Abstract
Infection with human immunodeficiency virus type 2 (HIV-2) occurs mainly in West Africa, but an increasing number of cases have been recognized in Europe, India, and the United States. In this era of global integration, clinicians must be aware of when to consider the diagnosis of HIV-2 infection and how to test for this virus. Although there is debate regarding when therapy should be initiated and which regimen should be chosen, recent trials have provided important information on treatment options for HIV-2 infection. In this review, we present information on recent clinical advances in our understanding of HIV-2 infection and highlight remaining diagnostic and therapeutic challenges.
Although human immunodeficiency virus type 1 (HIV-1) infection is responsible for most of the global AIDS pandemic, HIV type 2 (HIV-2) is an important cause of disease in a number of regions of the world. HIV-2 was initially found in West Africa but has spread to other parts of Africa, Europe, India, and the United States. As a result, it is more important than ever to consider the diagnosis of HIV-2 infection and to test at-risk individuals or people in whom an HIV-1 western blot exhibits an unusual indeterminate result. Because quantitative HIV-2 RNA assays are not commercially available in the United States, infected patients are generally monitored by measurement of CD4 cell counts. When treatment is initiated, it is important to consider the fact that HIV-2 is intrinsically resistant to some commonly used classes of antiretroviral medications.
This review provides information on the epidemiology, diagnosis, and clinical manifestations of HIV-2 infection. In addition, we review the natural history of HIV-2 infection and how it may shed light on AIDS pathogenesis. Finally, we conclude by examining treatment options for patients with HIV-2 infection and highlight challenges in improving care of infected patients.
EPIDEMIOLOGY
HIV-2 infection is predominantly found in West African nations, such as Guinea-Bissau, The Gambia, Senegal, Cape Verde, Cote d'Ivoire, Mali, Sierra Leone, and Nigeria. In the late 1980s, each of these countries had a reported prevalence of >1% of the national population [1]. An estimated 1 to 2 million people in West Africa are infected with HIV-2 [2]. However, in recent years, HIV-2 prevalence has been declining in several West African countries, particularly among younger people [3, 4]. For example, in a rural area of northwestern Guinea-Bissau, the HIV-2 prevalence dropped from 8.3% in 1990 to 4.7% in 2000; during the same period HIV-1 prevalence increased from 0.5% to 3.6% [5]. These trends may be related to the lower transmission efficiency of HIV-2 compared with HIV-1.
HIV-2 infection has also been reported in countries with historical and socio-economic ties to West Africa. For example, HIV-2 may have spread from Guinea-Bissau to Portugal during the war of independence [6]. HIV-2 has also been reported in former Portuguese colonies, such as Angola, Mozambique, and Brazil, and in parts of India with previous ties to Portugal, such as Goa and Maharashtra. In Portugal, HIV-2 is responsible for 4.5% of AIDS cases] [7]. In France, of 10,184 new HIV diagnoses between 2003 and 2006, 1.8% were infected by HIV-2 (1.6% HIV-2 monoinfection and 0.2% probable HIV-1/2 dual infections) [9].
In the United States, the first case of HIV-2 infection was diagnosed in 1987 in a West African woman who presented with central nervous system toxoplasmosis [10]. Although the total number of known cases of HIV-2 in the U.S. is small, in New York City alone there have been 62 confirmed or probable cases of HIV-2 infection diagnosed and reported since the implementation of named HIV reporting in 2000 [8]. Moreover, given the large number of immigrants from HIV-2 endemic areas living in the U.S., it is clear that the current number of cases is higher than older estimates [1]. According to the 2000 U.S. Census, of the estimated 31.1 million immigrants, more than a quarter of a million people are from Western African countries with a high prevalence of HIV-2 infection [11]. Although immigrants from West Africa are located throughout the country, there are areas of concentrated residence for specific groups. For example, of the estimated 200,000 Cape Verdeans in the United States, many have settled in New England states, such as Massachusetts and Rhode Island [12]. In addition, large numbers of Nigerians and Sierra Leoneans have settled in East Coast states, particularly the Washington DC area [13]. The Chicago area is home to a substantial number of Ghanaians [14]. Therefore, health care professionals practicing in the United States should have a high index of suspicion for HIV-2 infection in immigrants from high-risk areas and their sexual partners, and a low threshold for HIV-2 testing in such individuals.
RISK FACTORS AND TRANSMISSION
The modes of transmission for HIV-2 are the same as those for HIV-1, namely sexual contact, blood-borne exposure (blood transfusion, shared needles), and perinatal transmission. However, HIV-2 has a lower infectivity than HIV-1. For example, in a prospective cohort of female sex workers in Senegal, heterosexual spread of HIV-2 was slower than that of HIV-1 [15]. Among a prospective cohort of women in Ivory Coast in the early 1990s, the rate of perinatal transmission of HIV-2 was 1.2% compared with 24.7% for HIV-1 (relative risk 21-fold lower for HIV-2) [16]. More recently, in a study in The Gambia, the mother-to-child transmission rate of HIV-2 was 4%, 6-fold lower than the HIV-1 transmission rate of 24.4% [17]. The lower infectivity of HIV-2 is likely related to lower RNA levels. For example, in the Gambian study, the geometric mean antenatal plasma viral load in HIV-2–positive women was 410 copies/mL, which was 37-fold lower than the viral load in HIV-1–infected women (15,100 copies/mL); after adjusting for viral load, the odds of HIV-2 transmission were similar to that of HIV-1 [17].
NATURAL HISTORY
HIV-2 infection is notable for a longer asymptomatic phase and slower progression to AIDS than HIV-1 infection [18–20]. In a cohort of Senegalese female commercial sex workers, the probability of AIDS-free survival 5 years after seroconversion was 100% in the HIV-2–positive patients compared with only 67% for HIV-1–infected patients [19]. Furthermore, the rate of progression to AIDS in HIV-2–infected patients is highly variable. Whereas some HIV-2–infected patients develop advanced immunodeficiency and AIDS-related complications, similar to HIV-1–infected patients, others seem to have normal survival or progress much more slowly [21, 22]. Distinguishing patients who are likely to progress from those who may have a more indolent course is important for deciding how best to monitor HIV-2–infected patients.
In addition, HIV-2 infection is characterized by higher CD4 cell counts and lower viral RNA levels than that seen in HIV-1 infection [18, 20]. Viral loads are on average 28-fold lower in recent HIV-2–seroconvertors than in recent HIV-1–seroconvertors [23]. However, once advanced immunodeficiency develops, HIV-2–infected individuals have a high mortality: in a study from the Gambia, HIV-1– and HIV-2–infected patients with CD4 cell counts of <200/mm3 had a similarly high mortality rate [24]. Why the 2 viruses have distinct natural histories is not known, although differences in immune activation or viral proteins may play a role [25–28].
Because HIV-2 seems to be a less virulent virus than HIV-1, there has been interest in the possibility that HIV-2 infection might protect against HIV-1 infection—a form of acquired immunity. A prospective study of Senegalese female commercial sex workers suggested HIV-2 offered protection against subsequent acquisition of HIV-1 [29]. Peripheral blood mononuclear cells from HIV-2–infected patients in this cohort were found to be resistant to in vitro infection by HIV-1, perhaps through a beta-chemokine-mediated mechanism [30]. However, other cohort-based studies have concluded that HIV-2 does not protect against acquisition of HIV-1 infection [31–34]. Additional studies, including work on the mechanism of interaction between HIV-1 and HIV-2, may shed light on the controversy.
CLINICAL MANIFESTATIONS
As noted above, there is a prolonged asymptomatic phase among patients with HIV-2 infection. However, if patients do not receive treatment and their CD4 cell count declines, they develop illnesses similar to those seen in HIV-1 patients. For example, conditions such as tuberculosis, esophageal candidiasis, wasting syndrome, cerebral toxoplasmosis, disseminated Mycobacterium avium intracellulare, cryptococcosis, cryptosporidiosis, cytomegalovirus disease, Kaposi's sarcoma, AIDS dementia complex, recurrent bacterial pneumonia, and progressive multifocal leukoencephalopathy have been reported in HIV-2–infected patients [35–38]. A study from The Gambia comparing HIV-1– and HIV-2–infected AIDS patients revealed similar patterns of AIDS-defining events, with wasting syndrome and pulmonary tuberculosis occurring most frequently [39].
There have also been reports of other less common clinical manifestations of HIV-2 infection, including multiple cranial neuropathy [40] and non-immune thrombocytopenia in the setting of NK/T cell lymphoma [41]; these complications may respond to antiretroviral therapy (ART). A case of demyelinating encephalitis, presenting with lesions of the optic nerves, spinal cord, and brain, was recently reported in an HIV-2–infected patient with a CD4 cell count of 30/mm3 [42]. There is a suggestion that encephalitis might occur more frequently in patients with HIV-2 infection than in those with HIV-1 infection, although it is unclear if this finding is due to longer survival of HIV-2–infected patients, or because HIV-2 itself is more neurotropic [42]. Although HIV-associated nephropathy (HIVAN) occurs in about 7% of patients with HIV-1 infection [43], this complication has been only rarely reported in HIV-2–infected individuals [44].
TESTING AND DIAGNOSIS
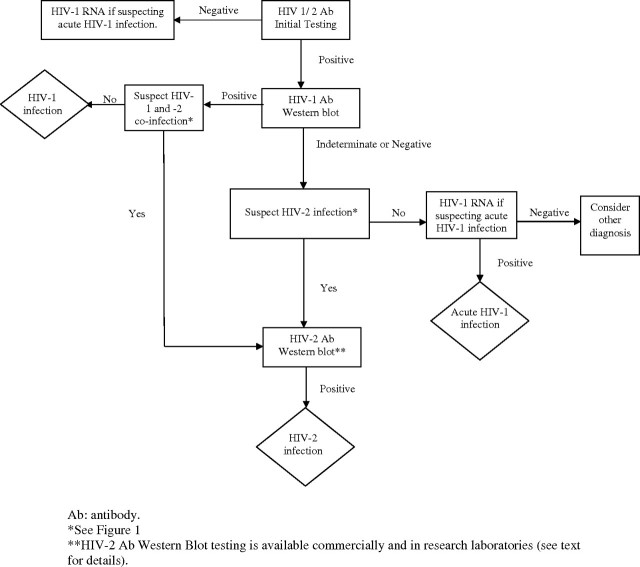
The CDC recommends testing for HIV-2 infection in persons who are at risk based on exposure history, individuals with an illness that suggests HIV infection but whose HIV-1 test result is not positive, and people who have unusual HIV-1 western blot patterns (Figure 1). Current U.S. Food and Drug Administration (FDA)-approved diagnostic tests capable of detecting HIV-2 infection use enzyme-linked immunosorbent and chemiluminescent immunoassay methods. While these immunoassays detect both HIV-1 and HIV-2, they do not differentiate between the 2 types of HIV infection. The only FDA-approved rapid antibody test that detects and differentiates HIV-2 infection is Bio-Rad Laboratories’ Multispot HIV-1/HIV-2 Rapid Test [45]. The Multispot test, which uses an immunoconcentration method, incorporates peptide sequences representing HIV-1 and HIV-2 envelope proteins and allows differentiation between HIV-1 and HIV-2 infections. A reactive result for initial serologic HIV-2 antibody test should be confirmed with a supplemental HIV-2 antibody test, such as an immunoblot. Currently, there is no supplemental HIV-2 antibody test approved by the FDA for in vitro diagnostic use in the United States. However, supplemental HIV-2 antibody tests are commercially available at reference testing laboratories, such as Focus Diagnostics, Inc./Quest Diagnostics Inc. (HIV-2 antibody immunoblot). In addition, laboratory-developed HIV-2 western blot assays are performed by the California Department of Health Services and by various research laboratories. Supplemental HIV-2 antibody assays that are available commercially in other countries include the INNO- LIA HIV I/II Score (Innogenetics NV) and HIV-2 Blot, version 1.2 (MP Biomedicals, LLC). A suggested HIV-2 testing algorithm is presented in Figure 2.
Figure 1.
Center for Disease Control and Prevention (CDC) recommendations for HIV-2 testing.
Figure 2.
Proposed HIV-1 and HIV-2 testing algorithm.
There are currently no FDA-approved assays for quantification of HIV-2 RNA. Some HIV-1 RNA assays, such as the Roche AMPLICOR HIV-1 Monitor [46] and NucliSens EasyQ (version 1.1), may at times detect HIV-2 RNA. For example, in one study, the NucliSens detected HIV-2 in 34 (45%) of 75 specimens; no virus was detected in the other samples [47]. When HIV-2 RNA stock material was quantified by the NucliSens assay or by electron microscopy, the values were lower on the NucliSens assay [47]. These commercial HIV-1 assays are not approved for quantification of HIV-2 RNA, nor have they been validated according to current U.S. clinical laboratory regulations for testing clinical specimens. In addition, in one study only approximately 50% of HIV-2–infected patients had detectable HIV-2 RNA on reverse transcriptase polymerase chain reaction (PCR) testing; in patients with a detectable RNA, the median viral load was 1000 copies/mL [35].
Because of the lack of a commercially-available test, HIV-2 RNA levels cannot generally be monitored in patients who are started on treatment. The development of an FDA-approved quantitative HIV-2 RNA test is needed to improve the care of HIV-2-infected patients in the U.S.
TREATMENT AND ANTIRETROVIRAL RESISTANCE
When to Start Treatment
The lack of large observational or randomized treatment studies in HIV-2–infected patients makes it difficult to be certain when therapy should be started. Given the poor CD4 cell count recovery following treatment initiation in many HIV-2–infected patients (see below), it is important to start therapy before advanced immunodeficiency develops. Although the most recent U.S. Department of Health and Human Services (DHHS) guidelines recommend HIV-1–infected patients with a CD4 cell count <500/mm3 should initiate therapy [48], they do not specifically comment on when ART should be initiated in HIV-2–infected patients. The 2008 French guidelines recommend ART initiation for all symptomatic patients; for HIV-infected pregnant women (to prevent transmission to the infant); and for asymptomatic patients who have a CD4 cell count <350/mm3. Furthermore, these guidelines suggest therapy should be considered when the CD4 cell count is <500/mm3 [49].
Once a decision has been made to treat HIV-2 infection, the next question is what medications to use. Both in vitro data and clinical trials information may help inform this decision.
In vitro Studies
NRTIs are active against HIV-2, but due to naturally occurring polymorphisms in reverse transcriptase, there may be variation in the potency of different agents [50]. Although some studies find that similar concentrations of NRTIs are needed to inhibit HIV-1 and HIV-2 replication, others find that higher concentrations of zidovudine are required to suppress HIV-2 [50]. In addition, the NRTI mutations M184V and Q151M were found in individuals in Burkina Faso who were reported to be treatment-naive, suggesting the possibility of naturally occurring polymorphisms or transmitted drug resistance [51, 52].
Protease inhibitors (PI), although active against HIV-2, also show varying degrees of activity due to natural protease polymorphisms. Comparing the potency of different PIs against HIV-2 using kinetic inhibition assays, Brower et al [53] found that lopinavir, saquinavir, tipranavir, and darunavir were the most potent against HIV-2 protease. Desbois et al [54] found that saquinavir, lopinavir, and darunavir are potent inhibitors of HIV-2 isolates, whereas other protease inhibitors were less active in vitro. Given the possibility of reduced activity, the 2008 French guidelines state that atazanavir, fosamprenavir, and tipranavir should be used with caution in patients with HIV-2 infection [49].
Because HIV-2 is intrinsically resistant to first-generation nonnucleoside reverse transcriptase inhibitors (NNRTIs) and the fusion inhibitor enfuvirtide [55], these agents should not be used.
Among the newer antiretroviral agents, HIV-2 appears to be susceptible to the 2 integrase strand transferase inhibitors, raltegravir and elvitegravir [56]. Given the fact that HIV-2 can use several coreceptors—including CXCR4, CCR5, CCR1-5, GPR15, and CXCR6—to enter cells in vitro, the potency of the CCR5 antagonist maraviroc against HIV-2 is uncertain [50].
Clinical Studies
A major limitation in our knowledge of how to treat HIV-2 infection is the absence of randomized treatment trials [2]. One reason for this dearth of information is because there are relatively few HIV-2–infected patients in the United States and Europe. In addition, because a large proportion of HIV-2–infected patients do not have detectable plasma viremia, this measure should not be the sole primary endpoint for HIV-2 treatment studies [57].
Many of the published studies of HIV-2 treatment have been relatively small observational trials. The ANRS CO5 HIV-2 Cohort assessed treatment response in 29 HIV-2–infected patients starting lopinavir/ritonavir in combination with NRTIs [58]. Prior to treatment, the median CD4 cell count was 142 cells/mm3, and only 16 patients had a detectable HIV-2 RNA (in these individuals, the median viral load was 2189 copies/mL). The median increase in CD4 cell count after treatment initiation was 71 cells/mm3 at week 24 (n = 28) and 122 cells/mm3 at week 48 (n = 19). At week 24, 20 patients had an undetectable HIV-2 RNA, and 5 patients reportedly had virologic failure (the virologic status of the other patients is not specified).
Another observational study found that viral suppression may be achieved using a regimen of 2 NRTIs and ritonavir-boosted indinavir [59]. However, nelfinavir-containing regimens seem to have limited virologic benefit in HIV-2 –infected patients [60].
What to Start
The World Health Organization (WHO) 2010 treatment guidelines state that a triple nucleoside regimen may be considered in patients with HIV-2 infection [61]. However, the guideline notes that there are concerns about the potency of a triple nucleoside regimen; for this reason many experts favor using 2 NRTI plus a ritonavir-boosted PI to treat HIV-2 infection. The U.S. Department of Health and Human Services HIV treatment guidelines suggest starting a boosted-PI regimen [48] but do not specify which drugs should be used. Based on in vitro and clinical trial information outlined above, ritonavir-boosted lopinavir or darunavir is a reasonable choice for the PI-component [71]. Tenofovir plus either emtricitabine or lamivudine could be used as the NRTI-component of the regimen (Table 1).
Table 1.
Potential Initial Treatment Regimens for HIV-2 Infection
Initial therapy for treatment-naïve patients with HIV-2 infection should include a ritonavir-boosted protease inhibitor plus 2 nucleoside reverse transcriptase inhibitors unless drug resistance is detected or suspected. Therapy for drug-resistant HIV-2 infection must be individualized.
| Ritonavir-boosted Protease Inhibitor | Nucleoside reverse transcriptase inhibitors |
| Lopinavir/ritonavir or Darunavir/ritonavir |
Tenofovir + emtricitabine or lamivudine or Zidovudine + lamivudine (this combination may have more toxicity than tenofovir-based therapy) |
NOTE. Adapted with permission from the following reference: British HIV Association guidelines for antiretroviral treatment of HIV-2-positive individuals 2010. HIV Med. 11:611–9.
Monitoring of Treatment Response and HIV-2 Drug Resistance
Response to treatment is usually assessed using a composite of both immunologic and virologic criteria. Data from the ANRS CO5 HIV-2 Cohort Study Group showed lower than expected CD4 cell recovery in HIV-2–treated patients, despite the fact that most patients achieved virologic suppression [62]. The CD4 cell response to therapy in HIV-2–infected patients appears to be lower than the response in HIV-1–infected patients [63].
Monitoring for drug resistance is difficult because assays are not commercially available; as a result, studies have used research assays. Descamps et al [64] noted a high frequency of Q151M and K65R mutations in NRTI-treated HIV-2–infected patients. (K65R has been also reported in patients with non-clade B HIV-1 infection failing first-line antiretroviral medications, including regimens that did not contain tenofovir [65]). Integrase gene resistance mutations have been reported during treatment with raltegravir [66]. Emergence of multiclass drug resistance has also been detected: In a cohort of 23 patients in Senegal, Gottlieb et al [67] found a large proportion (30%) who developed multiclass drug resistance mutations (including M184V, Q151M, and multiple PI-associated mutations). Therapy for drug-resistant HIV-2 must be individualized and may include newer agents such as raltegravir [68].
HIV-1/2 COINFECTION
In West Africa, 0.3%–1% of HIV-infected patients are estimated to be dually infected with both HIV-1 and HIV-2 [69]. The mortality rate in HIV-1/2 coinfected patients is similar to that of HIV-1 patients in all CD4 cell count strata [25]. Treatment failures have been noted in coinfected patients who were placed on HIV-1 targeted therapy and were later found to have HIV-2 that was resistant to the antiretroviral regimen [69, 70]. Treatment-naive coinfected patients should be treated with a ritonavir-boosted protease inhibitor plus 2 NRTIs. If treatment failure ensues, the resistance patterns of both viruses should be evaluated.
CONCLUSION
HIV-2 infection, originally discovered in West Africa, has now been found in many countries throughout the world; given widespread immigration to the United States and Europe, increased surveillance is warranted. Although HIV-2 is less pathogenic than HIV-1, some HIV-2–infected patients develop advanced immunodeficiency and AIDS-related conditions, which can lead to death. Little is known about optimal management of HIV-2 infection, and there is a need for randomized controlled trials to compare different antiretroviral treatment options. In addition, development of a commercial quantitative HIV-2 RNA test is a high priority so that the effectiveness of therapy can be appropriately monitored. Recent efforts to develop an international consortium to study HIV-2 infection and its management may result in improved care of HIV-2–infected patients [57].
Acknowledgments
We would like to thank Drs Kimon Zachary and Joseph Yao for their review of and comments on sections of this paper and Dr Eric Rosenberg for information regarding HIV-1 and HIV-2 diagnostic testing. We would like to acknowledge Betsy Wonderly and Rishabh Phukan for assistance in preparing this paper. R.T.G. has received grant support from Tibotec and Gilead.
Financial support. R.T.G. is supported by NIH R01 AI066992-04A1 and NIH G08LM008830-01 and by grants to the AIDS Clinical Trials Group (NIH U01 AI 694722) and the Harvard University Center for AIDS Research (NIH 2P30 AI060354-06).
Potential conflicts of interest. All other authors: no conflicts.
References
- 1.Centers for Disease Control. Factsheet HIV Type 2. Available at: http://wonder.cdc.gov/wonder/prevguid/m0038078/m0038078.asp. Accessed 24 June 2010. [Google Scholar]
- 2.Gottlieb GS, Eholié SP, Nkengasong J, et al. A call for randomized controlled trials of antiretroviral therapy for HIV-2 infection in West Africa. AIDS. 2008;22:2069. doi: 10.1097/QAD.0b013e32830edd44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.da Silva Z, Oliveira I, Andersen A, et al. Changes in prevalence and incidence of HIV-1, HIV-2 and dual infections in urban areas of Bissau, Guinea-Bissau: is HIV-2 disappearing? AIDS. 2008;22:1195–202. doi: 10.1097/QAD.0b013e328300a33d. [DOI] [PubMed] [Google Scholar]
- 4.van der Loeff MF, Awasana A, Sarge-Njie R, et al. Sixteen years of HIV surveillance in a West African research clinic reveals divergent epidemic trends of HIV-1 and HIV-2. Int J Epidemiol. 2006;35:1322–28. doi: 10.1093/ije/dyl037. [DOI] [PubMed] [Google Scholar]
- 5.van Tienen C, van der Loeff MS, Zaman S, et al. Two distinct epidemics: the rise of HIV-1 and decline of HIV-2 infection between 1990 and 2007 in rural Guinea-Bissau. J Acquir Immune Defic Syndr. 2010;53:640–7. doi: 10.1097/QAI.0b013e3181bf1a25. [DOI] [PubMed] [Google Scholar]
- 6.de Silva T, Cotton M. Rowland-Jones S HIV-2: the forgotten AIDS virus. Trends Microbiol. 2008;16:588–95. doi: 10.1016/j.tim.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Soriano V, Gomes P, Heneine W, et al. Human immunodeficiency virus type 2 (HIV-2) in Portugal: clinical spectrum, circulating subtypes, virus isolation, and plasma viral load. J Med Virol. 2000;61:111–6. [PubMed] [Google Scholar]
- 8.Torian L, et al. HIV Type 2 in New York City, 2000–2008. CID. 2010;51:1334–42. doi: 10.1086/657117. [DOI] [PubMed] [Google Scholar]
- 9.Barin F, Cazein F, Lot F, et al. Prevalence of HIV-2 and HIV-1 group O infections among new HIV diagnoses in France: 2003–2006. AIDS. 2007;21:2351–53. doi: 10.1097/QAD.0b013e3282f15637. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control. Epidemiologic notes and reports AIDS due to HIV-2 infection—New Jersey. MMWR Morb Mortal Wkly Rep. 1988;37:33–5. [PubMed] [Google Scholar]
- 11.US Census 2000 Report. Available at: http://www.census.gov/population/www/socdemo/foreign/STP-159-2000tl.html. Accessed 24 June 2010. [Google Scholar]
- 12.Smith J. Cape Verde, rising, with emigres’ help; deepening ties bind islands, Bay State. Available at: http://www.boston.com/news/local/massachusetts/articles/2009/04/26/cape_verde_rising_with_emigres_help/?page=2. The Boston Globe April 26 2009. Accessed 24 June 2010. [Google Scholar]
- 13.Kent MM. Immigration, and America's black population. Vol 62. Population Bulletin; 2007. 13. Available at: http://www.prb.org/pdf07/62.4immigration.pdf. Accessed 24 June 2010. [Google Scholar]
- 14.Settegren A. History of Ghanaians in Chicago. http://www.chicagosistercities.com/cities/h_accra.php. Accessed 24 June 2010. [Google Scholar]
- 15.Kanki P, Travers K, Mboup S, et al. Slower heterosexual spread of HIV-2 than HIV-1. Lancet. 1994;343:943–6. doi: 10.1016/s0140-6736(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 16.Adjorlolo-Johnson G, De Cock K, Ekpini E, et al. Prospective comparison of mother-to-child transmission of HIV-1 and HIV-2 in Abidjan, Ivory Coast. JAMA. 1994;272:462–6. [PubMed] [Google Scholar]
- 17.O'Donovan D, Ariyoshi K, Milligan P, et al. Maternal plasma viral RNA levels determine marked differences in mother-to-child transmission rates of HIV-1 and HIV-2 in the Gambia. AIDS. 2000;14:441–8. doi: 10.1097/00002030-200003100-00019. [DOI] [PubMed] [Google Scholar]
- 18.Popper S, Sarr A, Travers K, et al. Lower Human Immunodeficiency Virus (HIV) Type 2 viral load reflects the difference in pathogenicity of HIV-1 and HIV-2. J Infect Dis. 1999;180:1116–21. doi: 10.1086/315010. [DOI] [PubMed] [Google Scholar]
- 19.Marlink R, Kanki P, Thior I, et al. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science. 1994;265:1587–90. doi: 10.1126/science.7915856. [DOI] [PubMed] [Google Scholar]
- 20.MacNeil A, Sarr A, Sankale J, Meloni S, Mboup S, Kanki P. Direct evidence of lower viral replication rates in vivo in Human Immunodeficiency Virus type 2 (HIV-2) infection than in HIV-1 infection. J Virol. 2007;81:5325–30. doi: 10.1128/JVI.02625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry N, Jaffar S, van der Loeff M, et al. Low level viremia and high CD4% predict normal survival in a cohort of HIV Type-2-infected villagers. AIDS Res and Human Retroviruses. 2002;18:1167–73. doi: 10.1089/08892220260387904. [DOI] [PubMed] [Google Scholar]
- 22.van der Loeff MF, Larke N, Kaye S, et al. Vndetectable plasma viral load predicts normal survival in HIV-2 infected people in a West African village. Retrovirology. 2010;7:46. doi: 10.1186/1742-4690-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson S, Norregren H, da Silva Z, et al. Plasma viral load in HIV-1 and HIV-2 singly and dually infected individuals in Guinea-Bissau, West Africa: significantly lower plasma virus set point in HIV-2 infection than in HIV-1 infection. Arch Intern Med. 2000;160:3286–93. doi: 10.1001/archinte.160.21.3286. [DOI] [PubMed] [Google Scholar]
- 24.Schimm van der Loeff M, Jaffar S, Aveika AA, et al. Mortality of HIV-1, HIV-2 and HIV-1/HIV-2 dually infected patients in a clinic-based cohort in the Gambia. AIDS. 2002;16:1775–83. doi: 10.1097/00002030-200209060-00010. [DOI] [PubMed] [Google Scholar]
- 25.Hanson A, Sarr A, Shea A, et al. Distinct profile of T cell activation in HIV type 2 compared to HIV type 1 infection: differential mechanism for immunoprotection. AIDS Res Hum Retroviruses. 2005;21:791–8. doi: 10.1089/aid.2005.21.791. [DOI] [PubMed] [Google Scholar]
- 26.Michel P, Balde A, Roussilhon C, Aribot G, Sarthou JL, Gougeon ML. Reduced immune activation and T cell apoptosis in human immunodeficiency virus type 2 compared with type1: correlation of T cell apoptosis with of β2–microglobulin concentration and disease evolution. J Infect Dis. 2000;181:64–75. doi: 10.1086/315170. [DOI] [PubMed] [Google Scholar]
- 27.Leligdowicz A, Feldman J, Jaye A, et al. Direct relationship between virus load and systemic immune activation in HIV-2 infection. J Infect Dis. 2010;201:114–22. doi: 10.1086/648733. [DOI] [PubMed] [Google Scholar]
- 28.Schindler M, Münch J, Kutsch O, et al. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell. 2006;125:1055–67. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 29.Travers K, Mboup S, Marlink R, et al. Natural protection against HIV-1 infection provided by HIV-2. Science. 1995;268:1612–15. doi: 10.1126/science.7539936. [DOI] [PubMed] [Google Scholar]
- 30.Kokkotou E, Sankale JL, Mani I, et al. In vitro correlates of HIV-2 mediated HIV-1 protection. Proc Natl Acad Sci U S A. 2000;97:6797–802. doi: 10.1073/pnas.97.12.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiktor S, Nkengasong J, Ekpini E, et al. Lack of protection against HIV-1 infection among women with HIV-2 infection. AIDS. 1999;13:695–9. doi: 10.1097/00002030-199904160-00010. [DOI] [PubMed] [Google Scholar]
- 32.Schim van der Loeff M, Aaby P, Aryioshi K, et al. HIV-2 does not protect against HIV-1 infection in a rural community in Guinea-Bissau. AIDS. 2001;15:2303–10. doi: 10.1097/00002030-200111230-00012. [DOI] [PubMed] [Google Scholar]
- 33.Norregren H, Andersson S, Biague A, et al. Trends and interaction of HIV-1 and HIV-2 in Guinea-Bissau, West Africa: no protection of HIV-2 against HIV-1 infection. AIDS. 1999;13:701–7. doi: 10.1097/00002030-199904160-00011. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg A. Possible protective effect of HIV-2 against incident HIV-1 infection: review of available epidemiological and in vitro data. AIDS. 2001;15:2319–21. doi: 10.1097/00002030-200111230-00015. [DOI] [PubMed] [Google Scholar]
- 35.Matheron S, Pueyo S, Damond F, et al. Factors associated with clinical progression in HIV-2 infected patients. AIDS. 2003;17:2593–601. doi: 10.1097/00002030-200312050-00006. [DOI] [PubMed] [Google Scholar]
- 36.Bienaime A, Colson P, Moreau J, Zandotti C, Pellissier J-F, Brouqui P. Progressive multifocal leukoencephalopathy in HIV-2 infected patient. AIDS. 2006;20:1342–43. doi: 10.1097/01.aids.0000232249.89404.d6. [DOI] [PubMed] [Google Scholar]
- 37.Stoner GL, Agostini HT, Ryschkewitch CF, et al. Detection of JC virus in two African cases of progressive multifocal leukoencephalopathy including identification of JCV type 3 in a Gambian AIDS patient. J Med Microbiol. 1998;47:733–42. doi: 10.1099/00222615-47-8-733. [DOI] [PubMed] [Google Scholar]
- 38.Rubsamen-Waigman H, Adamski M, Von Briesen H. Lethal progressive multifocal leukoencephalopathy in an HIV 2 infected person as the only AIDS manifestation. AIDS Forsch. 1987;2:572–5. [Google Scholar]
- 39.Martinez-Steel E, Awasana A, Corrah T, et al. Is HIV-2-induced AIDS different from HIV-1-associated AIDS? Data from a West African clinic. AIDS. 2007;21:317–24. doi: 10.1097/QAD.0b013e328011d7ab. [DOI] [PubMed] [Google Scholar]
- 40.Calado S, Canas N, Viana-Baptista M, Ribeiro C, Mansinho K. Multiple cranial neuropathy and HIV-2. J Neurol Neurosurg Psychiatry. 2004;75:660–1. doi: 10.1136/jnnp.2003.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torres JR, Torres-Viera MA, Schupbach J, Rangel HR, Pujol FH. Non-immune thrombocytopenia responsive to antriretroviral therapy and HIV-2 infection. J Infect. 2007;54:e21–4. doi: 10.1016/j.jinf.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 42.Moulignier A, Lascoux C, Bourgarit A. HIV type 2 demyelinating encephalomyelitis. Clin Infect Dis. 2006;42:e89–91. doi: 10.1086/503909. [DOI] [PubMed] [Google Scholar]
- 43.Shahinian V, Rajaraman S, Borucki M, Grady J, Hollander WM, Ahuja TS. Prevalence of HIV-associated nephropathy in autopsies of HIV-infected patients. Am J Kidney Dis. 2000;35:884–8. doi: 10.1016/s0272-6386(00)70259-9. [DOI] [PubMed] [Google Scholar]
- 44.Izzedine H, Damond F, Brocheriou I, Ghosn J, Lassal H, Deray G. HIV-2 infection and HIV-associated nephropathy. AIDS. 2006;20:949–58. doi: 10.1097/01.aids.0000218566.05274.2b. [DOI] [PubMed] [Google Scholar]
- 45.Greenwald J, Burstein G, Pincus J, Branson B. A rapid review of rapid HIV antibody tests. Curr Infect Dis Rep. 2006;8:125–31. doi: 10.1007/s11908-006-0008-6. [DOI] [PubMed] [Google Scholar]
- 46. Roche Amplicor HIV-1 Monitor Test Summary. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/PremarketApprovalsPMAs/UCM093319.pdf. [Google Scholar]
- 47.Rodés B, Sheldon J, Toro C, et al. Quantitative detection of plasma HIV type 2 subtype A RNA by the Nuclisens Easy Q Assay (version 1.1) J Clin Microbiol. 2007;45:88–92. doi: 10.1128/JCM.01613-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panel on Antiretroviral Guidelines for Adults, and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2009. pp. 1–161. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 24 June 2010. [Google Scholar]
- 49.Prise en Charge Médicale des Personnes Infectées par le VIH Ministry of Health France. 2008. http://www.sante-sports.gouv.fr/IMG/pdf/Recommandations_du_groupe_d_experts_2008_-_Points_forts_et_recommandations.pdf. Accessed 24 June 2010. [Google Scholar]
- 50.Ntemgwa ML, Toni T, Brenner B, Camacho R, Wainberg W. Antiretroviral drug resistance in human immunodeficiency virus type 2. Antimicrob Agents Chemother. 2009;53:3611–19. doi: 10.1128/AAC.00154-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruelle J, Sanou M, Liu H, Vandenbroucke A, Duquenne A, Goubau P. Genetic polymorphisms and resistance mutations Of HIV type 2 in antiretroviral-naive patients in Burkina Faso. AIDS Res Hum Retroviruses. 2007;23:955–64. doi: 10.1089/aid.2007.0034. [DOI] [PubMed] [Google Scholar]
- 52.Ruelle J, Roman F, Vandenbroucke A, et al. Transmitted drug resistance, selection of resistance mutations and moderate antiretroviral efficacy in HIV-2: analysis of the HIV-2 Belgium and Luxembourg database. BMC Infect Dis. 2008;8:21. doi: 10.1186/1471-2334-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brower ET, Bacha UM, Kawasaki Y, Freire E. Inhibition of HIV-2 protease by HIV-1 protease inhibitors in clinical use. Chem Biol Drug Des. 2008;71:298–305. doi: 10.1111/j.1747-0285.2008.00647.x. [DOI] [PubMed] [Google Scholar]
- 54.Desbois D, Roquebert D, Peytavin G, et al. In vitro phenotypic susceptibility of human immunodeficiency virus type 2 clinical isolates to protease inhibitors. Antimicrob Agents Chemother. 2008;52:1545–8. doi: 10.1128/AAC.01284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Witvrouw M, Pannecouque C, Switzer WM, et al. Susceptibility of HIV-2, SIV and SHIV to various anti-HIV-1 compounds: implications for treatment and postexposure prophylaxis. Antivir Ther. 2004;9:57–65. [PubMed] [Google Scholar]
- 56.Roquebert B, Damond F, Collin G, et al. HIV-2 integrase gene polymorphism and phenotypic susceptibility of HIV-2 clinical isolates to the integrase inhibitors raltegravir and elvitegravir in vitro. J Antimicrob Chemother. 2008;62:914–20. doi: 10.1093/jac/dkn335. [DOI] [PubMed] [Google Scholar]
- 57.Matheron S. HIV-2 infection: a call for controlled trials. AIDS. 2008;22:2073–4. doi: 10.1097/QAD.0b013e32830edd44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benard A, Damond F, Campa P, et al. Good response to lopinavir/ritonavir-containing antiretroviral regimens in antiretroviral-naïve HIV-2 infected patients. AIDS. 2009;23:1171–179. doi: 10.1097/QAD.0b013e32832949f0. [DOI] [PubMed] [Google Scholar]
- 59.van der Ende M, Prins J, Brinkman K, et al. Clinical, immunological and virological response to different antiretroviral regimens in a cohort of HIV-2 infected patients. AIDS. AIDS. 2003;(Suppl 3):S55–61. doi: 10.1097/00002030-200317003-00008. 2003 Jul;17 (Suppl 3):S55–61. [DOI] [PubMed] [Google Scholar]
- 60.Adje-Toure C, Cheingsong R, Garcia-Lerma J, et al. Antiretroviral therapy in HIV-2-infected patients: changes in plasma viral load, CD4+ cell counts, and drug resistance profiles of patients treated in Abidjan, Cote d'Ivoire. AIDS. 2003;17(Suppl 3):S49–54. [PubMed] [Google Scholar]
- 61.World Health Organization. 2010 Guidelines on antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. Available at: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. Accessed 24 August 2010. [PubMed] [Google Scholar]
- 62.Matheron S, Damond F, Benard A, et al. CD4 cell recovery in treated HIV-2 infected adults is lower than expected: results from the French ANRS CO5 HIV-2 cohort. AIDS. 2006;20:459–62. doi: 10.1097/01.aids.0000199829.57112.2f. [DOI] [PubMed] [Google Scholar]
- 63.Drylewicz J, Matheron S, Lazaro E, et al. Comparison of viro-immunological marker changes between HIV-1 and HIV-2-infected patients in France. AIDS. 2008;22:457–68. doi: 10.1097/QAD.0b013e3282f4ddfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Descamps D, Damond F, Matheron S, et al. High frequency of selection of K65R and Q151M mutations in HIV-2 infected patients receiving nucleoside reverse transcriptase inhibitors containing regimen. J Med Virol. 2004;74:197–201. doi: 10.1002/jmv.20174. [DOI] [PubMed] [Google Scholar]
- 65.Hawkins C, Chaplin B, Idoko J, et al. Clinical and genotypic findings in HIV-infected patients with the K65R mutation failing first line antiretroviral therapy in Nigeria. J Acquir Immune Defic Syndr. 2009;52:228–34. doi: 10.1097/QAI.0b013e3181b06125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salgado M, Toro C, Simón A, et al. Mutation N155H in HIV-2 integrase confers high phenotypic resistance to raltegravir and impairs replication capacity. J Clin Virol. 2009;46:173–5. doi: 10.1016/j.jcv.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 67.Gottlieb G, Badiane N, Hawes S, et al. Emergence of multiclass drug-resistance in HIV-2 in antiretroviral-treated individuals in Senegal: implications for HIV-2 treatment in resource-limited West Africa. Clin Infect Dis. 2009;48:476–83. doi: 10.1086/596504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Damond F, Lariven S, Roquebert B, et al. Virological and immunogical response to HAART regimen containing integrase inhibitors in HIV-2-infected patients. AIDS. 2008;22:665–6. doi: 10.1097/QAD.0b013e3282f51203. [DOI] [PubMed] [Google Scholar]
- 69.Landman R, Damond F, Gerbe J, Brun-Vezinet F, Yeni P, Matheron S. Immunovirological and therapeutic follow up of HIV-1/HIV-2 dually seropositive patients. AIDS. 2009;23:423–43. doi: 10.1097/QAD.0b013e328321305a. [DOI] [PubMed] [Google Scholar]
- 70.Rodés B, Toro C, Jiménez V, Soriano V. Viral response to antiretroviral therapy in a patient coinfected with HIV type 1 and type 2. Clin Infect Dis. 2005;41:e19–21. doi: 10.1086/431204. [DOI] [PubMed] [Google Scholar]
- 71.Gilleece Y, Chadwick DR, Breuer J, Hawkins D, Smit E, McCrae LX, Pillay D, Smith N, Anderson J. BHIVA Guidelines Subcommittee. British HIV Association guidelines for antiretroviral treatment of HIV-2-positive individuals 2010. doi: 10.1111/j.1468-1293.2010.00889.x. HIV Med. 11:611–9. [DOI] [PubMed] [Google Scholar]