Abstract
Objectives
To describe therapeutic antibiotic use patterns in dogs at a small animal teaching hospital.
Methods
A retrospective case analysis of randomly sampled antibiotic prescriptions in dogs from May 20, 2008 – May 20, 2009, deemed to be for therapeutic use, was performed. Records were reviewed to determine if there was documentation of confirmed, suspected or no evidence of infection. The five most frequently prescribed antibiotics were identified and analyzed for their distribution in these categories.
Results
In 17% of therapeutic antibiotic prescriptions there was confirmed infection, in 45% suspected infection, and in 38% there was no documented evidence of infection. Amoxicillin-clavulanate was the most frequently prescribed antibiotic, followed by cefazolin/cephalexin, enrofloxacin, ampicillin/amoxicillin and doxycycline. Doxycycline was the most frequently prescribed with no documented evidence of infection, and amoxicillin-clavulanate was the most frequently prescribed with either confirmed or suspected evidence of infection.
Discussion
Clinicians use a variety of tools when deciding whether or not to prescribe an antibiotic and which antibiotic to use. As in human medicine, there is likely overuse and inappropriate use of antibiotics in veterinary medicine. Veterinarians should engage in discussions regarding clinically applicable guidelines for appropriate antibiotic use.
Introduction
Antibiotic resistance is of considerable and growing concern in human and veterinary medicine because of the difficulties in treating antibiotic resistant infections. Resistance among bacterial populations can occur through several mechanisms, including introduction of resistant bacteria into a formerly susceptible population, genetic mutation that confers resistance, transfer of a genetic mutation from a resistant strain, selection of resistant strains through antibiotic pressure, or dissemination of resistant bacteria caused by poor infection control.1 Widespread antibiotic use contributes to development of resistant populations by selecting for resistant bacteria. Resistant bacteria in turn can transfer resistance genes to other previously naïve bacterial populations.
In order to effectively prevent and control resistance, medical communities need to monitor and limit antibiotic use.2 Once a resistant strain has emerged, re-developing susceptibility to antimicrobial therapy is a difficult and lengthy process. 3 Therefore, efforts should be focused on preventing the emergence of resistant strains through prudent use of antibiotics, rather than trying to reduce the prevalence of resistant strains once they have emerged. Lack of standardized infection control practices and lack of adherence to recommended antibiotic use practices are two major areas of focus in this effort. A recent article surveyed veterinary teaching hospitals about infection control programs and showed that most institutions acknowledged the importance of biosecurity and the potential risks inherent in working at a veterinary hospital. Despite this, few had formal training programs for staff, long-term surveillance programs, or plans to ensure and enforce basic biosecurity measures.4
A large number of published studies propose a causal link between antimicrobial use in animals and antibiotic resistant bacterial strains in humans. Most of the literature focuses on how antibiotic use on farms affects the development and spread of resistant bacteria via entry into the human food supply. Several case studies have documented the presence of antibiotic-resistant bacterial strains in small animal veterinary medicine,5–7 including some that have shown that resistant strains (mostly methicillin-resistant staphylococcus aureus (MRSA)) can be transferred between health care providers and patients and also between owners and their pets.8,9 In the case of MRSA, veterinarians may serve as reservoirs of resistant infections, potentially infecting patients in their care.10
Several US institutions and international organizations including the American Veterinary Medical Association (AVMA), Centers for Disease Control and Prevention (CDC), World Health Organization (WHO), and the Food and Drug Administration (FDA) have issued broad statements about antibiotic use in human and veterinary patients. Although there is acknowledgement that increased awareness about the prudent use of antibiotics is necessary, there has been very little research or surveillance on the current use of antibiotics in small animal veterinary hospitals. Use of broad-spectrum antibiotics in humans indiscriminately amplifies reservoirs of resistant strains of bacteria, so it is reasonable to conclude that indiscriminate use of antibiotics in veterinary medicine contributes to similar problems of resistant strains in animal populations.11 Furthermore, because there is a large overlap in antibiotics used in human and in small animal veterinary medicine, and because of the close and continuous contact many pet owners have with their dogs and cats, it is especially important to extend the focus of antimicrobial research to include use in small animal medicine.12
Few studies to date have documented antibiotic prescription patterns in veterinary small animal hospitals and none have documented whether the AVMA guidelines (or others) on judicious use of antibiotics are being followed at a clinical level. One study in Finland found that 27% of dogs admitted to a veterinary hospital were treated with oral antibiotics. The authors of this study concluded that antibiotic use in dogs is likely more liberal than in food animals because compared to agricultural use, antibiotic use in companion animals is not strictly regulated and is driven by people’s emotional attachment to their individual pets’ needs.13 A second study looked at the application of guidelines for clinical use of antibiotics established by the Ontario Veterinary College Veterinary Teaching Hospital. The authors concluded that there is little objective data to guide small animal clinicians about prudent and rational use of antibiotics, and the general policies laid out by national and international organizations are difficult to apply in a clinical setting.14
The goal of the present study was to describe therapeutic antibiotic use patterns in dogs at a small animal teaching hospital and to determine, based on the American Veterinary Medical Associations Guidelines for Judicious Therapeutic Use of Antimicrobials (Appendix 1), the proportion of encounters for which antibiotics were prescribed where antibiotic use was indicated.15
Methods
The pharmacy database at a small animal teaching hospital was searched for all antibiotic prescriptions in dogs between May 20, 2008 and May 20, 2009. If a dog was prescribed the same antibiotic multiple times within a 30-day period, subsequent prescriptions were eliminated on the assumption that they likely represented treatment of the same infection or set of clinical signs. A random number generator was then used to select 15% of remaining prescriptions. The day of the randomly generated prescription was defined as the “medical encounter day”, and all antibiotics prescribed on that day were included in further analysis.
Each prescription was then classified as either therapeutic or non-therapeutic use by reviewing the medical charts. Non-therapeutic use was defined as drugs used to treat chemotherapy and radiation patients without documented infection, surgical prophylaxis and/or non-antimicrobial use of metronidazole for diarrhea or portosystemic shunts. Therapeutic use included all other reasons for which antibiotics were prescribed (Table 1).
Table 1.
Non-infectious disease use, confirmed, suspect, no evidence of infection criteria.
| Non-infectious use (excluded from analysis) |
Confirmed diagnosis of infection: |
Suspect infection: | No evidence of infection: |
|---|---|---|---|
Prophylactic use:
|
|
|
|
Non-antimicrobial use of metronidazole:
| |||
Therapeutic use prescriptions were further categorized by classification of infection status as ‘confirmed’, ‘suspected’ or ‘no evidence’ of infection. In instances where a dog was prescribed multiple antibiotics for the same diagnosis on the day of the medical encounter all prescriptions were grouped as a single data point for analysis of infection status. ‘Confirmed infection’ was defined as an encounter with documentation of a positive culture, fluid analysis with presence of bacteria, positive tick titer or a SNAP test with clinical signs of tick-borne disease. ‘Suspected infection’ was defined as an encounter with documentation of an open wound, presence of neutrophilic fluid with no bacteria seen, radiographs identifying pneumonia but without positive transtracheal wash and culture, purulent skin disease without culture and sensitivity, purulent discharge from an orifice without culture and sensitivity, or visualization of gastrointestinal perforation, in the absence of ‘confirmed infection’. ‘No evidence of infection’ was defined as an encounter that lacked documentation of confirmed or suspected infection or of an alternative reason for antibiotic. This category included encounters that documented negative titers or cultures, did not document any titers or cultures, ‘preventative’ (as written in record), or systemic antibiotic use after clean eye or orthopedic surgery (Table 1).
For each encounter included in the study, the following variables were recorded from electronic and paper records: breed, sex, age of animal, and in or outpatient status. We also recorded the temperature of the animal on the day the antibiotic was prescribed, dose, method of delivery, and dose length. Dose length was defined as the number of days of antibiotic administration in hospital plus the number of days for which antibiotics were prescribed at discharge. Amoxicillin and ampicillin as well as cefazolin and cephalexin were combined into one category because they cover the same general spectrum and are frequently inter-changed during a single treatment. We also determined when possible the condition for which the antibiotic was initially prescribed and the final diagnosis of the patient. For each case, we recorded whether a culture was performed and if so, which fluid was cultured. For cases of suspected Lyme disease it was determined whether a tick titer, SNAP test, or no test was performed.
We used Excel 11.5.5 to determine the percent of encounters in each diagnosis category and the most prescribed antibiotics. In addition, because multiple antibiotics were prescribed at some encounters, we tabulated the frequency of use of each antibiotic overall and within each therapeutic use category. The Institutional Review Board (IRB) determined that this study met the criteria for exemption.
Results
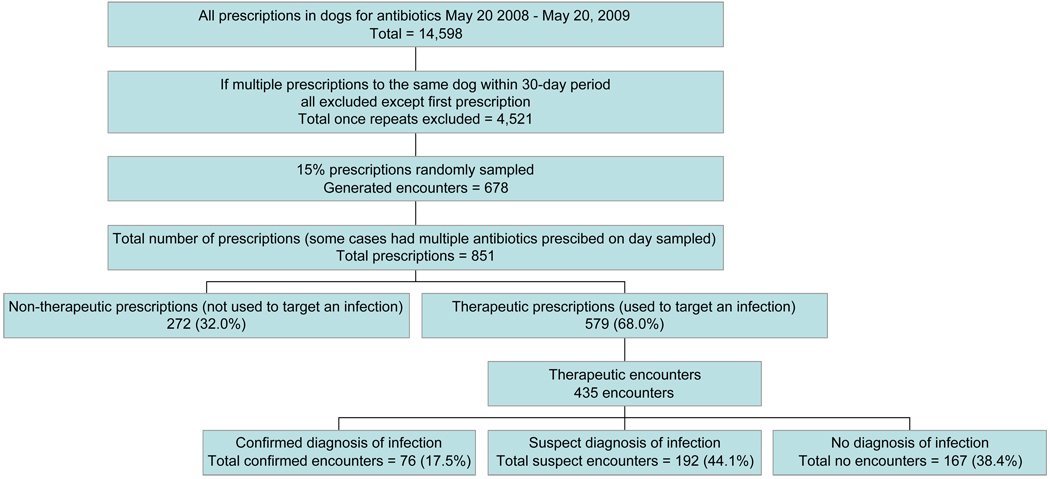
A total of 14,598 prescriptions for antibiotics were written for dogs between May 20, 2008 and May 20, 2009. Eliminating prescriptions of antibiotics to the same dog within 30 days of a first encounter left 4,521 prescriptions in the database. Fifteen percent (15%) of these were randomly sampled, generating 678 encounters. Adding in all antibiotics prescribed at each encounter left 851 prescriptions in the final dataset. 579 (68%) prescriptions fell into the therapeutic use category and 272 (32%) fell into the non-therapeutic use category (Fig 1). The 579 prescriptions in the therapeutic use category represented 435 unique encounters.
Figure 1.
Flow chart of case selection for analysis including results from each analysis.
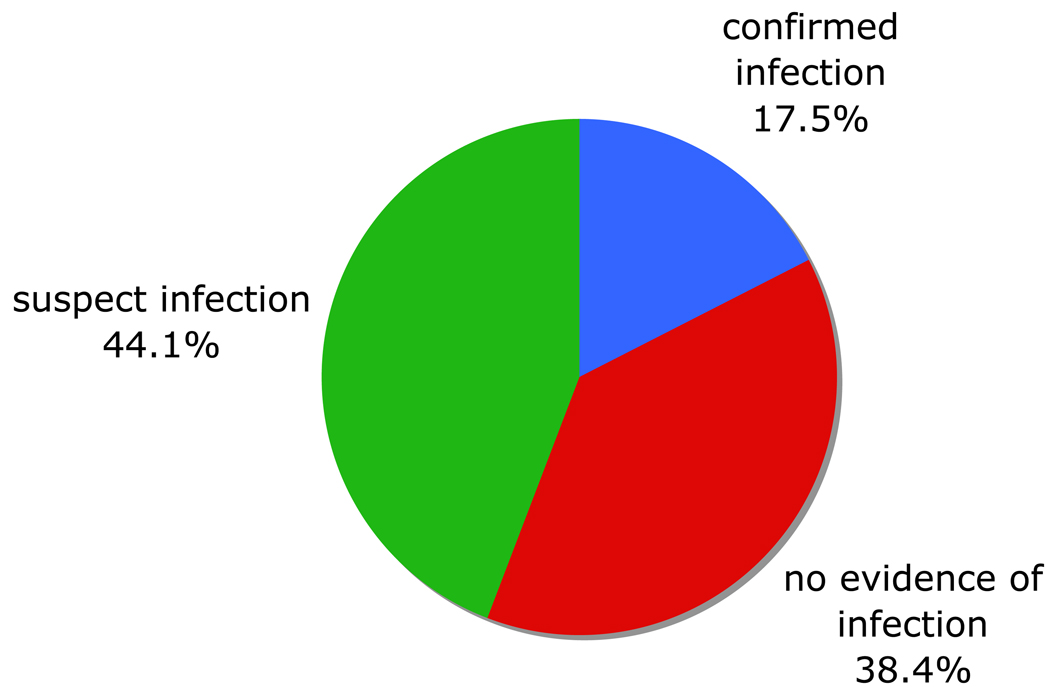
Of the 435 encounters analyzed, 76 (17.5%) met criteria for confirmed infection, 192 (44.1%) for suspected infection, and 167 (38.4%) for no documented evidence of infection (Fig 2).
Figure 2.
Distribution of encounters by category in which a therapeutic antibiotic was prescribed between May 20, 2008 and May 20, 2009 (N=435).
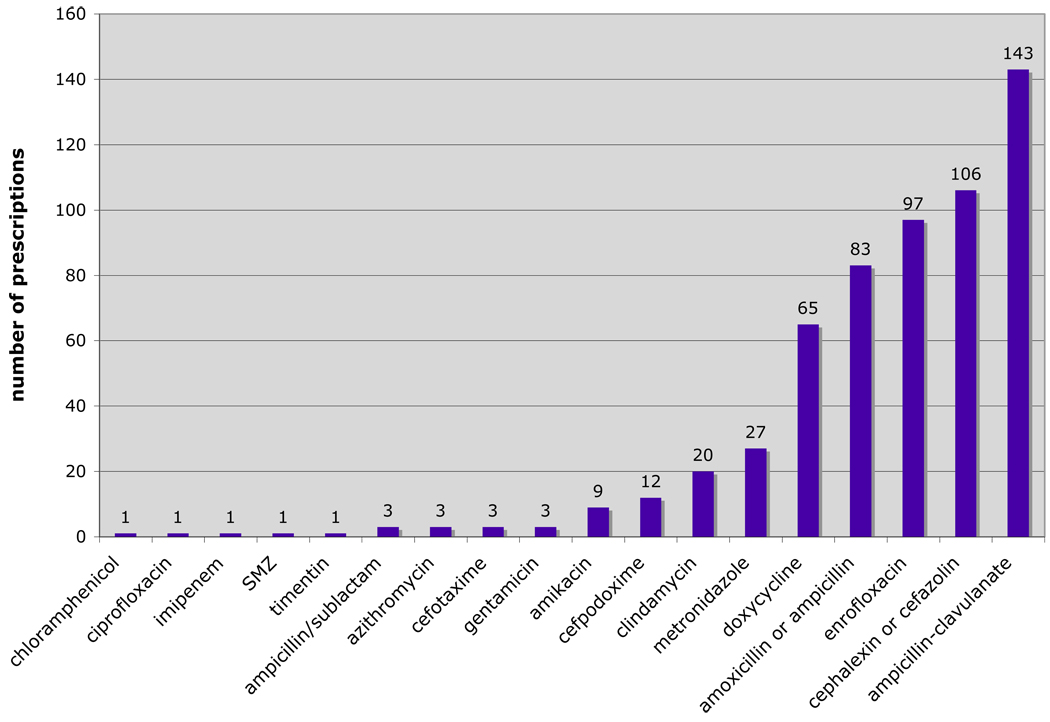
Antibiotics prescribed most frequently for therapeutic reasons were amoxicillin-clavulanate (143 prescriptions, 24.7%), cefazolin/cephalexin (106 prescriptions, 18.3%) enrofloxacin (97 prescriptions, 16.7%), ampicillin /amoxicillin (83 prescriptions, 14.9%), and doxycycline (65 prescriptions, 11.2%) (Fig 3).
Figure 3.
Distribution of antibiotic prescriptions at 435 encounters targeting infectious disease randomly sampled between May 20, 2008 and May 20, 2009 (N= 579).
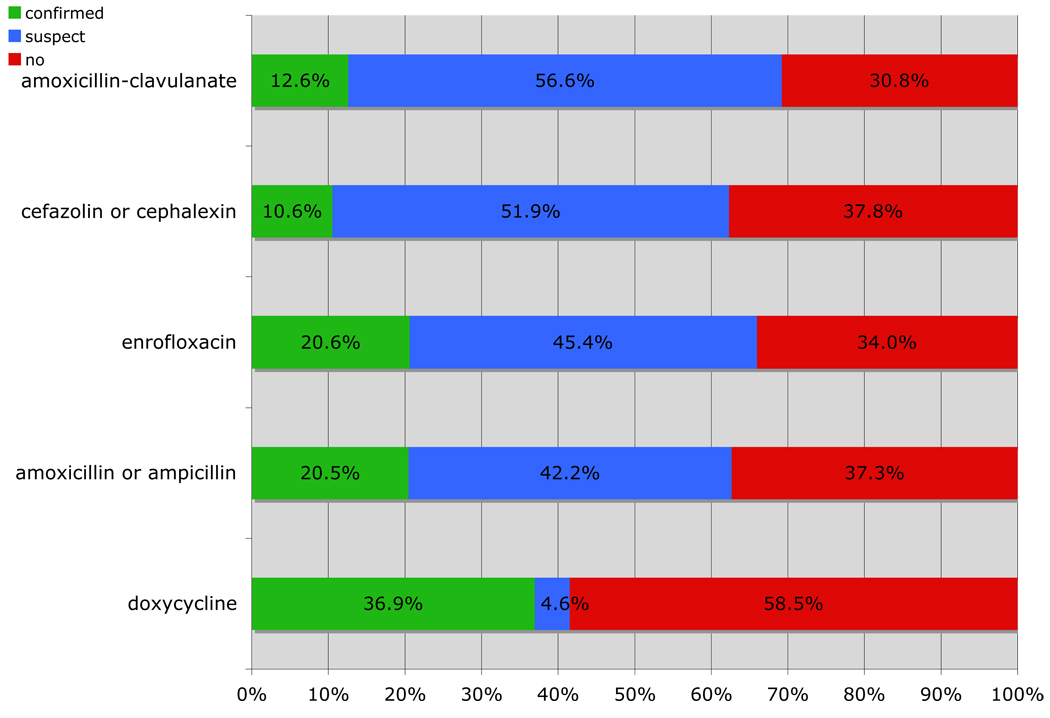
Doxycycline was the antibiotic that had the largest proportion of prescriptions with no documented evidence of infection (58.5%) Cefazolin/cephalexin had 37.8% of prescriptions with no documented evidence of infection, followed by ampicillin/amoxicillin (37.3%), enrofloxacin (34.0%), and amoxicillin-clavulanate (30.8%) (Fig 4).
Figure 4.
Percent distribution of prescriptions targeting infections for confirmed, suspect or no evidence of infection by antibiotic of the top five most prescribed antibiotics between May 20, 2008 and May 20, 2009.
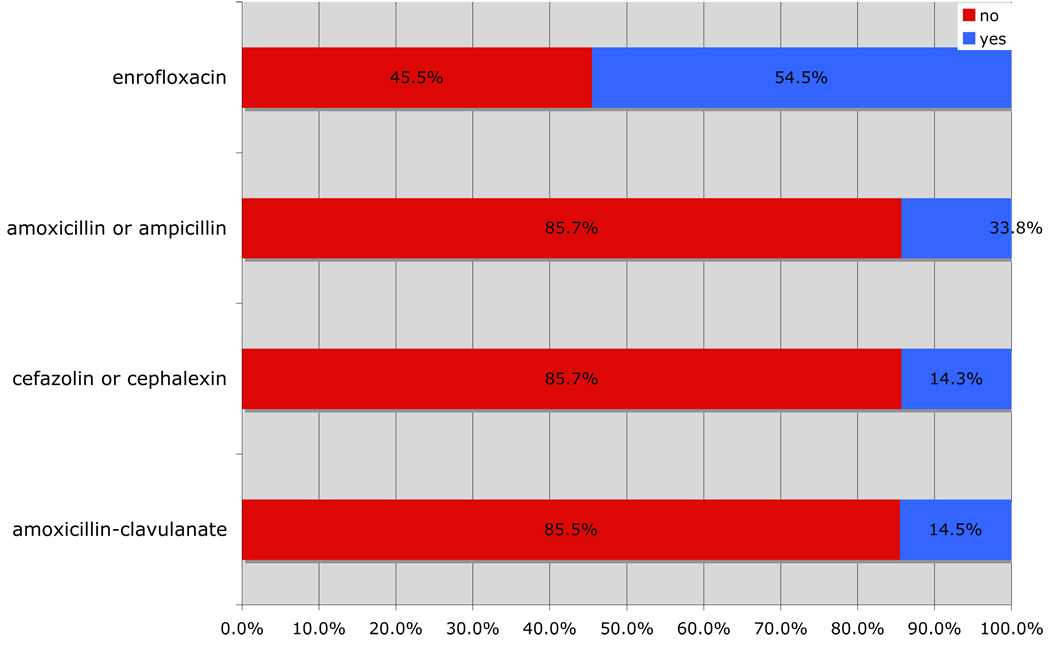
Excluding doxycycline, which is frequently prescribed for tick-borne infection, and for which no culture is available, amoxicillin-clavulanate (85.5%) and cefazolin/cephalexin (85.7%) contributed the largest percentage of cases for which a culture was not performed. Enrofloxacin contributed the largest percentage of cases for which a culture was performed (54.5%) (Fig 5).
Figure 5.
Percent cases for which a culture was or was not performed for the most prescribed antibiotics (excluding doxycycline) between May 20, 2008 and May 20, 2009.
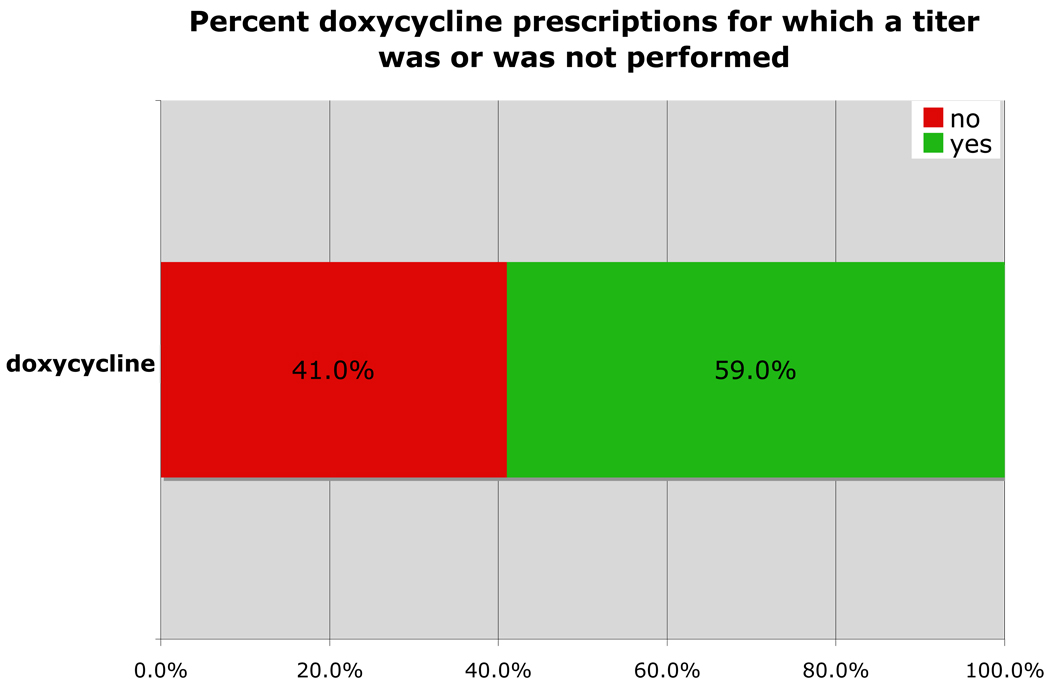
Tick titer panels or a SNAP test were performed in 59.0% of doxycycline prescriptions (Fig 6).
Figure 6.
Percent cases in which doxycycline was prescribed in which a titer was or was not performed between May 20, 2008 and May 20, 2009.
Discussion
Results of this study indicate that based on specified criteria, in 38.4% of medical encounters antibiotics were dispensed with no evidence of infection, in 44.1% of encounters antibiotics were dispensed with just suspected evidence of infection, and in only 17.5% of encounters antibiotics were dispensed for an infection that was confirmed and documented in the medical record. These observations conflict with the American College of Veterinary Internal Medicine (ACVIM) Consensus Statement on Antimicrobial Drug Use, which does not support therapeutic use without justification. These observations suggest that clinicians are frequently prescribing antibiotics without proper documentation in the medical record or without indication for their use. In addition, since this analysis was by medical encounter day and not total prescriptions, these percentages likely underestimate the number of antibiotics prescribed without evidence of infection. Cultures allow more targeted antibiotic use and therefore would potentially reduce the number of inappropriate antibiotic prescriptions.
Research on use of antibiotics in human medicine has documented variability among individual clinicians’ dispensing criteria, and suggests that prescriptions based solely on clinical signs are often inappropriate, and can lead to overuse.16, 17 One study found that among hospitalized patients treated with antibiotics, one third of antimicrobial days were unnecessary.18 A second study of general practitioners’ prescribing patterns for patients with lower respiratory tract infections found that one third of prescriptions were ‘definitely not indicated’.19 Both studies noted that the general over-prescription of antibiotics in human medicine has not significantly changed since the 1970s. One potential reason for over-prescriptions is perceived pressure from patients, and presumably many of these same influences exist in the veterinary-client relationship.20–23
The American College of Veterinary Internal Medicine Consensus Statement on Antimicrobial Drug Use notes that prophylactic administration of antibiotics is justified in some cases. In this study, prophylactic surgical use was excluded from analysis, but antibiotics prescribed for ‘preventative’ measures without documentation of infection were classified as ‘no documented evidence of infection’. The primary focus in preventing infection should be on using aseptic technique and minimizing the possibility of infection wherever possible.22
In agreement with previous studies, we found that amoxicillin-clavulanate was the most commonly prescribed antibiotic.24, 25 and in this series was the antibiotic prescribed most frequently when an infection was suspected, but not confirmed. Amoxicillin-clavulanate is a broad-spectrum inexpensive drug with few side effects, and its use as a first choice for wounds or other suspected infections without culture and sensitivity testing is considered inappropriate. Only 14% of the amoxicillin-clavulanate prescriptions examined here had a confirmed positive culture. Narrow spectrum antibiotics are generally recommended whenever possible to decrease development of microbial resistance, so perhaps an alternate choice of antibiotic would have been more prudent in some of these cases.15
Cefazolin/cephalexin was the second most frequently prescribed antibiotic, and like amoxicillin-clavulanate, was used in a high proportion of encounters without a culture (85.7%). The highest number of prescriptions was for ‘unknown target infection’, followed by wound or abscess. Although this could indicate overuse, first generation cephalosporins such as cefazolin and cephalexin are relatively narrow-spectrum antibiotics, which follows AVMA recommendations.
Enrofloxacin was the third most prescribed antibiotic, and of the top five most prescribed antibiotics was the antibiotic associated most frequently with a documented positive culture. Enrofloxacin is a wide-spectrum, bactericidal antibiotic used for treating mixed infections. The comparatively high percentage of cultures performed in this study indicates a higher level of documented appropriate use of enrofloxacin than other antibiotics. Minimizing the introduction and spread of enrofloxacin resistance is important, especially since enrofloxacin resistance has been linked to multi-drug resistance.5, 24, 26
Ampicillin/amoxicillin was the fourth most prescribed antibiotic and also had a high proportion of prescriptions lacking a documented positive culture (85.5%). The most common indications for ampicillin/amoxicillin use were for an “unknown target infection” or for pneumonia. Ampicillin and amoxicillin are relatively safe, inexpensive, narrow spectrum antibiotics, which makes them a relatively good choice for empirical use.
Doxycycline was the fifth most common antibiotic prescribed, primarily for treatment of presumed or confirmed tick-borne infections. Tick titers or SNAP tests were performed in 58.5% of these cases. Although a SNAP test identifies exposure rather than disease, we chose to consider a positive SNAP test as ‘confirmed’ infection because there is no easily available method to culture for these organisms. As a result the number of actual confirmed infections treated with doxycycline may be an overestimate. It is likely that is some cases tick titer panels or SNAP tests may not have been performed because the owner declined because of financial or other concerns.
When antibiotics were first introduced, there was intense study of their effectiveness with attention to the possibility of resistance development.22 Unfortunately, long-term surveillance on the development and spread of resistance within veterinary populations and between companion animal populations and humans has not yet been established. At present, the only wide-scale standardized surveillance system for companion animals is through the National Companion Animal Surveillance Program at Banfield Hospitals. This system was established initially to monitor zoonotic disease through a partnership with the Centers for Disease Control and Prevention (CDC), but also collects valuable information about disease and treatment patterns in dogs and cats seen at Banfield clinics.27 Although Banfield is a national veterinary medical provider, the data it collects is not widely available for public use. Additionally, the Banfield clientele and veterinarians may not be representative of other US veterinary hospitals. Many private practitioners are currently switching to electronic systems for data entry and/or billing services, which provides and excellent opportunity to encourage adoption and use of standardized medical encounter-based electronic record-keeping systems that can be integrated into population-based surveillance programs. It would greatly benefit veterinary medical practice and research to have such information available for improving the health of veterinary patients.
Inconsistent and poor record keeping is a pervasive problem in veterinary medicine as well as human medicine. An editorial published in JAVMA stated that although medical records are an essential component of successful practice, few practitioners emphasize good record keeping.28 Although veterinary medical practices are moving increasingly toward eflectronic record keeping, there are very few “standards, methodologies for integrating disparate systems…and system usability”.29 Similarly, studies in human medicine have documented variable and inconsistent record keeping, despite a shift toward electronic records.30 It is possible that some of the antibiotics in this study were prescribed for reasons not documented in the medical record. Although we examined all hard-copy data from referring hospitals, information on tests performed before referral may have been transmitted by phone or other method and thus not recorded in the medical record. It is also possible that cytology examinations or other tests performed by a clinician “in house” without formal submission to the outside, contracted laboratory were not recorded.
Our data are subject to several limitations. The study was conducted in dogs at a single tertiary care veterinary medical hospital in the northeast, and prescribing patterns may be different in other small animals, or at other facilities and/or regions of the country. We relied on chart documentation at our facility to determine whether an infection was definitely or probably present, and in at least some of the cases sampled, additional tests may have been done at other primary care facilities and not recorded in the records at the time of the encounter. Finally, antibiotics categorized as non-therapeutic were not included in the analysis, even though their use may contribute to the emergence of resistance by the same mechanisms as antibiotics used to treat infections.
Despite these limitations our findings strongly suggest that veterinary medical clinicians frequently prescribe antibiotics without corresponding documentation or indication of infection. This study also demonstrates the challenges in evaluating the appropriateness of antibiotic use in specific clinical cases and the need for standardized, data-driven definitions of appropriate use. Computerized records and national surveillance systems have the potential to lead to effective implementation of guidelines for appropriate antibiotic use as one part of a larger effort to limit the emergence of antibiotic resistance. Antibiotic resistance in human medicine is already a grave concern. Although instances of antibiotic resistance in veterinary medicine have been reported, the problem has not yet reported to be widespread and the veterinary field has the opportunity to stem widespread antibiotic resistance by setting reasonable and clinically applicable best practice guidelines for prescribing antibiotics.7–10
Acknowledgments
Supported in part by NIH Short-term Training Grant (T35 DK07635)
Appendix 1
Judicious Therapeutic Use of Antimicrobials in Cats and Dogs
Preventive strategies, such as appropriate husbandry and hygiene, routine health monitoring, and vaccinations should be emphasized. Routine preventative health care in cats and dogs includes the following:
Adhere to the American Association of Feline Practitioner guidelines for feline vaccinations, and American Animal Hospital Association guidelines for canine vaccinations.
Parasite control, nutritional counseling and dental health care.
Client education and involvement to successfully adopt good preventative health care programs.
Appropriate hygiene and husbandry is especially important in multiple pet households.
Therapeutic antimicrobial use should be confined to appropriate clinical indications.
The definitive diagnosis should be established whenever possible, and empirical use avoided.
Practitioners should strive to rule out those viral infections, parasitism, mycotoxicosis, nutritional imbalances, and other ailments that will not respond to antimicrobial therapies.
Antimicrobial therapy is not indicated in most viral upper respiratory (feline herpesvirus or calicivirus and canine influenza) infections not suspected to be complicated by secondary bacterial infection.
Most cases of pancreatitis in dogs and cats do not have bacterial involvement.
Most cases of feline lower urinary tract disease do not involve bacterial infection and in such cases antimicrobials are not indicated.
Therapeutic alternatives should be considered prior to antimicrobial therapy. This includes supportive care, such as correction of fluid and electrolyte abnormalities, maintaining acid-base balance, and ensuring adequate nutrition. Surgical intervention may be necessary in some cases. The use of antimicrobials to prevent infection can only be justified in cases where bacterial infection is likely to occur.
Culture and susceptibility results aid in the appropriate selection of antimicrobials.
In suspected urinary tract infection (UTI), urine collected by cystocentesis can help distinguish infection from contamination.
It is important to note that dilute urine is a risk factor for UTI, and infection may exist despite the lack of pyuria and bacteriuria on microscopic examination. Urine culture may be the only way to identify infection in such cases.
Ideally, minimum inhibitory concentrations (MIC) sensitivities should be done to identify the best choice of antimicrobials.
Gram stains can help determine appropriate antimicrobial choice while awaiting culture results.
Since certain antimicrobials are more effective against gram positive or gram negative organisms, interim antimicrobial decisions can be based on gram stain and the site of infection.
Use narrow spectrum antimicrobials whenever appropriate. It is best to choose an antimicrobial with a narrow spectrum that is effective against the organism.
Antimicrobials considered important in treating refractory infections in human or veterinary medicine should be used in animals only after careful review and reasonable justification.
Consider using other antimicrobials for initial therapy.
Drug side effects or interactions should be considered when choosing an appropriate antimicrobial.
Treat for the shortest effective period possible in order to minimize therapeutic exposure to antimicrobials.
Culture and sensitivity at the conclusion of therapy will determine if additional therapy is necessary.
Rechecking complete blood counts and urine analyses may also be indicated.
For specific conditions, refer to appropriate resources.
References
- 1.Gould IM. A review of the role of antibiotic policies in the control of antibiotic resistance. J Antimicrob Chemoth. 1999;43:459–465. doi: 10.1093/jac/43.4.459. [DOI] [PubMed] [Google Scholar]
- 2.Levy SB. Antibiotic resistance: An ecological imbalance. In: Chadwick DJ, Goode J, editors. Antibiotic Resistance: Origins, Evolution, Selection and Spread. West Sussex, England: Wiley, Chichester (Ciba Foundation Symposium 207); 1997. pp. 1–14. [DOI] [PubMed] [Google Scholar]
- 3.Singer RS. Antibiotic resistance – the interplay between antibiotic use in animals and human beings. The Lancet Infectious Diseases. 2003;3:47. doi: 10.1016/s1473-3099(03)00490-0. [DOI] [PubMed] [Google Scholar]
- 4.Benedict KM, Morley PS, Metre DC. Characteristics of biosecurity and infection control programs at veterinary teaching hospitals. J Am Vet Med Assoc. 2008;233(5):767–773. doi: 10.2460/javma.233.5.767. [DOI] [PubMed] [Google Scholar]
- 5.Warren A, et al. Multi-drug resistant Escherichia coli with extended spectrum β-lactamase activity and flouroquinolone resistance isolated from clinical infections in dogs. Aus Vet J. 2001;79(9):621–623. doi: 10.1111/j.1751-0813.2001.tb10783.x. [DOI] [PubMed] [Google Scholar]
- 6.Prescott JF, Hanna WJ, Reid-Smith R, Drost K. Antimicrobial drug use and resistance in dogs. Can Vet J. 2002;43:107–116. [PMC free article] [PubMed] [Google Scholar]
- 7.Faires MC, Tater KC, Weese JS. Methicillin-resistant Staphylococcus aureus colonization in people and pets. J of Am Vet Med Assoc. 2009;235(5):540–543. doi: 10.2460/javma.235.5.540. [DOI] [PubMed] [Google Scholar]
- 8.Loeffler A, et al. Prevalence of methicillin-resistant Staphylococcus aureus among staff and pets in a small animal referral hospital in the UK. J Antimicrob Chemoth. 2005;56:692–697. doi: 10.1093/jac/dki312. [DOI] [PubMed] [Google Scholar]
- 9.Duijkeren E, et al. Human-to-dog transmission of methicillin-resistant Staphylococcus aureus. Emerg Infec Dis. 2004;10(12):2235–2237. doi: 10.3201/eid1012.040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLean CL, Ness MG. Methicillin-resistant Staphylococcus aureus in a veterinary orthopedic referral hospital: staff nasal colonization and incidence of clinical cases. J Small Anim Pract. 2008;49:170–177. doi: 10.1111/j.1748-5827.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 11.Levy SB. The challenge of antibiotic resistance. Sci Am. 1998 Mar;:32–39. doi: 10.1038/scientificamerican0398-46. [DOI] [PubMed] [Google Scholar]
- 12.Guardabassi L, Schwarz S, Lloyd DH. Pet animals as reservoirs of antimicrobial-resistant bacteria. J Antimicrob Chemoth. 2005;54:321–332. doi: 10.1093/jac/dkh332. [DOI] [PubMed] [Google Scholar]
- 13.Rantala M, Holso K, Lillas A, et al. Survey of condition-based prescribing of antimicrobial drugs for dogs at a veterinary teaching hospital. Vet Rec. 2004:259–262. doi: 10.1136/vr.155.9.259. [DOI] [PubMed] [Google Scholar]
- 14.Weese J. Investigation of antimicrobial use and the impact of antimicrobial use guidelines in a small animal veterinary teaching hospital. J of Am Vet Med Assoc. 2006;228(4):553–558. doi: 10.2460/javma.228.4.553. [DOI] [PubMed] [Google Scholar]
- 15.American Veterinary Medical Association. Judicious Use of Antimicrobials. 2007 June [Google Scholar]
- 16.Wigton RS, Darr CA, Corbett KK, et al. How do community practitioners decide whether to prescribe antibiotics for acute respiratory tract infections. J Gen Intern Med. 2008;23(10):1615–1620. doi: 10.1007/s11606-008-0707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinman M, Landefeld S, Gonzales R. Predictors of broad-spectrum antibiotic prescribing for acute respiratory tract infections in adult primary care. JAMA. 2003;289:719–725. doi: 10.1001/jama.289.6.719. [DOI] [PubMed] [Google Scholar]
- 18.Hecker MT, Aron DC, Patel NP, et al. Unnecessary Use of Antimicrobials in Hospitalized Patients: Current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Intern Med. 2003;163:972–978. doi: 10.1001/archinte.163.8.972. [DOI] [PubMed] [Google Scholar]
- 19.Macfarlane J, Lewis SA, Macfarlane R, Holmes W. Contemporary use of antibiotics in 1089 adults presenting with acute lower respiratory tract illnesses in general practice in the U.K.: implications for developing management guidelines. Respir Med. 1997;91:427–434. doi: 10.1016/s0954-6111(97)90258-4. [DOI] [PubMed] [Google Scholar]
- 20.Macfarlane J, Holmes W, Macfarlane R, Britten N. Influence of patients’ expectations on antibiotic management of acute lower respiratory tract illness in general practice: a questionnaire study. BMJ. 1997;315:1211–1214. doi: 10.1136/bmj.315.7117.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott JG, Cohen D, Dicicco-Bloom B, et al. Antibiotic use in acute respiratory infections and the ways patients pressure physicians for a prescription. J Fam Pract. 2001;50(12):853–858. [PubMed] [Google Scholar]
- 22.Morley PS, Apley MD, Besser TE, et al. Antimicrobial Drug Use in Veterinary Medicine: ACVIM Consensus Statement. J Vet Intern Med. 2005;19:617–629. doi: 10.1892/0891-6640(2005)19[617:aduivm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Britten N, Ukoumunne O. The influence of patients’ hopes of receiving a prescription on doctors’ perceptions and decision to prescribe: a questionnaire survery. BMJ. 1997;315:1506–1510. doi: 10.1136/bmj.315.7121.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weese J. Investigation of antimicrobial use and the impact of antimicrobial use guidelines in a small animal veterinary teaching hospital. J of Am Vet Med Assoc. 2006;228(4):553–558. doi: 10.2460/javma.228.4.553. [DOI] [PubMed] [Google Scholar]
- 25.Black DM, Rankin SC, King LG. Antimicrobial therapy and aerobic bacteriologic culture patterns in canine intensive care unit patients: 74 dogs (January–June 2006) J Vet Emerg Crit Care. 2009;19(5):489–495. doi: 10.1111/j.1476-4431.2009.00463.x. [DOI] [PubMed] [Google Scholar]
- 26.Cooke CL, Singer RS, Jang SS, et al. Enrofloxacin resistance in Escherichia coli isolated from dogs with urinary tract infections. J of Am Vet Med Assoc. 2002;220(2):190–192. doi: 10.2460/javma.2002.220.190. [DOI] [PubMed] [Google Scholar]
- 27.Glickman LT, Moore GM, Glickman NW, et al. Purdue University-Banfield National Companion Animal Surveillance Program for Emerging and Zoonotic Diseases. Vector Borne Zoonotic Dis. 2006;6(1):14–23. doi: 10.1089/vbz.2006.6.14. [DOI] [PubMed] [Google Scholar]
- 28.McCurdy DM. The paperless practice. J of Am Vet Med Assoc. 2001;218(11):1776–1777. doi: 10.2460/javma.2001.218.1776. [DOI] [PubMed] [Google Scholar]
- 29.Smith-Akin KA, Bearden CF, Pittenger ST, et al. Toward a veterinary informatics research agenda: An analysis of the PudMed-indexed literature. Int J Med Inf. 2007;76:306–312. doi: 10.1016/j.ijmedinf.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Mann R, Williams J. Standards in medical record keeping. Clin Med. 2003;3:329–332. doi: 10.7861/clinmedicine.3-4-329. [DOI] [PMC free article] [PubMed] [Google Scholar]