for pragmatic, evidence-based interventions that are cost-effective and amenable to widespread dissemination. Such TLC+ programs will require improved integration across agencies and service providers, and barriers between HIV testing/prevention and clinical/supportive service funders and providers must be broken down. Furthermore, iterative evaluation and surveillance of program effectiveness in linking patients to and retaining patients in HIV care is essential. Although electronic medical records and health information technology may facilitate efforts across this feedback loop, there is an essential human element at each step that is vital.
With the reality that substantive increases in funding are unlikely to be appropriated despite continued increases in patient volume, the capacity of the health care system in the United States to improve engagement in HIV care can only be addressed through critical appraisal and appropriate redistribution of funds within existing programs [48]. Funding paradigms for HIV service delivery were largely established in the early 1990s, when much of the focus was on helping patients, most of whom resided in large urban cities, to die with dignity. Today, the epidemic has changed significantly, equilibrating to include smaller and intermediate communities and rural areas where HIV-related stigma is rampant and inadequate access to medical services is pervasive. Moreover, HIV care in the present day is increasingly focused on helping HIV-infected patients live long, healthy, and productive lives while effectively managing multiple comorbidities and health complications that occur with aging. Many patients face the dilemma of losing medical benefits currently provided by programs such as Medicaid, Medicare, and the Ryan White Program if they gain full employment and have income levels surpassing eligibility levels. It is imperative that HIV-infected patients are supported and encouraged to seek employment and productivity in the work place without the risk of jeopardizing their health and access to medical treatment.
The programmatic distribution of funds between and within federal agencies supporting HIV testing, prevention, treatment, and supportive services needs to reflect the current patient profile and geographic distribution of the epidemic. Iterative assessment and redistribution of funds to areas of greatest need are essential to optimize efficiency and effectiveness of the federal dollars appropriated to domestic HIV services to maximize the individual and public health benefits that may be achieved by fostering sustained engagement in quality HIV care.
In conclusion, considerable health care system and policy factors serve as impediments to successful engagement in HIV care activities in our fragmented health care system. However, with strong political will and leadership in local communities and across federal agencies, opportunities exist to increase emphasis and improve efforts in this area. The success of such efforts is intimately reliant on breaking down barriers between and integrating surveillance and service efforts and resources across local and national agencies that act across the spectrum of engagement. Decisive and purposeful action is urgently needed to foster improved engagement in HIV medical care. We cannot settle for the status quo; it is just not good enough.
Acknowledgments
We are grateful to a number of individuals for sharing their insights and/or critical review of our manuscript including John Brooks (CDC), Laura Cheever (Health Resources and Services Administration), Allen Gifford (Veterans Affairs), Robert Greenwald (Harvard University), Michael Horberg (Kaiser Permanente), Peter Leone (University of North Carolina-Chapel Hill), Faye Malitz (Health Resources and Services Administration), Rochelle Walensky (Harvard University), and Andrea Weddle (HIV Medical Association).
Financial support. The authors received no direct financial support for this manuscript, but support for related work was provided by National Institute of Mental Health (grant K23MH082641 to M.J.M.), the University of Alabama at Birmingham Center for AIDS Research (P30AI27767), and CFAR-Network of Integrated Clinical Systems (R24AI067039-1).
Supplement sponsorship. This article was published as part of a supplement entitled, “Linkage, Engagement, and Retention in HIV Care,” supported by Bristol-Myers Squibb, Positive Charge Initiative. Editorial support for the supplement was provided by J. Turner at PAREXEL and was funded by Bristol-Myers Squibb, Positive Charge.
Potential conflicts of interest. M.M. has received grant support and/or consulted for Bristol-Myers Squibb, Gilead Sciences, Pfizer, and Tibotec Therapeutics. M.S. has received research support and/or has consulted for Achillion Pharmaceuticals, Avexa, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Merck, Monogram Biosciences, Panacos, Pfizer, Progenics, Roche, Serono, Tanox, Tibotec, Trimeris, and Vertex. W.N.: no conflicts.
References
- 1.Aberg JA, Kaplan JE, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 2.Cheever LW. Engaging HIV-infected patients in care: their lives depend on it. Clin Infect Dis. 2007;44:1500–1502. doi: 10.1086/517534. [DOI] [PubMed] [Google Scholar]
- 3.Health Resources and Services Administration, HIV/AIDS Bureau. Outreach: engaging people in HIV care, summary of a HRSA/HAB 2005 consultation on linking PLWH into care. 2006 ftp://ftp.hrsa.gov/hab/HIVoutreach.pdf. Accessed 13 May 2010. [Google Scholar]
- 4.Ulett KB, Willig JH, Lin HY, et al. The therapeutic implications of timely linkage and early retention in HIV care. AIDS Patient Care STDS. 2009;23:41–49. doi: 10.1089/apc.2008.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giordano TP, Gifford AL, White AC, Jr, et al. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 6.Horstmann E, Brown J, Islam F, Buck J, Agins BD. Retaining HIV-infected patients in care: Where are we? Where do we go from here? Clin Infect Dis. 2010;50:752–761. doi: 10.1086/649933. [DOI] [PubMed] [Google Scholar]
- 7.Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48:248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 9.Dieffenbach CW, Fauci AS. Universal voluntary testing and treatment for prevention of HIV transmission. JAMA. 2009;301:2380–2382. doi: 10.1001/jama.2009.828. [DOI] [PubMed] [Google Scholar]
- 10.Giordano TP, White AC, Jr., Sajja P, et al. Factors associated with the use of highly active antiretroviral therapy in patients newly entering care in an urban clinic. J Acquir Immune Defic Syndr. 2003;32:399–405. doi: 10.1097/00126334-200304010-00009. [DOI] [PubMed] [Google Scholar]
- 11.Project Inform. TLC+: testing, linkage to care and treatment. http://www.projectinform.org/tlc+/. Accessed 13 May 2010. [Google Scholar]
- 12.McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q. 1988;15:351–377. doi: 10.1177/109019818801500401. [DOI] [PubMed] [Google Scholar]
- 13.Brookmeyer R. Measuring the HIV/AIDS epidemic: approaches and challenges. Epidemiol Rev. 2010;32:26–37. doi: 10.1093/epirev/mxq002. [DOI] [PubMed] [Google Scholar]
- 14.Bozzette SA, Berry SH, Duan N, et al. The care of HIV-infected adults in the United States. HIV Cost and Services Utilization Study Consortium. N Engl J Med. 1998;339:1897–1904. doi: 10.1056/NEJM199812243392606. [DOI] [PubMed] [Google Scholar]
- 15.Fleming P, Byers R, Sweeney P, Daniels D, Karon J, Jansen R. Program and abstracts of the 9th Conference on Retroviruses and Opportunistic Infections, Seattle. HIV prevalence in the United States, 2000 [abstract 11] pp. 24–28. February 2002. [Google Scholar]
- 16.McNaghten AD, Wolfe MI, Onorato I, et al. Improving the representativeness of behavioral and clinical surveillance for persons with HIV in the United States: the rationale for developing a population-based approach. PLoS One. 2007;2:e550. doi: 10.1371/journal.pone.0000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Morb Mortal Wkly Rep. 2006;55:1–17. quiz CE1–4. [PubMed] [Google Scholar]
- 18.Metsch LR, Pereyra M, Messinger S, et al. HIV transmission risk behaviors among HIV-infected persons who are successfully linked to care. Clin Infect Dis. 2008;47:577–584. doi: 10.1086/590153. [DOI] [PubMed] [Google Scholar]
- 19.Evaluating the contribution of ancillary services in improving access to primary care in the United States under the Ryan White CARE Act. AIDS Care. 2002;14:3–136. [Google Scholar]
- 20.Katz MH, Cunningham WE, Fleishman JA, et al. Effect of case management on unmet needs and utilization of medical care and medications among HIV-infected persons. Ann Intern Med. 2001;135:557–565. doi: 10.7326/0003-4819-135-8_part_1-200110160-00006. [DOI] [PubMed] [Google Scholar]
- 21.Mugavero MJ, Lin HY, Allison JJ, et al. Failure to establish HIV care: characterizing the “no show” phenomenon. Clin Infect Dis. 2007;45:127–130. doi: 10.1086/518587. [DOI] [PubMed] [Google Scholar]
- 22.Schneider J, Kaplan SH, Greenfield S, Li W, Wilson IB. Better physician-patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. J Gen Intern Med. 2004;19:1096–1103. doi: 10.1111/j.1525-1497.2004.30418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelen GD, Rothman RE. Emergency department-based HIV testing: too little, but not too late. Ann Emerg Med. 2009;54:65–71. doi: 10.1016/j.annemergmed.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 24.Walensky RP, Weinstein MC, Smith HE, Freedberg KA, Paltiel AD. Optimal allocation of testing dollars: the example of HIV counseling, testing, and referral. Med Decis Making. 2005;25:321–329. doi: 10.1177/0272989X05276955. [DOI] [PubMed] [Google Scholar]
- 25.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 26.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department Health Hum Serv. 1 December 2009; 1–161. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 22 March 2010. [Google Scholar]
- 27.Centers for Disease Control and Prevention. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. quiz CE1–4. [PubMed] [Google Scholar]
- 28.Justice AC. HIV and aging: time for a new paradigm. Curr HIV/AIDS Rep. 2010;7:69–76. doi: 10.1007/s11904-010-0041-9. [DOI] [PubMed] [Google Scholar]
- 29.Saag MS. Which policy to ADAP-T: waiting lists or waiting lines? Clin Infect Dis. 2006;43:1365–1367. doi: 10.1086/508664. [DOI] [PubMed] [Google Scholar]
- 30.National Alliance of State & Territorial AIDS Directors (NASTAD) The ADAP watch. 30 July 2010. http://www.nastad.org/Docs/Public/InFocus/2010730_NASTAD%20ADAP%20Watch%20-%20July%202010.pdf. Accessed 9 September 2010. [Google Scholar]
- 31.Mugavero MJ, Saag MS. HIV care at a crossroads: the emerging crisis in the US HIV epidemic. Med Gen Med. 2007;9:58. [PMC free article] [PubMed] [Google Scholar]
- 32.Weddle A. Program and abstracts of the 2008 National Summit on HIV Diagnosis, Prevention, and Access to Care, Session 3: Workforce needs and challenges in delivery of HIV care, Arlington, Virginia. HIVMA-FCHR survey on workforce needs [presentation] pp. 19–21. November 2008. [Google Scholar]
- 33.Chen RY, Accortt NA, Westfall AO, et al. Distribution of health care expenditures for HIV-infected patients. Clin Infect Dis. 2006;42:1003–1010. doi: 10.1086/500453. [DOI] [PubMed] [Google Scholar]
- 34.Mayer KH, Chaguturu S. Penalizing success: is comprehensive HIV care sustainable? Clin Infect Dis. 2006;42:1011–1013. doi: 10.1086/500463. [DOI] [PubMed] [Google Scholar]
- 35.Penner M, Leone PA. Integration of testing for, prevention of, and access to treatment for HIV infection: state and local perspectives. Clin Infect Dis. 2007;45:S281–S286. doi: 10.1086/522551. [DOI] [PubMed] [Google Scholar]
- 36.Saag MS. Ryan White: an unintentional home builder. AIDS Read. 2009;19:166–168. [PubMed] [Google Scholar]
- 37.Greenberg AE, Hader SL, Masur H, et al. Fighting HIV/AIDS in Washington, D.C. Health Aff (Millwood) 2009;28:1677–1687. doi: 10.1377/hlthaff.28.6.1677. [DOI] [PubMed] [Google Scholar]
- 38.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19:423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 39.Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr. 2008;47:597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 40.Bradford JB, Coleman S, Cunningham W. HIV system navigation: an emerging model to improve HIV care access. AIDS Patient Care STDS. 2007;21:S49–S58. doi: 10.1089/apc.2007.9987. [DOI] [PubMed] [Google Scholar]
- 41.Wylie R, Marler M, Lin H, et al. Program and abstracts of the 4th International Conference on HIV Treatment Adherence. Miami. Project CONNECT: overcoming barriers to establishing HIV care; pp. 5–7. April 2009. [Google Scholar]
- 42.Mugavero MJ. Improving engagement in HIV care: what can we do? Top HIV Med. 2008;16:156–161. [PubMed] [Google Scholar]
- 43.Horberg M, Hurley L, Towner W, et al. Program and abstracts of the 5th International Conference on HIV Treatment Adherence, Miami. Antiretroviral therapy (ART) adherence and HIV RNA (VL) control as HIV quality measures in Kaiser Permanente (KP) [abstract# 61795] pp. 23–25. May 2010. [Google Scholar]
- 44.Goetz MB, Hoang T, Bowman C, et al. A system-wide intervention to improve HIV testing in the Veterans Health Administration. J Gen Intern Med. 2008;23:1200–1207. doi: 10.1007/s11606-008-0637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Office of the White House. National HIV/AIDS Strategy for the United States. July 2010. http://www.whitehouse.gov/sites/default/files/uploads/NHAS.pdf. Accessed 9 September 2010. [Google Scholar]
- 46.Swensen SJ, Meyer GS, Nelson EC, et al. Cottage industry to postindustrial care—the revolution in health care delivery. N Engl J Med. 2010;362:e12. doi: 10.1056/NEJMp0911199. [DOI] [PubMed] [Google Scholar]
- 47.El-Sadr WM, Mayer KH, Hodder SL. AIDS in America—forgotten but not gone. N Engl J Med. 2010;362:967–970. doi: 10.1056/NEJMp1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linas BP, Losina E, Rockwell A, Walensky RP, Cranston K, Freedberg KA. Improving outcomes in state AIDS drug assistance programs. J Acquir Immune Defic Syndr. 2009;51:513–521. doi: 10.1097/QAI.0b013e3181b16d00. [DOI] [PMC free article] [PubMed] [Google Scholar]