HCV incidence from 1996-2008 among HIV-infected men in U.S. HIV therapeutic trials was 0.51 per 100 person-years. Incident HCV occurred primarily through non-parenteral means; 75% of seroconverters reported no drug injection. At-risk HIV-infected persons should have access to HCV surveillance
Abstract
Background. Outbreaks of sexually transmitted hepatitis C virus (HCV) infection have been reported among human immunodeficiency virus (HIV)–infected men who have sex with men in Europe, Australia, and New York. Whether this is occurring across the United States is unknown.
Methods. We determined incidence of HCV infection during 1996–2008 among male participants of the AIDS Clinical Trial Group Longitudinal Linked Randomized Trials cohort, a long-term study of HIV-infected persons randomized into selected US-based clinical trials. We evaluated associations with self-reported injection drug use (IDU), time-varying CD4+ cell count, and HIV RNA level with use of multivariate Poisson regression. No sexual or non-IDU risk factor data was available.
Results. A total of 1830 men had an initial negative HCV antibody test result and at least 1 subsequent HCV antibody test result, contributing >7000 person-years. At the time of the initial negative HCV antibody test result, 94% of men were receiving highly active antiretroviral therapy (HAART) and 6% reported current or prior IDU. Thirty-six seroconverted, with overall incidence of .51 cases per 100 person-years (95% confidence interval, .36–.70). Mean age at seroconversion was 46 years. Seroconversion was associated with IDU (25% of seroconverters reported IDU history vs 5% of nonseroconverters; P < .001), whereas 75% (n = 27) of seroconverters reported no IDU (incidence, 2.67 cases per 100 person-years among IDUs, .40 cases per 100 person-years among non-IDUs). Seroconversion was associated with HIV RNA level >400 copies/mL (44% at time of antibody positivity vs 21% at time of last negative antibody test result; P = .02) but not with CD4+ cell count.
Conclusions. Incident HCV infection occurs in HIV-infected men involved in US HIV therapeutic trials, primarily through nonparenteral means, despite engagement in care and HAART. HCV antibody development was not related to immune status but was associated with inadequate HIV suppression. At-risk HIV-infected persons should have access to HCV surveillance.
Because of shared transmission routes, human immunodeficiency virus (HIV) and hepatitis C virus (HCV) coinfection is common, impacting up to 30% of HIV-infected persons in the United States and 4–5 million persons worldwide [1,2]. Chronic HCV infection has become a leading cause of non-AIDS related morbidity and mortality for HIV-infected persons in the highly active antiretroviral therapy (HAART) era [3]. With aggressive HCV disease progression, low potential for spontaneous viral clearance, and poorly tolerated therapy with marginal efficacy in the context of HIV infection, new approaches to the diagnosis and treatment of HCV infection are imperative.
The principal route of HCV spread in the United States is injection drug use (IDU) [4]. Because of the low rates of transmission between discordant heterosexual couples, HCV has not been considered to be a sexually transmitted virus [5]. However, recent reports indicated outbreaks of acute HCV infection, defined as the initial 6 months of infection, among HIV-infected men who have sex with men (MSM) in association with traumatic sexual practices (eg, unprotected anal intercourse, the use of sex toys, multiple partners, and manual insertion) and sexually transmitted infections (STIs) in the absence of IDU in Europe, Australia, New York, and California [6–11]. The extent to which this pattern is occurring throughout the United States is unknown. Until recently, HCV has been thought to precede HIV infection in most cases; thus, current US Public Health Service HIV guidelines endorse only HCV antibody testing at the time of HIV diagnosis. HIV-infected, HCV-seronegative patients are rarely screened subsequently [12]. However, HCV treatment leading to a sustained virologic response reduces liver-related mortality and morbidity and is more likely to be effective in the context of acute HCV infection. [13–16]. Thus, it is vital to diagnose incident HCV infection in HIV-infected persons.
This report describes behavioral and demographic factors associated with HCV antibody seroconversion in HIV-1–seropositive male participants of a large North American cohort, the AIDS Clinical Trial Group (ACTG) Longitudinal Linked Randomized Trials (ALLRT) study.
METHODS
The ACTG, funded by the US Department of Health and Human Services, National Institute of Allergy and Infectious Diseases, and the National Institutes of Health Division of AIDS, was founded in 1987 and is the largest HIV clinical trials organization in the world. ALLRT (Protocol A5001) is a prospective cohort study of HIV-1–seropositive persons randomized into selected US-based clinical trials conducted by the ACTG. Initiated in 2000, ALLRT examines long-term immunologic, virologic, pharmacologic, and clinical outcomes associated with HAART. Persons at least 13 years of age enrolled in an approved parent ACTG study and willing to participate in a long-term observational investigation are eligible for enrollment in ALLRT. At the time of its inception in 2000, persons actively involved in ongoing parent trials were also eligible for enrollment in ALLRT if they met the aforementioned requirements. ALLRT excludes ACTG parent studies with a primary focus other than treatment-related outcomes or with results not amenable to cross-protocol comparisons [17]. At the time of this analysis, 26 ACTG parent studies were included in ALLRT.
Clinical, laboratory, and self-reported data are collected on each ALLRT participant at regular intervals. The database includes results generated by parent studies and from the ALLRT protocol. Baseline evaluation occurs at a participants's enrollment in his or her parent study, but follow-up in ALLRT extends beyond parent study closure. Standardized visits in ALLRT occur every 16 weeks, with individual outcomes assessed at different intervals [17].
Laboratory data included in ALLRT were generated by both ACTG and non-ACTG laboratories that are Clinical Laboratory Improvement Amendments (CLIA) certified or the equivalent. HCV antibody tests and measurement of CD4+ cell count levels were performed at non-ACTG, CLIA-certified laboratories. ALLRT HIV-1 RNA levels were generated using the Ultrasensitive Roche Amplicor Monitor assay (Roche Molecular Systems), primarily at the ACTG central testing laboratory at Johns Hopkins University [17].
We determined incidence of HCV infection during 1996–2008 among male study participants. HCV antibody testing at ALLRT entry began in 2002, with retesting every 96 weeks added in 2006. Seventeen ACTG parent and/or coenrolled studies also performed HCV antibody testing during 1996–2002, with retesting at different intervals. For analysis, the date of seroconversion was assigned as halfway between the date of the last negative and first positive HCV antibody test result [18,19].
We evaluated associations of HCV seroconversion with self-reported IDU, calendar year, geographical region, time-varying CD4+ cell count, and HIV RNA level with use of single- and multivariable Poisson regression that accounts for subjects’ differential durations of HCV antibody follow-up [20]. Poisson regression was also used to compare the duration of HCV antibody testing intervals. Previous, current, or never IDU was assessed using a self-reported standardized baseline survey used across studies at parent study entry and again at ALLRT entry. No participant had missing IDU data, because this field was required for participant registration. CD4+ cell counts and plasma HIV-1 RNA levels were obtained at baseline and at 16-week intervals. No sexual or non-IDU risk factor data was available. We compared the prevalent and incident cohorts for the same baseline factors.
RESULTS
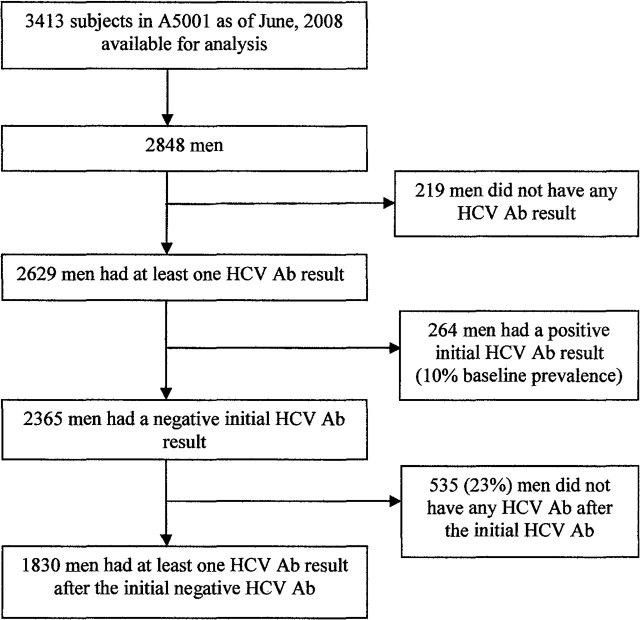
As of June 2008, data derived from 26 ACTG parent studies were included in ALLRT (n = 4641). Data derived from 24 of these studies were available for analysis (n = 3413), because 2 parent studies remained ongoing (Figure 1). A total of 2848 of these participants were male, 2629 had at least 1 HCV antibody test result, and 264 had a positive initial HCV antibody test result, for a baseline prevalence of 10% (264 of 2629). A total of 2365 men had an initial negative HCV antibody test result, and 1830 of these had at least 1 subsequent HCV antibody test and were included in this analysis, contributing >7000 person-years. Participants attended 55 ACTG clinical sites in 22 states across the United States, the District of Columbia, and Puerto Rico, primarily in urban centers (Supplementary Table 1).
Figure 1.
Participants in the analysis
Of 1830 men, 57% were white, 22% were black, 18% were Hispanic, 2% were Asian-Pacific Islander, 1% were Native American, and 70% attended college (Table 1a). At the time of the initial negative HCV antibody test result, the mean age was 42 years (range, 17–79 years; 52% were aged >40 years), 94% were receiving HAART, and 6% reported current or prior IDU at either parent study or ALLRT entry (previously, 5.3%, or currently, .5%).
Table 1a.
Characteristics and Risk Factors for Participants with and without Incident Hepatitis C Virus (HCV) Infection
| HCV status at the final HCV Ab test |
||||
| Characteristic | HCV negative (n= 1794) | HCV positive (n = 36) | Total (n = 1830) | |
| Race/Ethnicity | White Non-Hispanic | 1023 (57%) | 20 (56%) | 1043 (57%) |
| Black Non-Hispanic | 398 (22%) | 9 (25%) | 407 (22%) | |
| Hispanic (Regardless of Race) | 319 (18%) | 6 (17%) | 325 (18%) | |
| Asian, Pacific Islander | 35 (2%) | 1 (3%) | 36 (2%) | |
| American Indian, Alaskan Native | 14 (1%) | 0 (0%) | 14 (1%) | |
| Other | 5 (0%) | 0 (0%) | 5 (0%) | |
| Injection drug use | Never | 1698 (95%) | 27 (75%) | 1725 (94%) |
| Currently/previously | 96 (5%) | 9 (25%) | 105 (6%) | |
| Education level | [<12 years] Less than high school graduate | 193 (11%) | 3 (8%) | 196 (11%) |
| [12 years] High school graduate with no college | 342 (19%) | 7 (19%) | 349 (19%) | |
| [12–15 years] Some college | 608 (34%) | 13 (36%) | 621 (34%) | |
| [16 years] Bachelor degree with no post-graduate | 377 (21%) | 8 (22%) | 385 (21%) | |
| [>16 years] Post-graduate education | 274 (15%) | 5 (14%) | 279 (15%) | |
| Age at the initial negative HCV Ab test, years | Mean (SD) | 41.6 (9.3) | 43.1 (9.8) | 41.6 (9.3) |
| Range | 17–79 | 22–67 | 17–79 | |
| Age at the final HCV Ab,a years | Mean (SD) | 45.5 (9.7) | 45.6 (9.8) | 45.5 (9.7) |
| Range | 18–84 | 22–69 | 18–84 | |
| Geographical Region | Northeast | 319 (18%) | 9 (25%) | 328 (18%) |
| South | 484 (27%) | 9 (25%) | 493 (27%) | |
| West | 408 (23%) | 6 (17%) | 414 (23%) | |
| Midwest | 583 (32%) | 12 (33%) | 595 (33%) | |
| CD4 cell count at the initial negative HCV Ab test, cells/uL | 0–200 | 471 (26%) | 10 (28%) | 481 (26%) |
| 201–350 | 445 (25%) | 7 (19%) | 452 (25%) | |
| >350 | 878 (49%) | 19 (53%) | 897 (49%) | |
| HIV RNA level at the initial negative HCV Ab test, copies/mL | <400 | 839 (47%) | 15 (42%) | 854 (47%) |
| ≥400 | 955 (53%) | 21 (58%) | 976 (53%) | |
| HIV RNA level at the final HCV Ab test,a copies/mL | <400 | 1409 (79%) | 20 (56%) | 1429 (78%) |
| ≥400 | 385 (21%) | 16 (44%) | 401 (22%) | |
Abbreviations: Ab, antibody; SD, standard deviation.
The timepoint halfway between the last seronegative date and the date of first positive HCV Ab test result for seroconverters; the date of last available negative HCV Ab test result for participants who did not seroconvert.
Participants had varying durations of follow-up, with 47% contributing >4 years of person-time. The mean period between HCV antibody testing was 2.8 years in seroconverters and 2.6 years in nonseroconverters (P = .5). Ninety percent of testing intervals were <4.8 years; 50% were <3 years. The median number of HCV antibody tests among seroconverters was 2 (range, 2–4 tests) and 2 (range, 2–6 tests) among the nonseroconverters.
Thirty-six men seroconverted, with an overall incidence of .51 cases per 100 person-years (95% confidence interval [CI], .36 - .70). Mean age at the assigned time of seroconversion was 46 years (range, 22–69 years; 72% were aged >40 years). Seroconversion was associated with IDU (25% of seroconverters reported IDU history vs 5% of nonseroconverters; P < .001), whereas 75% (n = 27) of seroconverters reported no IDU (Tables 1a and 2). The incidence of seroconversion was 2.67 cases per 100 person-years among IDUs, compared with .40 cases per 100 person-years among non-IDUs.
Table 2.
Unadjusted and Adjusted Relative Risk of the Association Between Baseline, Immunologic, Virologic, and Other Risk Factors and Hepatitis C Virus (HCV) Antibody (Ab) Seroconversion (N = 1830)
| Unadjusted |
Adjustedb |
|||
| Variable | Relative Risk (95% CI) | P | Relative Risk (95% CI) | P |
| Injection drug use | ||||
| Nevera | 1 | 1 | ||
| Currently/previously | 6.65 (3.13–14.1) | <.001 | 6.12 (2.86–13.1) | <.001 |
| Time updated HIV RNA level, copies/mL | ||||
| <400a | 1 | 1 | ||
| ≥400 | 2.66 (1.38–5.13) | .004 | 2.28 (1.13–4.61) | .02 |
| Calendar year | ||||
| Before or during 2002a | 1 | .2c | 1 | |
| 2003–2005 | 1.39 (.57–3.40) | 1.76 (.72, 4.33) | .2 | |
| 2006–2008 | 0.53 (.16–1.74) | 0.68 (.21, 2.24) | .5 | |
| Time updated CD4 cell count, cells/μL | ||||
| ≤350a | 1 | 1 | ||
| >350 | 0.55 (.29–1.06) | .08 | 0.75 (.37–1.52) | .4 |
Abbreviation: CI, confidence interval.
Reference level.
Adjusted for the other variables in the table.
Based on the test for the linear trend.
Seroconversion was also associated with HIV RNA level >400 copies/mL (44% of seroconverters had an HIV RNA level >400 copies/mL at the assigned time of antibody positivity; 21% of nonseroconverters had an HIV RNA level >400 copies/mL at the time of the last negative antibody test result; P = .02, adjusting for IDU, calendar year, and CD4+ cell count) (Table 2). For the 16 seroconverters (44%) with HIV RNA levels >400 copies/mL at the assigned time of seroconversion, 1 was in the initial period after starting HAART, 11 were receiving a failing HAART regimen that was not their initial regimen, and 4 had discontinued HAART. Seroconversion was not associated with race, ethnicity, education, or CD4+ cell count. Seroconversion rates were similar across geographical region (Northeast, .71; Midwest, .51; South, .50; West, .36 cases per 100 person-years; P = .63).
Compared with men with initial HCV antibody positivity, seroconverters were more likely to be white and less likely to be black and were more likely to have never injected drugs and to have attended college (Table 1b).
Table 1b.
Characteristics and Risk Factors for 264 Participants with Initial Positive Hepatitis C Virus (HCV) Antibody (Ab) Test Results
| Total | ||
| Race/Ethnicity | White Non-Hispanic | 105 (40%) |
| Black Non-Hispanic | 103 (39%) | |
| Hispanic (Regardless of Race) | 48 (18%) | |
| Asian, Pacific Islander | 3 (1%) | |
| American Indian, Alaskan Native | 4 (2%) | |
| Subj does not know or other/unknown | 1 (0%) | |
| Injection drug use | Never | 116 (44%) |
| Currently/previously | 148 (56%) | |
| Education level | [<12 years] Less than high school graduate | 62 (23%) |
| [12 years] High school graduate with no college | 65 (25%) | |
| [12–15 years] Some college < Bachelor degree | 101 (38%) | |
| [16 years] Bachelor degree w/no post-grad | 25 (9%) | |
| [>16 years] Post-graduate education | 11 (4%) | |
| Age at the initial HCV Ab test, years | Mean (standard deviation) | 44.7 (7.2) |
| Range | 26–68 |
DISCUSSION
Incident HCV infection occurs in an older, well-educated US HIV-infected male population across the country, primarily through nonparenteral means, despite patients’ engagement in care and access to HAART. Twenty-four (75%) of 36 seroconverters reported no IDU. Although study participants may have underreported IDU, in view of the accumulating evidence of HCV transmission through mucosal traumatic sex among HIV-infected MSM, sexual transmission is likely to be responsible for incident HCV infection. In an analogous study of HIV-infected participants, Swiss HIV Cohort Study investigators found comparable incidence of HCV infection (.64 cases per 100 person-years overall and .7 cases per 100 person-years among MSM who reported history of unsafe sex but no IDU) [21]. Among HIV-infected MSM, associations between incident HCV infection and reporting unsafe sex, inconsistent condom use, and positive syphilis serologic findings supported HCV sexual transmission. In a recent Australian study of HIV-infected and HIV-uninfected MSM, incident HCV infection was rare but more attributable to sexual risk behavior than IDU, while prevalent HCV was primarily related to IDU [22]. Similarly, our prevalent HCV infection was more attributable to IDU, whereas incident infection was not, supporting a changing epidemiology over time. The phylogenetic analysis of Van de Laar et al [23] of an international HCV transmission network among HIV-infected MSM delineated increasing permucosal HCV transmission caused by traumatic sexual practices since the late 1990s. These investigators note that this timeline corresponds with the availability of HAART, prevention fatigue [24], and the subsequent increase in sexual risk behaviors and STI rates. Further study is needed to elucidate specific behaviors associated with HCV acquisition and precise modes of transmission in this population and predictors of HCV antibody seroconversion. HCV seroconversion indicates behavior that signifies risk for other STIs and/or blood borne pathogens; thus, seroconversion should trigger evaluation for co-occurring infections and discussion of measures to reduce risk.
HCV seroconversion was associated with inadequate HIV suppression. This may reflect greater engagement in HCV transmission behaviors among persons who are less adherent to HAART, leading to detectable HIV RNA [25]. There is evidence supporting a relationship between nonadherence to HAART and high-risk sexual practices [26,27]. Another contributing factor may be that there is more HAART intolerance during acute HCV infection, leading to treatment interruption or poor compliance with antiretroviral regimens.
Most seroconverters were >40 years of age, consistent with the literature to date on sexually transmitted HCV infection among HIV-infected MSM. Older age at time of HCV infection, defined as being >40 years of age, has been associated with a decreased rate of spontaneous viral clearance and accelerated fibrosis progression [28,29]. It has been reported that men acquiring HCV after HIV develop hepatic fibrosis more rapidly than do their HIV-seronegative counterparts [30], although this rate may be mitigated by HAART-associated HIV suppression [31,32]. Older age at the time of infection necessitates HCV treatment initiation at an older age; being aged ≥40 years has a detrimental effect on sustained virologic response for individuals infected with HCV genotypes 1 or 4 [33,34].
Several study limitations should be noted. Participants were screened for HCV antibody at varied intervals, with only half of the participants with intervals of <3 years. Because we were unable to determine exactly when seroconversion occurred and because HIV-infected patients may have a delayed HCV antibody response [35], we are not able to report the trend of incidence over time. Because 23% of the study participants did not undergo repeat HCV antibody screening, potential selection bias could be present, at least around the screening interval, if not for the entire screening process. However, comparison of baseline demographic and behavioral (history of IDU) factors did not reveal any differences between those screened 1 time and those screened >1 time over the course of the study. Comparison of baseline clinical variables revealed that CD4+ cell count and plasma HIV RNA levels at the initial negative HCV antibody test result differed between those screened 1 time and those screened >1 time (CD4+ cell count, 344 cells/μL vs 399 cells/μL; HIV RNA level ≥400 copies/mL, 53% vs 42%), but this was not consistent with a substantial clinical difference. Thus, it is likely that the HCV seroconversions represent the true incidence among HIV-infected patients in care. No data on HIV transmission risk factors were available. No sexual risk behaviors or non-IDU data were available for analysis to delineate nonparenteral risk factors for HCV acquisition. The baseline survey including previous or current IDU did not include information on injection practices, nor were IDU behaviors updated as the study progressed. Future investigations should consider behaviors, such as serosorting, the practice of engaging in unprotected sex with others of the same HIV status, which has been proposed as an explanation for increasing rates of HCV infection among HIV-infected MSM more than HIV-uninfected MSM; the contributions of crystal methamphetamines and other noninjection drugs; and reuse of condoms, gloves, and sex toys during traumatic sex practices, which have been postulated to facilitate HCV transmission. Other limitations include the lack of HCV RNA data at this time. It is possible that seroconversion represents a false-positive antibody reaction or that the participants were HCV RNA positive but seronegative, because HCV antibody testing was performed during the window period of recent infection before seroconversion [35]. These issues will be explored in an upcoming study of stored serum samples. Previous studies have demonstrated long-term stability of HCV RNA [36]. Lastly, information about specific HCV antibody tests was not collected.
CONCLUSION
We identified incident HCV infection among HIV-infected men involved in HIV therapeutic trials in the United States. The question of whether rates of HCV infection are truly increasing among HIV-infected men or whether the perception of increasing incidence is attributable to enhanced ascertainment warrants further investigation. In the interim, irrespective of perceived risk, at-risk HIV-infected persons should have access to ongoing HCV surveillance. This may ensure that incident cases are not missed for this disease that is clinically silent in most infected individuals until late stages. Diagnosis of HCV infection during the acute stage (first 6 months of infection) may permit more successful treatment [16] and provide opportunity for intervention to mitigate disease spread among drug-using or sexual partners and education to limit liver damage. Although the optimal interval of routine HCV antibody surveillance remains to be determined, the European AIDS Clinical Society's Coinfection Guidelines endorse serologic testing for HCV at entry into HIV care and annually thereafter for patients with prior negative antibody test results [37]. Similarly, the New York State Department of Health AIDS Institute recommends annual HCV antibody testing for HIV-infected patients who have continued high-risk drug and/or sexual behaviors but are seronegative for HCV [38]. Implementing these guidelines ensures diagnosis in contexts in which patients and providers may not appreciate or adequately discuss nonclassic HCV risk factors and may provide answers with regard to whether HCV disease burden is increasing.
Supplementary Material
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/).
Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
We thank the HIV-infected individuals who participated in the ALLRT Protocol A5001. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Financial support. This study was supported in part by the AIDS Clinical Trials Group funded by the National Institute of Allergy and In- 335 fectious Diseases (AI 38858, AI 68636, AI 38855, AI 68634); National Institute on Drug Abuse (to L.E.T.); Lifespan/Tufts/Brown Center for AIDS Research, an NIH funded program (P30 AI42853); Center for Drug Abuse and AIDS Research, an NIH funded program (P30 DA013868); and NIH (5U 01 AI069474 to J.A.D.). The Miriam Hospital is one of the Clinical 340 Research Sites under Harvard/Partners Clinical Trials Unit NIH funded program (U01 AI069472).
Potential conflicts of interest: L.T. Has received grant support from Roche and Vertex, has served on the speakers’ bureau for Genentech, and has received consulting fees from Vertex. D.W. Has received research grants paid to UCSD by Vertex and Merck. K.S. has been a member of the advisory board for Vertex, BMS, Merck, SciClone, GlaxoSmithKline, Three Rivers, J&J, and Regulus. R.B. has been a member of the advisory board for Tibotec. All other authors: no conflicts.
References
- 1.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006;44(suppl 1):S6–9. doi: 10.1016/j.jhep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002;288:199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 4.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47(RR-19):1–39. [PubMed] [Google Scholar]
- 5.Orlando R, Lirussi F. Hepatitis C virus infection: sexual or non-sexual transmission between spouses? A case report and review of the literature. Infection. 2007;35:465–8. doi: 10.1007/s15010-007-6188-7. [DOI] [PubMed] [Google Scholar]
- 6.Fisher M, Richardson D, Sabin C. Program and abstracts of the14th Conference on Retroviruses and Opportunistic Infections. Los Angeles, CA: 2007. Acute hepatitis C in men who have sex with men is not confined to those infected with HIV and their number continues to increase. [Google Scholar]
- 7.Ghosn J, Pierre-Francois S, Thibault V, et al. Acute hepatitis C in HIV-infected men who have sex with men. HIV Med. 2004;5:303–6. doi: 10.1111/j.1468-1293.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 8.Luetkemeyer A, Hare CB, Stansell J, et al. Clinical presentation and course of acute hepatitis C infection in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41:31–6. doi: 10.1097/01.qai.0000191281.77954.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher S. Sexual transmission of hepatitis C and early intervention. J Assoc Nurses AIDS Care. 2003;14(suppl 5):87S–94. doi: 10.1177/1055329003255815. [DOI] [PubMed] [Google Scholar]
- 10.Gilleece YC, Browne RE, Asboe D, et al. Transmission of hepatitis C virus among HIV-positive homosexual men and response to a 24-week course of pegylated interferon and ribavirin. J Acquir Immune Defic Syndr. 2005;40:41–6. doi: 10.1097/01.qai.0000174930.64145.a9. [DOI] [PubMed] [Google Scholar]
- 11.Serpaggi J, Chaix ML, Batisse D, et al. Sexually transmitted acute infection with a clustered genotype 4 hepatitis C virus in HIV-1-infected men and inefficacy of early antiviral therapy. AIDS. 2006;20:233–40. doi: 10.1097/01.aids.0000200541.40633.56. [DOI] [PubMed] [Google Scholar]
- 12.Aberg JA, Kaplan JE, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:651–81. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 13.Berenguer J, Alvarez-Pellicer J, Martin PM, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50:407–13. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- 14.Bruno S, Stroffolini T, Colombo M, et al. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–87. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 15.Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–8. doi: 10.1016/j.cgh.2009.11.018. 8 e1. [DOI] [PubMed] [Google Scholar]
- 16.Matthews GV, Hellard M, Haber P, et al. Characteristics and treatment outcomes among HIV-infected individuals in the Australian Trial in Acute Hepatitis C. Clin Infect Dis. 2009;48:650–8. doi: 10.1086/596770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smurzynski M, Collier AC, Koletar SL, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials. 2008;9:269–82. doi: 10.1310/hct0904-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tassiopoulos KK, Seage G, 3rd, Sam N, et al. Predictors of herpes simplex virus type 2 prevalence and incidence among bar and hotel workers in Moshi, Tanzania. J Infect Dis. 2007;195:493–501. doi: 10.1086/510537. [DOI] [PubMed] [Google Scholar]
- 19.Hanson J, Posner S, Hassig S, Rice J, Farley TA. Assessment of sexually transmitted diseases as risk factors for HIV seroconversion in a New Orleans sexually transmitted disease clinic, 1990–1998. Ann Epidemiol. 2005;15:13–20. doi: 10.1016/j.annepidem.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Holford TR. The analysis of rates and of survivorship using log-linear models. Biometrics. 1980;36:299–305. [PubMed] [Google Scholar]
- 21.Rauch A, Rickenbach M, Weber R, et al. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV Cohort Study. Clin Infect Dis. 2005;41:395–402. doi: 10.1086/431486. [DOI] [PubMed] [Google Scholar]
- 22.Jin F, Prestage GP, Matthews G, et al. Prevalence, incidence and risk factors for hepatitis C in homosexual men: data from two cohorts of HIV-negative and HIV-positive men in Sydney, Australia. Sex Transm Infect. 2010;86:25–8. doi: 10.1136/sti.2009.038182. [DOI] [PubMed] [Google Scholar]
- 23.van de Laar T, Pybus O, Bruisten S, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136:1609–17. doi: 10.1053/j.gastro.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valdiserri RO. Mapping the roots of HIV/AIDS complacency: implications for program and policy development. AIDS Educ Prev. 2004;16:426–39. doi: 10.1521/aeap.16.5.426.48738. [DOI] [PubMed] [Google Scholar]
- 25.Vanable PA, Ostrow DG, McKirnan DJ. Viral load and HIV treatment attitudes as correlates of sexual risk behavior among HIV-positive gay men. J Psychosom Res. 2003;54:263–9. doi: 10.1016/s0022-3999(02)00483-x. [DOI] [PubMed] [Google Scholar]
- 26.Schackman BR, Dastur Z, Ni Q, Callahan MA, Berger J, Rubin DS. Sexually active HIV-positive patients frequently report never using condoms in audio computer-assisted self-interviews conducted at routine clinical visits. IDS Patient Care STDS. 2008;22:123–9. doi: 10.1089/apc.2007.0037. [DOI] [PubMed] [Google Scholar]
- 27.Remien RH, Exner TM, Morin SF, et al. Medication adherence and sexual risk behavior among HIV-infected adults: implications for transmission of resistant virus. AIDS Behav. 2007;11:663–75. doi: 10.1007/s10461-006-9201-8. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Samaniego J, Rodriguez M, Berenguer J, et al. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. Am J Gastroenterol. 2001;96:179–83. doi: 10.1111/j.1572-0241.2001.03374.x. [DOI] [PubMed] [Google Scholar]
- 29.Giordano TP, Kramer JR, Souchek J, Richardson P, El-Serag HB. Cirrhosis and hepatocellular carcinoma in HIV-infected veterans with and without the hepatitis C virus: a cohort study, 1992–2001. Arch Intern Med. 2004;164:2349–54. doi: 10.1001/archinte.164.21.2349. [DOI] [PubMed] [Google Scholar]
- 30.Fierer DS, Uriel AJ, Carriero DC, et al. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J Infect Dis. 2008;198:683–6. doi: 10.1086/590430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brau N, Salvatore M, Rios-Bedoya CF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Grunhage F, Wasmuth JC, Herkenrath S, et al. Transient elastography discloses identical distribution of liver fibrosis in chronic hepatitis C between HIV-negative and HIV-positive patients on HAART. Eur J Med Res. 2010;15:139–44. doi: 10.1186/2047-783X-15-4-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy KR, Messinger D, Popescu M, Hadziyannis SJ. Peginterferon alpha-2a (40 kDa) and ribavirin: comparable rates of sustained virological response in sub-sets of older and younger HCV genotype 1 patients. J Viral Hepat. 2009;16:724–31. doi: 10.1111/j.1365-2893.2009.01122.x. [DOI] [PubMed] [Google Scholar]
- 34.Antonucci G, Longo MA, Angeletti C, et al. The effect of age on response to therapy with peginterferon alpha plus ribavirin in a cohort of patients with chronic HCV hepatitis including subjects older than 65 yr. Am J Gastroenterol. 2007;102:1383–91. doi: 10.1111/j.1572-0241.2007.01201.x. [DOI] [PubMed] [Google Scholar]
- 35.Thomson EC, Nastouli E, Main J, et al. Delayed anti-HCV antibody response in HIV-positive men acutely infected with HCV. AIDS. 2009;23:89–93. doi: 10.1097/QAD.0b013e32831940a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jose M, Gajardo R, Jorquera JI. Stability of HCV, HIV-1 and HBV nucleic acids in plasma samples under long-term storage. Biologicals. 2005;33:9–16. doi: 10.1016/j.biologicals.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Rockstroh JK, Bhagani S, Benhamou Y, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med. 2008;9:82–8. doi: 10.1111/j.1468-1293.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 38. New York State Department of Health AIDS Institute. HIV Clinical Resource: Hepatitis C Virus Guidelines. Available at: http://www.hivguidelines.org/clinical-guidelines/adults/hepatitis-c-virus/. Accessed 6 January 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.