Abstract
Diphenylmethane diisocyanate (MDI), the chemical commonly used as a cross-linking agent in commercial polyurethane production, is a well-recognized cause of asthma. Reaction products between MDI and “self” proteins are hypothesized to act as antigens capable of inducing airway inflammation and asthma; however, such MDI antigens remain incompletely understood. We used a variety of analytical methods to characterize the range of MDI–albumin reaction products that form under physiological conditions. Sites of MDI conjugation on antigenic MDI–albumin products, as defined by serum immunoglobulin G (IgG) from MDI-exposed workers, were determined by high-performance liquid chromatography (HPLC) followed by tandem mass spectrometry (MS/MS). The data identified 14 MDI conjugation sites (12 lysines and 2 asparagines) on human albumin and highlight reaction specificity for the second lysine in dilysine (KK) motifs, and this may be a common characteristic of “immune-sensitizing” chemicals. Several of the MDI conjugation sites are not conserved in albumin from other species, and this may suggest species differences in epitope specificity for self protein (albumin)–isocyanate conjugates. The study also describes new applications of contemporary proteomic methodology for characterizing and standardizing MDI–albumin conjugates destined for use in clinical research.
Keywords: Diphenylmethane diisocyanate, Human, Albumin, Lysine, Serology, IgG, Isocyanate, Asthma, Polyurethane
Diisocyanates are small chemicals, with two reactive N=C=O groups that serve as the obligate “cross-linker” for commercial polyurethane production [1]. The most commonly used diisocyanates, diphenylmethane diisocyanate (MDI),1 toluene diisocyanate (TDI), and hexamethylene diisocyanate (HDI), are a major cause of occupational asthma worldwide [1,2]. For numerous reasons, MDI is fast overtaking the isocyanate consumer market (e.g., foam, furniture/bedding, insulation, mining/“rock-lock”, construction), leading to increased exposure and new cases of occupational asthma [3–6].
The form of isocyanates that stimulates the human immune system (e.g., antigen) has been challenging to define due to the chemicals' high electrophilicity and spontaneous reactivity with proteins [7,8]. In vivo, it is believed that isocyanates react with “self” proteins, resulting in conformational changes (neoepitopes) recognized by the immune system [8–11]. Such isocyanate–protein conjugates have been shown to trigger both innate and adaptive immune responses and have been hypothesized to represent the antigenic basis for isocyanate asthma [7,11–14].
To date, only one self protein, albumin, is known to create a pathogenic antigen, specifically recognized by human antibodies, on reactivity with isocyanate in vivo [9,15,16]. The human humoral immune response to isocyanate exposure is highly specific and requires human albumin. Isocyanate conjugated to other carrier proteins, even proteins with strong primary sequence homology to human albumin (e.g., albumin from other species), is not recognized by human immunoglobulin epsilon (IgE) or immunoglobulin gamma (IgG) [9,15,16]. Human albumin's evolutionarily distinct amino acid sequence and unique capacity to serve as an isocyanate “carrier,” compared with albumin from other species, provide potential clues that may help to identify isocyanate binding sites and epitopes recognized by the human immune system.
To better characterize and understand isocyanate–albumin conjugates, we recently developed a combination of contemporary “proteomic” approaches (electrophoresis, enzymatic digestion, and tandem mass spectrometry [MS/MS]). In prior studies, we investigated the reactivity of human albumin with the aliphatic isocyanate, HDI, and identified two sites of conjugation [9]. One of the HDI conjugation sites, Lys414, forms part of a dilysine motif (KK413–414) not conserved in albumin from most other common laboratory animal species.
The conjugation of MDI to albumin is less well understood. MDI's larger size and aromatic (vs. aliphatic) configuration are likely to affect its reactivity with albumin and the structure of MDI–albumin reaction products. In this study, we investigate MDI–human albumin reactivity under physiological exposure conditions and characterize the structure and antigenicity of the resulting (MDI–albumin) reaction products using a variety of analytical methods. The results provide new insight into the structure of MDI–albumin conjugates that helps to explain the specificity of human antibodies induced by occupational exposure. The data also highlight specific sites on albumin that may be common targets for reactivity with chemical allergens.
Materials and methods
Chemicals and reagents
Low endotoxin certified human albumin was obtained from Sigma Chemical (St. Louis, MO, USA), 4′4-diphenyl methane diisocyanate was obtained from Aldrich (St. Louis, MO, USA), and acetone was obtained from J.T. Baker (Phillipsburg, NJ, USA).
Reactivity of MDI with human albumin
Human albumin was solubilized in phosphate-buffered saline (PBS: 20 mM phosphate and 140 mM NaCl, pH 7.2) at 0.5% (w/v) to achieve a concentration (~74 μM) similar to human airway epithelial lining fluid [17]. A 50% (w/v) stock solution of MDI in acetone was prepared immediately before use and further diluted so that the addition of 250 μl of MDI in acetone to 25 ml of albumin solution yielded final MDI/albumin ratios ranging from 2 to 1000 μg MDI/mg albumin. The final acetone concentration was kept constant at 0.1% (v/v). Control albumin samples were similarly exposed to 0.1% acetone without MDI (i.e., “mock”). Reactions were performed in polypropylene centrifuge tubes from BD Biosciences (Franklin Lakes, NJ, USA) at 25 °C for 2 h with end-over-end rotation followed by 0.2-μM filtration and dialysis against PBS with four buffer exchanges. Samples were aliquoted and stored at −20 °C until analysis. In some experiments, MDI conjugates were prepared with bovine, murine, canine, and leporine albumin (Sigma Chemical) using 200 μg MDI/mg albumin.
Electrophoresis
Albumin and MDI–albumin samples were mixed with commercial sample buffers (2×) for reducing, nonreducing, and native gel electrophoresis from Bio-Rad (Hercules, CA, USA). Reducing and nonreducing buffers contained 62.5 mM Tris (pH 6.8), 40% glycerol, and 0.01% bromophenol blue. Reducing buffer also contained 5% 2-mercaptoethanol. Sodium dodecyl sulfate (SDS) acrylamide gels (4–15% gradient) were obtained precast from Bio-Rad. Native gels were prepared using a commercial acrylamide mixture from American Bioanalytical (Natick, MA, USA) that contained 30.8% (w/v) acrylamide/bis (37.5:1). Electrophoresis was performed as described previously, and gels were stained with commercial Coomassie-based buffer Imperial protein stain from Pierce (Rockford, IL, USA) [9].
Enzyme-linked immunosorbent assay
Nunc MaxiSorp microtiter plates, obtained through Thermo Fisher Scientific (Rochester, NY, USA), were coated with 100 μl/well albumin or MDI–albumin, diluted to 10 μg/ml in 0.1 M carbonate buffer (pH 9.5), by overnight incubation at 4 °C. Plates were “blocked” with 3% (w/v) bovine serum albumin from Sigma before human sera samples from MDI-exposed workers or control nonexposed individuals were added at a 1:200 dilution in blocking buffer. Sera were incubated for 1 h at 25 °C, followed by a 1:2000 dilution of peroxidase-conjugated goat anti-human IgG from BD Pharmingen (San Diego, CA, USA). Enzyme-linked immunosorbent assays (ELISAs) were developed for up to 40 min with TMB substrate from BD Bioscience, and the final reactions were terminated by the addition of 0.1 M HCl from Sigma. Optical density (OD) measurements were obtained on a Benchmark microtiter plate reader from Bio-Rad using dual wavelength absorbance (450–550 nm). All samples were tested in duplicate to obtain average value expressed in the figures.
Human subjects
Sera samples from 10 workers with self-reported exposure to MDI and 10 age/sex-matched unexposed control subjects were obtained with informed consent. Exposed subjects used MDI during their employment in a factory that manufactures polyurethane-coated fabrics.
High-performance liquid chromatography
Control and MDI–albumin samples were reduced and acetylated prior to trypsin digestion as described previously [18,19]. Peptide fragments were separated on a Hewlett–Packard 1090 high-performance liquid chromatography (HPLC) system equipped with an Isco model 2150 peak separator and a 1 mm × 25 cm Vydac C-18 reverse phase column (5 μm particle size, 300 pore size) equilibrated with 98% buffer A (0.06% trifluoroacetic acid [TFA]) and 2% buffer B (0.052% TFA and 80% acetonitrile) as described previously [18,19]. Peptides were eluted at 50 μl/min using a stepwise buffer gradient—0 to 60 min (2–37% B), 60 to 90 min (37–75% B), and 90 to 105 min (75–98% B)—and were detected by their absorbance at 210 nm and simultaneously scanned at 245 nm.
Tandem mass spectrometry
Individual HPLC fractions suspected to contain MDI-conjugated peptides, based on their absorbance at 245 nm, were further analyzed by MS/MS on a Waters Q-TOF Ultima mass spectrometer (Milford, MA, USA) with a Waters nanoAcquity ultra-performance liquid chromatography (UPLC) device using a 75 × 250-μM BEH C18 column. After analysis, the MS/MS spectra were searched using the Mascot distiller (to locate and combine MS/MS spectra) and the Mascot database search algorithm (www.matrixscience.com/search_form_select.html). When searching Mascot, two modifications were queried: one adding 224 Da (the mass of MDI in which one NCO (isocyanate) group had been hydrolyzed) and another adding 250 Da (the mass of MDI with both NCO groups present). The spectra for each peptide, with an intact mass that matched albumin peptides with either of the two MDI modifications, were analyzed manually. The amino acid sequences and the sites of MDI were deduced based on the ion spectra and the expected mass loss of either 224 or 250 Da (Table 1).
Table 1.
MDI conjugation sites on human albumin identified by MS/MS.
| Position | Peptide sequence | m/z [M+2H] and/or [M+3H] | Monoisotopic mass | Notes |
|---|---|---|---|---|
| 4 | DAHKSEVAHR | 467.2 | 1398.7 | Second NCO group not hydrolyzed |
| 137 | KYLYEIAR | 640.4 | 1278.7 | |
| 162 | YKAAFTECCQAADK | 629.6 | 1885.8 | |
| 199 | LKCASLQK | 586.3 | 1170.6 | |
| 212 | AFKAWAVAR | 622.3 | 1242.6 | Loss of MDI at x8, x7, y8, and y7 |
| 262 | ADLAKYICENQDSISSK | 722.7 | 2165.1 | |
| 351 | LAKTYETTLEK | 760.9 | 1519.8 | |
| 391 | QNCELFEQLGEYK | 926 | 1849.9 | Second NCO group not hydrolyzed, cysteine not acetylated |
| 414 | KVPQVSTPTLVEVSR | 932.5 (954.5) 622.0 |
1863.1 (1890.0) | Doubly and triply charged species observed (masses with second NCO present in different HPLC fractions) |
| 429 | NLGKVGSK | 513.8 | 1025.6 | |
| 432 | NLGKVGSK | 625.5 | 1249.6 | Peptide doubly conjugated with MDI on the asparagine; spectrum shows several losses of MDI masses |
| 436 | VGSKCCK | 531.8 | 1061.5 | Loss of MDI for M+H, y6, y5, and y4 |
| (?) | HPEAKR | 481.3 | 960.5 | Arg6/Lys5 not conjugated, but only very low b1 ions, suggesting possible conjugation at histidine |
| 525 | KQTALVELVK | 679.9 451.6 |
1351.8 | Doubly and triply charged species observed |
| 541 | ATKEQLK | 521.3 (534.3) | 1040.6 (1066.6) | See x6-MDI, x5-MDI, y6-MDI, and y5-MDI, acting like phosphorylated neutral loss (masses with second NCO present in different HPLC fractions) |
Results
Effects of MDI exposure on human albumin
A rapid reaction (X + Y → Z) occurs when human albumin (X) is mixed with diisocyanate (Y), a class of chemicals known to cause asthma. The products of this reaction (Z) are antigenic to the human immune system but remain incompletely understood. We characterized the different reaction products between 4,4′-MDI and human albumin that result when a range of different starting reactant concentrations are present under the biologically relevant reaction conditions (see Materials and methods). Human albumin was held constant at 74 μM or 0.5% (w/v), similar to levels in vivo in the airway epithelial lining fluid [17], although the concentration of MDI was varied between 2 and 1000 μg/mg albumin (e.g., 40 μM and 64 mM) to achieve molar reactant ratios of Y:X (MDI/albumin) ranging from approximately 0.5:1 to 800:1.
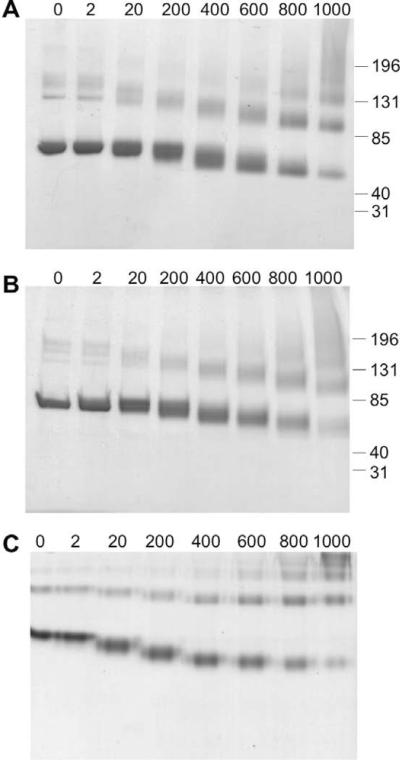
As shown in Fig. 1, MDI exposure caused a dose-dependent increase in albumin's electrophoretic mobility under reducing, nonreducing, and native conditions. Distinct reaction products formed depending on the starting ratio of the reactants and could be discerned as diffuse bands when stained with Coomassie dye. Increased migration of MDI-exposed albumin under native conditions suggests that MDI conjugation increases albumin's net negative charge. However, a similar increase in migration, in SDS gels, suggests that the altered electrophoresis pattern also reflects conformational changes.
Fig. 1.
MDI reactivity with human albumin changes its conformation and charge. Reaction products between MDI and human albumin were analyzed by electrophoresis under nonreducing/SDS conditions (A), reducing/SDS conditions (B), or native conditions (C). The relative amount of the reactants (MDI/albumin) at the beginning of the reactions was varied from 0 to 1000 μg MDI/mg albumin as indicated.
MDI–albumin conjugates for ELISA detection of exposure-induced IgG
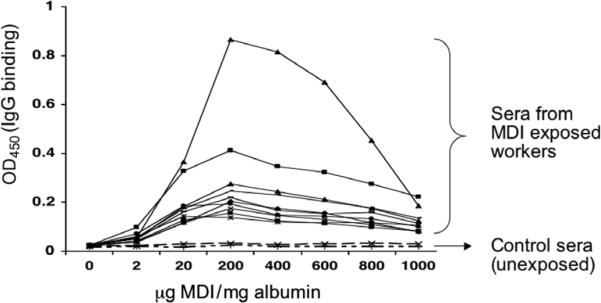
MDI–albumin-specific IgG, although not specifically associated with MDI asthma, has been shown to correlate with exposure and has been suggested as a possible exposure biomarker [10,20,21]. Thus, MDI-albumin reaction products may serve as antigens for detecting MDI (exposure-induced)-specific IgG by ELISA. To determine which MDI–albumin reaction products are most effective for specific IgG detection, we performed tests with microtiter plates coated with MDI–albumin reaction products prepared with different starting concentrations of MDI. As shown in Fig. 2, MDI–albumin reaction products prepared with a starting reactant ratio of 200 μg MDI/mg albumin consistently yielded the highest signal in MDI-specific IgG ELISAs using serum from a panel of MDI-exposed workers but not unexposed control subjects. There was a rapid decrease in the ELISA signal when MDI–albumin conjugates prepared with a higher reactants ratio (MDI/albumin ≥100:1 or 400 μg MDI/mg albumin) were used as “antigens.”
Fig. 2.
Variability in ELISA detection of specific IgG by MDI–albumin conjugates. MDI–albumin conjugates generated with different amounts of MDI, ranging from 0 to 1000 μg MDI/mg albumin, were used (as antigens) to coat microtiter plates in an ELISA for human IgG binding using serum samples from 10 MDI-exposed subjects and 2 representative unexposed individuals. All serum samples were diluted 1:200. The y axis reflects the amount of specific IgG binding. All samples were tested in duplicate, and the results shown are representative of three independent experiments.
HPLC–MS/MS analysis of antigenic MDI–albumin reaction products
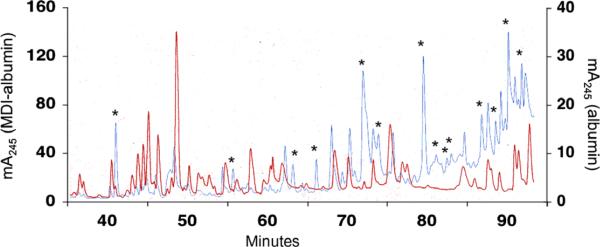
To better characterize those biochemical/biophysical changes in (MDI-exposed) human albumin associated with antigenicity, we performed further analysis using HPLC–MS/MS methods, focusing on MDI–albumin products prepared with 200 μg MDI/mg albumin. Prior to reverse-phase HPLC, MDI–albumin and, for comparison, control mock-exposed albumin were digested with trypsin. The A245 of HPLC elutions from MDI versus control albumin was used to identify fractions that likely contained MDI-conjugated peptides, as highlighted in Fig. 3. Further MS/MS analysis of these fractions confirmed 14 different sites of MDI conjugation to human albumin, as highlighted in Figs. 4 and 5. The majority of conjugation occurred on lysine residues (12 sites identified); however, MDI conjugation was also confirmed on 2 asparagines. Most of the MDI conjugation sites displayed evidence of hydrolysis of the second NCO group of MDI to the free amine; however, the molecular mass of two MDI-conjugated peptides indicate an intact NCO group, suggesting the likelihood of “intra(peptide)molecular” cross-linking. An additional two peptides were identified with and without the second NCO hydrolyzed (in different fractions), again suggesting either intra- or intermolecular cross-linking, possibly in combination with loss of MDI during mass spectrometry (MS). Several of the peptides were found in more than 1 HPLC fraction, and the corresponding unconjugated peptides were also sometimes observed, suggesting either heterogeneity among the MDI–albumin reaction products or partial loss of MDI during MS analysis.
Fig. 3.
Separation of MDI-conjugated (albumin) peptides by HPLC. Control (red) or MDI-conjugated (blue) albumin was trypsin digested and fractionated by HPLC as described in Materials and methods. The absorbance of the column elution at 245 nm (y axis) is plotted over time in minutes (x axis). Note different scales for the MDI–albumin samples (left axis) versus the control albumin samples (right axis). Data shown are representative of four independent experiments. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
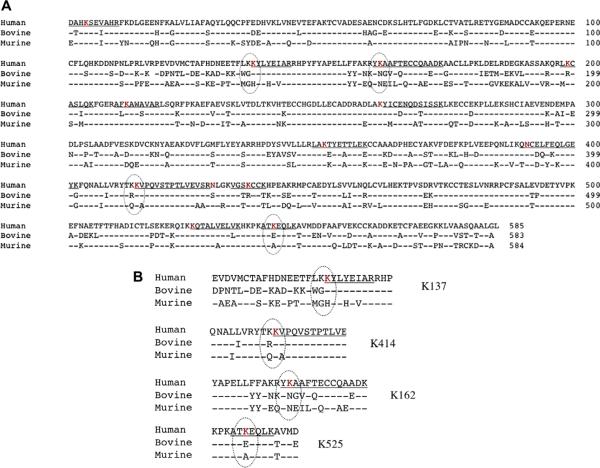
Comparison of human versus other species' albumin and mapping of MDI conjugation sites. (A) Primary amino acid sequence of the mature albumin protein from human, bovine, and murine species (GenBank Accession Nos. ABS29264.1, AAA51411.1, and AAH49971.1 respectively) aligned using the constraint-based multiple alignment tool from the National Center for Biotechnology Information. MDI conjugation sites in human albumin are depicted as red letters, and those not conserved in murine and bovine albumin are circled. (B) Unique MDI conjugation sites in human albumin. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5.
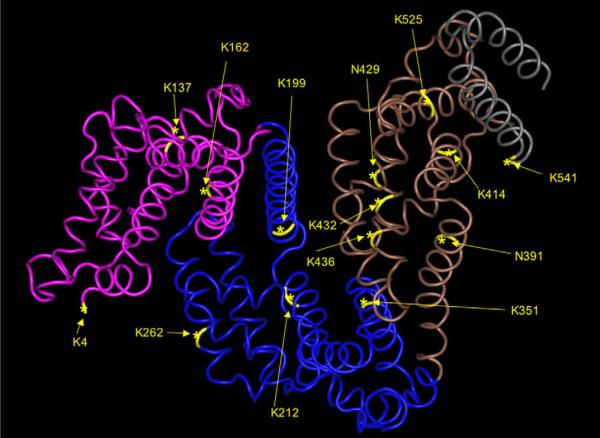
Localization of MDI conjugation sites on human albumin. The sites of MDI conjugation identified by HPLC–MS/MS are highlighted on a three-dimensional representation of human albumin (1UOR) obtained from the protein/molecular modeling database and are displayed using the Cn3D program from the National Center for Biotechnology Information.
Human albumin versus albumin from other species and immune specificity for MDI conjugates
In further ELISA experiments (Fig. 6), we found that human IgG that binds MDI–human albumin appears to exhibit minimal binding (<20%) to MDI conjugates prepared with albumin from other species (murine or bovine). Similar carrier specificity of the human response to aliphatic diisocyanates has been noted previously in studies with egg albumin [15,22]. The specificity of the human immune response for MDI–human albumin may be due in part to the chemical reactivity of MDI with specific amino acids in human albumin that are not present in albumin from other species (Fig. 4). For example, human albumin contains four dilysine motifs, of which we identified three as targets for MDI conjugation, in each case on the second of the two lysines. Two of these dilysine motifs, KK136–137 and KK413–414, are not conserved in the albumin gene of most other species. Similarly, K162 and K541 of human albumin, identified here as MDI conjugation sites, are also not found in albumin from most nonhuman species. On the other hand, murine and bovine albumins each contain dilysines (KK285–286/KK351–352 and KK131–132, respectively) not present in human albumin; they may be preferred targets for MDI conjugation and affect the specificity of the immune response to exposure in these different species.
Fig. 6.
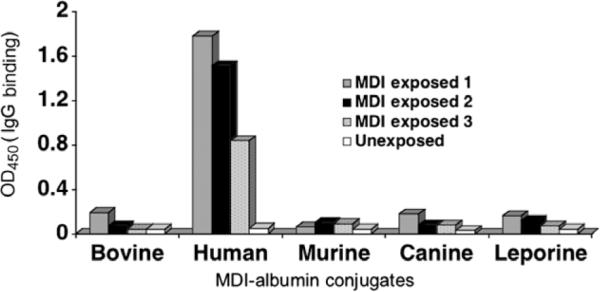
Specificity of the human immune response to MDI: Dependence on human albumin. MDI–albumin conjugates were prepared with albumin from different species as described in Materials and methods and were used as antigens in an ELISA for specific human IgG binding (y axis). Representative experiments performed with serum from 3 MDI-exposed subjects and 1 unexposed control subject are shown.
Discussion
This article has provided new insight into the antigenic changes that occur in the self protein, human albumin, when exposed to the occupational allergen MDI. Advances were made possible by combining several different analytical methods to characterize the range of MDI–albumin reaction products that form under different exposure conditions. Biologically relevant MDI–albumin reaction products produced in vitro were identified by serum IgG from MDI-exposed workers and further characterized through a series of protease digestion, HPLC, and MS/MS. The data define 14 sites of MDI conjugation on human albumin and begin to provide a structural basis for the human immune response to MDI exposure and possibly other chemicals known to bind albumin and cause allergy. The data also highlight the potential utility of the combined analytical methods (electrophoresis, protease digestion, HPLC, and MS) for characterizing and standardizing MDI–albumin reaction products for serology.
Several features of MDI–protein conjugation, some predictable and some unexpected, were highlighted by the current findings. Of the 14 MDI conjugation sites on human albumin, 12 are lysine residues, consistent with chemical theory suggesting that the ε-amine group of lysines' side chain likely consists of nucleophiles for addition reactions with isocyanate [23]. Dilysine motifs (KK) in albumin's primary sequence may be especially susceptible to conjugation with isocyanate. Three of the four dilysine motifs in human albumin were found to be conjugated with MDI, in each case on the second of the two lysines. Two of these dilysine (MDI) conjugation sites in human albumin are not found in most other species' albumin (e.g., murine, bovine), and one of these (Lys414) was previously identified as a preferred target for conjugation by the aliphatic isocyanate, HDI. Thus, lysine side chains, on the carboxyl side of another lysine (KK), appear to represent preferential conjugation targets for MDI onto human albumin.
Precise chemical specificity for the second lysine in the KK motifs of human albumin is emerging as a common characteristic of reactive low-molecular-weight chemicals that cause allergy. The strong “skin sensitizers” 2,4-dinitro-1-chlorobenzene (DNCB) and phenyl salicylate (PS) appear to exhibit the same specificity (as MDI) for K137, K414, and K525 but remarkably not for K136, K413, or K524 [24]. The lysine at position 414 warrants particular attention because it is located near the surface of albumin and the reactive tyrosine (Y) critical to albumin's indole binding domain [25].It has been hypothesized that a cationic group (Arg410) in this microdomain suppresses the pKa values of local side chains (Y411 and K414), and this should theoretically increase susceptibility to nucleophilic addition (e.g., MDI conjugation) [26]. Interestingly, dilysine motifs (KKXX–COOH) serve a well-recognized biological function as a signal motif during protein (e.g., antigen) processing; however, the relevance of this phenomenon to the current findings remains speculative [27].
Other lysine residues of human albumin, conjugated by MDI, have been identified as specific targets for covalent and noncovalent chemical binding. K199, which is the primary reaction target for penicillin conjugation, is unusually nucleophilic and is hypothesized to possess a relatively low pKa that should increase its reactivity with MDI [28,29]. K199 also reacts with acetylsalicyclic acid and p-nitrophenyl acetate, and it appears to be the target that differentiates strong (vs. weak) sensitizers among sultone isomers [30,31]. K199, along with K541 and K351, is also the major binding site for metabolites of the nonsteroidal antiinflammatory drug naproxen [32]. The lysine at position 212, which lies within the noncovalent drug binding site commonly called binding site 1, is a selective target for DNCB and 5-chloro-2-methylisothaizol-3-one but not for PS conjugation [24,33]. The side chains for at least five of the MDI-conjugated amino acids (K212, K351, K436, K525, and K541) are known to be accessible on the surface of albumin based on studies with succinimidyl cross-linkers [34]. Furthermore, two lysine residues (K73 and K240) located in unexposed/hydrophobic microdomains of albumin, theoretically unlikely to react with MDI, were not among the MDI conjugation sites identified here [35].
Although lysine is the major target (in human albumin) for MDI, conjugation was also confirmed on two different asparagines, both located within a stretch of 50 amino acids (391–441) that contains a substantial proportion (35%) of the identified MDI conjugation sites. A 15th potential MDI conjugation site may also exist within this region based on the mass of the intact peptide sequence HPEAKR (440–445); however, MS/MS data could not definitively resolve an MDI conjugation site (data not shown).
The majority of MDI conjugated to human albumin was found to be hydrolyzed to the free amine at the second NCO group. However, there were several exceptions where peptide masses were consistent with the presence of the second NCO group, suggesting intrapeptide cross-linking, as described previously for TDI [36].In all cases, the identified albumin peptide contained two potential sites for MDI conjugation. One of the peptides, DAHKSEA, was conjugated on the fourth position lysine and contains the N terminus of albumin, a likely target for isocyanate based on peptide studies with TDI [36]. Another peptide contains MDI conjugated to asparagine and a cysteine group, which surprisingly was not modified (reduced/acetylated) during sample processing (see technical issues below), suggesting that it may have reacted with MDI. Two other peptides, each containing two lysine residues, were sometimes observed without the second NCO group hydrolyzed, perhaps suggesting limited intra- or intermolecular cross-linking in combination with a loss of MDI during MS (see below).
Although the current data do not define the individual contribution of specific MDI conjugation sites, they do provide interesting speculation regarding MDI conjugation sites K162, K541, KK136–137, and KK413–414, which differ in human albumin versus albumin from other species (e.g., murine, bovine). The secondary/tertiary structure of MDI-conjugated albumin is likely another important characteristic that influences its recognition by human antibodies, as evidenced by the electrophoresis and ELISA data presented here as well as preliminary findings (not shown) that trypsin digestion abrogates antigenicity. The current data should facilitate molecular modeling of MDI–albumin conjugates and, ultimately, structural elucidation of the “neoepitopes” created when human albumin reacts with MDI.
Limitations of the current analytical methods should be recognized when interpreting the data, including issues common to all immunological and/or proteomic approaches as well as those specific to MDI's chemistry (e.g., reversibility/hydrolysis). Antigenicity in the current study was based largely on recognition by serum IgG using an ELISA format, which may be suboptimal for insoluble polymers or highly “haptenated” albumin reaction products (e.g., formed with high doses of MDI). Further experiments (e.g., competitive ELISA) may identify MDI–albumin reaction products with additional antibody binding and MDI conjugation sites. Proteomically, reduction and acetylation, often necessary to achieve efficient trypsin digestion prior to HPLC–MS/MS, could reverse MDI conjugation via thiol groups (e.g., Cys, Met). However, under native conditions, all Cys thiols except one are disulfide bonded and, thus, are not a likely target for MDI. The loss of MDI during MS analysis, however, was apparent in some of the ion m/z spectra; thus, some intermolecular cross-linked peptides could have been missed. The reliance on ultra-violet (UV) light absorbance to “track” MDI is another potential issue because aromatic amino acids also absorb UV light. However, only tryptophan exhibits substantial absorbance at 245 nm and was not part of any of the identified MDI-conjugated albumin peptides. Remarkably, the MDI–albumin ratio of the final reaction products based on UV light absorbance at 245 and 280 nm, and the respective molar extinction coefficients of MDI and albumin at these wavelengths, yields a value (13.7:1) very close to the number of MDI conjugation sites identified by HPLC–MS/MS [37]. Future characterization of MDI–albumin reaction products through additional approaches (e.g., modified thiol treatment, multiple reaction monitoring [MRM]–MS) should help to resolve lingering questions related to analytical methodology and provide more precise quantitation of MDI conjugation at different sites.
In summary, we have begun to define a structural basis for the human immune response to MDI exposure. We demonstrated that human albumin is unique in its ability to serve as an MDI carrier for recognition by human antibodies, and we identified a total of 14 different MDI conjugation sites, primarily on lysine residues, including 3 of the 4 dilysine loci. The findings highlight the importance of exposure conditions (e.g., reactant concentrations) on MDI's antigenicity, as measured by direct ELISA, and the potential utility of HPLC–MS/MS to standardize MDI–albumin conjugates for serology. The recognition that MDI conjugation to human albumin occurs at residues with important biological functional activity provides further interesting speculation about the potential health effects of MDI exposure.
Supplementary Material
Acknowledgments
We thank the Yale Keck Center for its assistance with the HPLC and MS studies. We are especially thankful to Tom Abbott, Mary LoPresti, Kathy Stone, and Walt McMurray for their helpful guidance and data analysis. Funding was provided by the National Institute of Environmental Health and Safety through Grants R42-ES016728 and R41-ES018021 (to A.V.W.) and by the National Institute of Occupational Health and Safety (to C.A.R.).
Footnotes
Abbreviations used: MDI, diphenylmethane diisocyanate; TDI, toluene diisocyanate; HDI, hexamethylene diisocyanate; IgE, immunoglobulin epsilon; IgG, immunoglobulin gamma; MS/MS, tandem mass spectrometry; PBS, phosphate-buffered saline; SDS, sodium dodecyl sulfate; ELISA, enzyme-linked immunosorbent assay; OD, optical density; HPLC, high-performance liquid chromatography; TFA, trifluoroacetic acid; UPLC, ultra-performance liquid chromatography; NCO, isocyanate; MS, mass spectrometry; DNCB, 2,4-dinitro-1-chlorobenzene; PS, phenyl salicylate; UV, ultraviolet; MRM, multiple reaction monitoring.
Appendix A. Supplementary data Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ab.2010.01.037.
References
- [1].Wisnewski A, Redlich C, Mapp C, Bernstein D. Polyisocyanates. Informa Healthcare; London: 2006. [Google Scholar]
- [2].Redlich CA, Bello D, Wisnewski AV. Health effects of isocyanates. In: Rom WN, editor. Environmental and Occupational Medicine. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 502–515. [Google Scholar]
- [3].Redlich CA, Bello D, Woskie SR, Streicher RP. Measurements of airborne methylene diphenyl diisocyanate concentration in the US workplace. J. Occup. Environ. Hyg. 2009;6:D82–D83. doi: 10.1080/15459620903256427. comment. Author reply, D83–D85. [DOI] [PubMed] [Google Scholar]
- [4].Robert A, Ducos P, Francin JM, Marsan P. Biological monitoring of workers exposed to 4,4′-methylenediphenyl diisocyanate (MDI) in 19 French polyurethane industries. Intl. Arch. Occup. Environ. Health. 2007;80:412–422. doi: 10.1007/s00420-006-0150-3. [DOI] [PubMed] [Google Scholar]
- [5].Sabbioni G, Wesp H, Lewalter J, Rumler R. Determination of isocyanate biomarkers in construction site workers. Biomarkers. 2007;12:468–483. doi: 10.1080/13547500701395636. [DOI] [PubMed] [Google Scholar]
- [6].Lesage J, Stanley J, Karoly WJ, Lichtenberg FW. Airborne methylene diphenyl diisocyanate (MDI) concentrations associated with the application of polyurethane spray foam in residential construction. J. Occup. Environ. Hyg. 2007;4:145–155. doi: 10.1080/15459620601133779. [DOI] [PubMed] [Google Scholar]
- [7].Liu Q, Wisnewski AV. Recent developments in diisocyanate asthma. Ann. Allergy Asthma Immunol. 2003;90:35–41. doi: 10.1016/s1081-1206(10)61647-x. [DOI] [PubMed] [Google Scholar]
- [8].Wisnewski AV, Srivastava R, Herick C, Xu L, Lemus R, Cain H, Magoski NM, Karol MH, Bottomly K, Redlich CA. Identification of human lung and skin proteins conjugated with hexamethylene diisocyanate in vitro and in vivo. Am. J. Respir. Crit. Care Med. 2000;162:2330–2336. doi: 10.1164/ajrccm.162.6.2002086. [DOI] [PubMed] [Google Scholar]
- [9].Wisnewski AV, Stowe MH, Cartier A, Liu Q, Liu J, Chen L, Redlich CA. Isocyanate vapor-induced antigenicity of human albumin. J. Allergy Clin. Immunol. 2004;113:1178–1184. doi: 10.1016/j.jaci.2004.03.009. [DOI] [PubMed] [Google Scholar]
- [10].Wisnewski AV. Developments in laboratory diagnostics for isocyanate asthma. Curr. Opin. Allergy Clin. Immunol. 2007;7:138–145. doi: 10.1097/ACI.0b013e3280895d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ye YM, Kim CW, Kim HR, Kim HM, Suh CH, Nahm DH, Park HS, Redlich CA, Wisnewski AV. Biophysical determinants of toluene diisocyanate antigenicity associated with exposure and asthma. J. Allergy Clin. Immunol. 2006;118:885–891. doi: 10.1016/j.jaci.2006.06.026. [DOI] [PubMed] [Google Scholar]
- [12].Bernstein JA, Munson J, Lummus ZL, Balakrishnan K, Leikauf G. T-cell receptor V beta gene segment expression in diisocyanate-induced occupational asthma. J. Allergy Clin. Immunol. 1997;99:245–250. doi: 10.1016/s0091-6749(97)70104-0. [DOI] [PubMed] [Google Scholar]
- [13].Redlich CA, Karol MH. Diisocyanate asthma: clinical aspects and immunopathogenesis. Intl. Immunopharmacol. 2002;2:213–224. doi: 10.1016/s1567-5769(01)00174-6. [DOI] [PubMed] [Google Scholar]
- [14].Wisnewski AV, Liu Q, Liu J, Redlich CA. Human innate immune responses to hexamethylene diisocyanate (HDI) and HDI–albumin conjugates. Clin. Exp. Allergy. 2008;38:957–967. doi: 10.1111/j.1365-2222.2008.02982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wass U, Belin L. Immunologic specificity of isocyanate-induced IgE antibodies in serum from 10 sensitized workers. J. Allergy Clin. Immunol. 1989;83:126–135. doi: 10.1016/0091-6749(89)90487-9. [DOI] [PubMed] [Google Scholar]
- [16].Baur X, Chen Z, Flagge A, Posch A, Raulf-Heimsoth M. EAST and CAP specificity for the evaluation of IgE and IgG antibodies to diisocyanate–HSA conjugates. Intl. Arch. Allergy Immunol. 1996;110:332–338. doi: 10.1159/000237325. [DOI] [PubMed] [Google Scholar]
- [17].Ishizaka A, Watanabe M, Yamashita T, Ogawa Y, Koh H, Hasegawa N, Nakamura H, Asano K, Yamaguchi K, Kotani M, Kotani T, Morisaki H, Takeda J, Kobayashi K, Ogawa S. New bronchoscopic microsample probe to measure the biochemical constituents in epithelial lining fluid of patients with acute respiratory distress syndrome. Crit. Care Med. 2001;29:896–898. doi: 10.1097/00003246-200104000-00043. [DOI] [PubMed] [Google Scholar]
- [18].Kim S, Merrill BM, Rajpurohit R, Kumar A, Stone KL, Papov VV, Schneiders JM, Szer W, Wilson SH, Paik WK, Williams KR. Identification of N(G)-methylarginine residues in human heterogeneous RNP protein A1: Phe/Gly-Gly-Gly-Arg-Gly-Gly-Gly/Phe is a preferred recognition motif. Biochemistry. 1997;36:5185–5192. doi: 10.1021/bi9625509. [DOI] [PubMed] [Google Scholar]
- [19].Williams KR, Stone KL. Enzymatic cleavage and HPLC peptide mapping of proteins. Mol. Biotechnol. 1997;8:155–167. doi: 10.1007/BF02752260. [DOI] [PubMed] [Google Scholar]
- [20].Pronk A, Preller L, Raulf-Heimsoth M, Jonkers IC, Lammers JW, Wouters IM, Doekes G, Wisnewski AV, Heederik D. Respiratory symptoms, sensitization, and exposure response relationships in spray painters exposed to isocyanates. Am. J. Respir. Crit. Care Med. 2007;176:1090–1097. doi: 10.1164/rccm.200702-215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Redlich CA, Stowe MH, Wisnewski AV, Eisen EA, Karol MH, Lemus R, Holm CT, Chung JS, Sparer J, Liu Y, Woskie SR, Appiah-Pippim J, Gore R, Cullen MR. Subclinical immunologic and physiologic responses in hexamethylene diisocyanate-exposed auto body shop workers. Am. J. Ind. Med. 2001;39:587–597. doi: 10.1002/ajim.1058. [DOI] [PubMed] [Google Scholar]
- [22].Baur X. Immunologic cross-reactivity between different albumin-bound isocyanates. J. Allergy Clin. Immunol. 1983;71:197–205. doi: 10.1016/0091-6749(83)90100-8. [DOI] [PubMed] [Google Scholar]
- [23].Urlich H. Chemistry and Technology of Isocyanates. John Wiley; West Sussex, UK: 1996. [Google Scholar]
- [24].Aleksic M, Pease CK, Basketter DA, Panico M, Morris HR, Dell A. Investigating protein haptenation mechanisms of skin sensitisers using human serum albumin as a model protein. Toxicol. In Vitro. 2007;21:723–733. doi: 10.1016/j.tiv.2007.01.008. [DOI] [PubMed] [Google Scholar]
- [25].Brown JR, Shockley P. Serum albumin: structure and characterization of its ligand binding sites. In: Jost PC, Griffith OH, editors. Lipid–Protein Interactions. 3rd ed. Vol. 1. John Wiley; New York: 1982. pp. 22–68. [Google Scholar]
- [26].Ahmed N, Dobler D, Dean M, Thornalley PJ. Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J. Biol. Chem. 2005;280:5724–5732. doi: 10.1074/jbc.M410973200. [DOI] [PubMed] [Google Scholar]
- [27].Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- [28].Yvon M, Wal JM. Identification of lysine residue 199 of human serum albumin as a binding site for benzylpenicilloyl groups. FEBS Lett. 1988;239:237–240. doi: 10.1016/0014-5793(88)80924-4. [DOI] [PubMed] [Google Scholar]
- [29].Gerig JT, Katz KE, Reinheimer JD. Reactions of 2,6-dinitro-4-trifluoromethyl-benzenesulfonate with human serum albumin. Biochim. Biophys. Acta. 1978;534:196–209. doi: 10.1016/0005-2795(78)90002-8. [DOI] [PubMed] [Google Scholar]
- [30].Walker JE. Lysine residue 199 of human serum albumin is modified by acetylsalicyclic acid. FEBS Lett. 1976;66:173–175. doi: 10.1016/0014-5793(76)80496-6. [DOI] [PubMed] [Google Scholar]
- [31].Awad-Elkarim A, Means GE. The reactivity of p-nitrophenyl acetate with serum albumins. Comp. Biochem. Physiol. B. 1988;91:267–272. doi: 10.1016/0305-0491(88)90141-1. [DOI] [PubMed] [Google Scholar]
- [32].Olsen J, Bjornsdottir I, Tjornelund J, Honore Hansen S. Identification of the amino acids of human serum albumin involved in the reaction with the naproxen acyl coenzyme A thioester using liquid chromatography combined with fluorescence and mass spectrometric detection. Anal. Biochem. 2003;312:148–156. doi: 10.1016/s0003-2697(02)00462-1. [DOI] [PubMed] [Google Scholar]
- [33].Kosa T, Maruyama T, Otagiri M. Species differences of serum albumins: I. Drug binding sites. Pharm. Res. 1997;14:1607–1612. doi: 10.1023/a:1012138604016. [DOI] [PubMed] [Google Scholar]
- [34].Huang BX, Dass C, Kim HY. Probing conformational changes of human serum albumin due to unsaturated fatty acid binding by chemical cross-linking and mass spectrometry. Biochem. J. 2005;387:695–702. doi: 10.1042/BJ20041624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huang BX, Kim HY, Dass C. Probing three-dimensional structure of bovine serum albumin by chemical cross-linking and mass spectrometry. J. Am. Soc. Mass Spectrom. 2004;15:1237–1247. doi: 10.1016/j.jasms.2004.05.004. [DOI] [PubMed] [Google Scholar]
- [36].Hettick JM, Ruwona TB, Siegel PD. Structural elucidation of isocyanate-peptide adducts using tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2009;20:1567–1575. doi: 10.1016/j.jasms.2009.04.016. [DOI] [PubMed] [Google Scholar]
- [37].Jin RZ, Karol MH. Intra- and intermolecular reactions of 4,4′-diisocyanato-diphenylmethane with human serum albumin. Chem. Res. Toxicol. 1988;1:281–287. doi: 10.1021/tx00005a005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.