Abstract
Aims
The aim of this study was to investigate the heritability as well as genetic and environmental correlations of left ventricular (LV) structural and functional traits in complex pedigrees of a Caucasian population.
Methods and results
We randomly recruited 459 white European subjects from 52 families (50% women; mean age 45 years). LV structure was measured by M-mode and 2D echocardiography and LV function was measured by conventional Doppler and tissue Doppler imaging (TDI). Other measurements included blood pressure, anthropometric, and biochemical measurements. We estimated the heritability of LV traits while adjusting for covariables, including sex, age, body height and weight, systolic and diastolic blood pressures, and heart rate. With full adjustment, heritability of LV mass was 0.23 (P= 0.025). The TDI-derived mitral annular velocities Ea and Aa showed moderate heritability (h2= 0.36 and 0.53, respectively), whereas the mitral inflow A peak had weak heritability (h2 = 0.25) and the E peak was not heritable (h2 = 0.11). We partitioned the total phenotypic correlation when it reached significance, into a genetic and an environmental component. The genetic correlations were 0.61 between the E and Ea peaks and 0.90 between the A and Aa peaks.
Conclusion
Our study demonstrated moderate heritability for LV mass as well as the mitral annular Ea and Aa peaks. We also found significant genetic correlations between the E and Ea peaks and between the A and Aa peaks. Our current findings support the ongoing research to map and detect genetic variants that contribute to the variation in LV mass and other LV structural and functional phenotypes.
Keywords: Echocardiography, Heritability, Left ventricular phenotypes, Population science
Introduction
Left ventricular (LV) mass and hypertrophy are well-established risk factors for cardiovascular morbidity and mortality.1–4 Phenotypic variation among individuals is due to genetic determinants, environmental factors, and lifestyle. Heritability refers to the phenotypic variation in a population that is attributable to the genetic variation among individuals. Heritability of LV mass estimated in studies of twins,5–9 hypertensive siblings,10–12 nuclear,13–15 and complex families16–20 ranged from 1517 to 84%.8
Tissue Doppler imaging (TDI) is an ultrasound technique that records the velocity of myocardium instead of blood flow. The TDI-derived velocities provide information on LV diastolic function over and beyond the Doppler indexes of transmitral blood flow. A few studies5,9,11,12 reported on the heritability of LV diastolic function as evaluated by transmitral blood flow. To our knowledge, no previous study reported on the heritability of the TDI-derived indexes of LV diastolic function or on the genetic and environmental correlations of these TDI indexes with other LV functional phenotypes. In the present study, we addressed these issues in complex pedigrees randomly recruited from a Caucasian population.
Methods
Study population
Recruitment for the Flemish Study on Environment, Genes, and Health Outcomes (FLEMENGHO) started in 1985. From August 1985 to November 1990, a random sample of the households living in a geographically defined area of Northern Belgium was investigated with the goal to recruit an equal number of participants in each of six subgroups by sex and age (20–39, 40–59, and ≥60 years). All household members with a minimum age of 20 years were invited to take part, provided that the quota of their sex-age group had not yet been fulfilled. From June 1996 to January 2004 recruitment of families continued using the former participants (1985–90) as index persons and also including siblings younger than 20 years. In all study phases, we employed the same standardized methods to measure phenotypes and administer questionnaires. The participation rate at enrolment was ∼70%. From May 2005 to June 2009, we re-invited 1184 of 2740 former participants21 for a follow-up examination including echocardiography. Of those, 18 had moved out of the study area, 26 had died, 27 were severely ill, 82 did not respond. Of the remaining 1031 former participants, 802 renewed their consent in writing and took part (77.8%). The Ethics Committee of the University of Leuven approved the study.
For the current analyses, we removed two parent–offspring relations based on Mendelian inconsistency in the ABO and Rhesus blood groups. Furthermore, we excluded subjects based on the following criteria: (i) age below 18 years (n= 4); (ii) clinically significant heart diseases, such as myocardial infarction or coronary revascularization (n= 26), valvular heart disease (n= 33), cardiac arrhythmias or pacemaker implantation (n= 12), cardiomyopathy (n= 1), or congenital heart disease (n= 1); (iii) and an echocardiogram of insufficient quality (n= 11) to obtain all of the structural and functional measurements. To avoid confounding by drugs influencing cardiovascular structure and function,20 we additionally excluded 160 patients on antihypertensive treatment. Finally, we removed 95 singletons without complete family information. Thus, the analysis of heritability of each trait under study included 459 subjects belonging to 52 complex families.
Echocardiography
The participants were refrained from smoking, heavy exercise, or drinking alcohol for at least 3 h before echocardiography. One experienced observer (T.K.)22 performed all echocardiograms according to the recommendations of the American Society of Echocardiography,23 using a Vivid 7 Pro (GE Vingmed, Horten, Norway) interfaced with a 2.5 MHz phased-array probe. All recordings were digitally stored for off-line analyses by the EchoPac software package, version 4.0.4 (GE Vingmed).
M-mode echocardiograms of the left ventricle were recorded from the parasternal long-axis view guided by two-dimensional (2D) image. We measured left ventricular internal diameter (LVID), interventricular septum (IVS), and posterior wall thickness (PWT) on this M-mode tracings, as described in the American Society of Echocardiography guidelines.23 When optimal orientation of the M-mode ultrasound beam could not be obtained, we performed linear measurements on correctly oriented two-dimensional images. We determined left atrial (LA) diameter and aortic root (AO) diameter on M-mode images. All the measurements of cavities and wall thickness were made by the leading-edge method24 and averaged over three cardiac cycles. The ejection fraction (EF) was calculated by using the Teichholz's formula25 from the M-mode measurements of LV end-diastolic and end-systolic diameter. We computed LV mass in grams as 0.8 × (1.04[(LVID + IVS + PWT)3 − LVID3]) + 0.6.23 The intra-observer intra-session variability of LV mass was 4.3%.22
From the apical window, the sonographer placed the Doppler sample volume between the tips of the mitral valve to obtain pulsed-wave Doppler images of mitral blood flow. From the transmitral flow signal, she measured peak early diastolic velocity (E) and peak late diastolic velocity (A) and calculated the E/A ratio. The observer acquired TDI images of the mitral annulus by recording the low-velocity, high-intensity myocardial signals at a frame rate of >190 per second, while adjusting the imaging angle to ensure parallel alignment of the beam. At the apical window, the observer placed a 5 mm Doppler sample at the sites of mitral annulus to acquire the myocardial signals. From these TDI recordings, we determined peak early (Ea) and peak late (Aa) diastolic mitral annular velocities, and calculated the Ea/Aa ratio. For the analysis, we averaged Ea and Aa measured from septal, lateral, posterior, and inferior annular sites.26
Other measurements
Trained nurses measured body height to the nearest 0.5 cm with a pliable measurer and the participant standing against the wall. Participants wore light indoor clothing without shoes for body weight measurement. Body mass index was weight in kilograms divided by the square of height in meters. After the subjects had rested for 5 min in the sitting position, the nurses obtained five consecutive blood pressure readings (phase V diastolic pressure) to the nearest 2 mmHg, using a standard mercury sphygmomanometer. Standard cuffs had a 12 × 24 cm inflatable portion, but if upper arm girth exceeded 31 cm, larger cuffs with 15 × 35 cm bladders were used. For the analysis, the five blood pressure readings were averaged. The nurses administered a standardized questionnaire to collect information on the participants’ medical history, smoking and drinking habits, and intake of medications. From the type and quantity of the alcoholic beverages used, we computed alcohol consumption in grams per day. We defined regular drinking as an alcohol consumption of at least 5 g per day.
The participants collected a 24 h urine sample in a wide-neck plastic container. Sodium and potassium in urine were determined by flame photometry, creatinine by an automated enzymatic method, and aldosterone concentration by radioimmunoassay.
Statistical methods
For database management and statistical analyses, we used SAS software (SAS Institute, Cary, NC, USA), version 9.1.3. Continuous data are presented as mean ± standard deviation and categorical data as frequencies and percentages. We searched for possible covariables of LV traits by a stepwise regression procedure with P-values for independent variables to enter and to stay in the models set at 0.15. The variables considered for entry into the models were sex, age, body height and weight, systolic and diastolic blood pressure, heart rate, smoking, intake of alcohol, and physical activity. We used multiple (R2) and partial (r2) coefficients of determination to assess the goodness of fit of regression models and the contribution of covariables to the variance of phenotypic traits. We ran collinearity diagnostics, using the COLLIN option as implemented in the PROC REG procedure in the SAS software.
To estimate heritability and to calculate the genetic and environmental correlations, we used S.A.G.E. (2009) Statistical Analysis for Genetic Epidemiology software package, release 6.0.1: http://darwin.cwru.edu/. The maximum likelihood method as implemented in the ASSOC procedure of S.A.G.E. was applied. We estimated heritability by assuming multivariate normality after a simultaneously estimated power transformation. ASSOC uses a multiple linear regression model, in which the residual variance is partitioned into the sum of an additive polygenic component, a sibling component and an individual-specific random component. Heritability (h2) was estimated as the polygenic component divided by the total residual variance (see Supplementary data online, Table S1).
We calculated the genetic and environmental correlations between LV structural and functional phenotypes with adjustments applied for covariables. Assuming no dominance variance and no interaction between the genetic and environmental variance components, the variance of a trait is given by: V = G + E, where G is the additive polygenic component and E is the environmental component. The total phenotypic correlation between two traits (ρp) can be partitioned into a genetic and an environmental component given by the equation:
where h12 and h22 represent the heritability of the two traits, and ρG and ρE are the genetic and environmental correlations, respectively. Significance of ρG and ρE suggests the influence of shared genes and shared environmental factors on two traits.27
Results
Characteristics of the participants
Our study sample (mean age ± SD, 44.8 ± 14.3 years; 49.9% women) included 459 subjects from 52 complex pedigrees with a size ranging from 3 to 151 individuals. The number of generations per pedigree amounted to 2 in 6 families, 3 in 16 families, 4 in 25 families, and 5 in 5 families. Our participants included 69 parents and 390 offspring. Table 1 lists the clinical and echocardiographic characteristics of the participants. In comparison with offspring, parents had higher (P= 0.012) systolic blood pressure and lower (P < 0.001) E/A and Ea/Aa ratios. Offspring more frequently reported regular alcohol intake than parents (44.9 vs. 30.4%; P = 0.025), while the proportions of smokers (25.9% vs. 26.1%; P = 0.97) were similar in both groups.
Table 1.
Characteristics of study subjects
| Clinical measurements |
Echocardiographic measurements |
||||
|---|---|---|---|---|---|
| Parents | Offspring | Parents | Offspring | ||
| Number | n= 69 | n= 390 | Number | n= 69 | n= 390 |
| Anthropometrics | M-mode/2D echocardiography | ||||
| Age (years) | 57.7 ± 10.1 | 42.6 ± 13.7 | LV mass (g) | 164.4 ± 40.1 | 162.2 ± 45.1 |
| Women, n (%) | 41 (59.4) | 188 (48.2) | LV internal diameter (mm) | 49.7 ± 4.6 | 50.7 ± 4.6 |
| Body height (cm) | 167.8 ± 9.6 | 170.6 ± 9.5 | Interventricular septum (mm) | 9.66 ± 1.50 | 9.31 ± 1.53 |
| Body weight (kg) | 73.4 ± 12.2 | 75.1 ± 15.0 | Posterior wall (mm) | 8.85 ± 1.32 | 8.43 ± 1.32 |
| Body mass index (kg/m2) | 26.0 ± 3.4 | 25.7 ± 4.4 | Left atrial diameter (mm) | 38.9 ± 5.2 | 38.3 ± 4.9 |
| Systolic blood pressure (mmHg) | 128.9 ± 17.7 | 123.2 ± 14.0 | Aortic root diameter (mm) | 30.3 ± 4.3 | 29.4 ± 4.3 |
| Diastolic blood pressure (mmHg) | 79.8 ± 7.6 | 78.3 ± 9.2 | Ejection fraction (%) | 69.6 ± 7.4 | 67.3 ± 6.4 |
| Heart rate (bpm) | 62.6 ± 8.8 | 60.8 ± 9.3 | Doppler echocardiography | ||
| Questionnaire data | E peak (cm/s) | 69.6 ± 13.6 | 79.3 ± 15.2 | ||
| Current smoking, n (%) | 18 (26.1) | 101 (25.9) | A peak (cm/s) | 68.2 ± 14.8 | 57.7 ± 15.2 |
| Drinking ≥5 g alcohol day, n (%) | 21 (30.4) | 175 (44.9) | E/A ratio | 1.06 ± 0.30 | 1.48 ± 0.50 |
| 24 h urinary measurements | Ea peaka (cm/s) | 10.6 ± 2.73 | 13.2 ± 3.47 | ||
| Sodium (mmol/24 h) | 165.9 ± 64.9 | 174.6 ± 77.1 | Aa peaka (cm/s) | 11.0 ± 1.80 | 9.50 ± 2.17 |
| Potassium (mmol/24 h) | 70.8 ± 24.2 | 75.0 ± 28.6 | Ea/Aa ratio | 1.00 ± 0.33 | 1.53 ± 0.69 |
| Aldosterone (nmol/24 h) | 22.6 ± 13.9 | 29.5 ± 19.6 | E/Ea ratio | 6.87 ± 1.88 | 6.28 ± 1.64 |
Values are mean±SD or number of subjects (%). LV indicates left ventricle. E and A indicate the peak velocities of the transmitral blood flow in early and late diastole. Ea and Aa refer to the peak velocities of the mitral annulus in early and late diastole.
aAverage of septal, lateral, posterior, and inferior mitral annular sites.
Determinants of LV structure and function
In stepwise regression, we searched for the independent correlates of the M-mode and 2D echocardiographic measurements (Supplementary data online, Table S2) and the Doppler measurements (Supplementary data online, Table S3). LV mass and LA diameter increased with age, body weight, and systolic blood pressure, but decreased with female sex and heart rate. All the covariables entering the regression model explained 58% of the total variance of LV mass, while body weight on its own explained 42% of the total variance of LV mass. The thickness of the IVS and the posterior wall also increased with age, body weight, and systolic blood pressure with lower values in women than men. LV internal diameter increased with weight and height and decreased with heart rate.
The E/A and Ea/Aa ratios (Supplementary data online, Table S2) decreased with age, because the associations were negative for the peak early diastolic velocities (E and Ea) and positive for the peak late diastolic velocities (A and Aa). The A and Aa peaks increased with weight and heart rate, so that the E/A and Ea/Aa ratios were independently associated with the same covariables, but in an opposite direction. Both the E/A and Ea/Aa ratios decreased with diastolic blood pressure. The E/Ea ratio increased with female sex, age, weight, and systolic blood pressure, and decreased with height and heart rate.
Smoking, alcohol intake, and physical activity did not enter any of the models presented in Supplementary data online, Tables S2 and S3. We did not detect a problem of collinearity in the models in Supplementary data online, Tables S2 and S3.
Heritability
We estimated heritability of LV structural and functional traits while adjusting for covariables (Table 2). Model 1 included only sex and age as covariables; model 2 was additionally adjusted for height and weight; model 3 also included systolic blood pressure and heart rate. LV diastolic functions were additionally adjusted for diastolic blood pressure28 in model 3. All LV traits showed significant heritability with the exception of the IVS, the mitral inflow E peak and the Ea/Aa ratio in model 1. Heritability ranged from 0.24 for the LA diameter to 0.63 for AO diameter. For comparison, the sex- and age-adjusted heritability was 0.81 for body height and 0.55 for body weight. In model 2 (Table 2), heritability was only borderline significant for the A peak and lost significance for LA diameter and the E/Ea ratio. With full adjustment (model 3; Table 2), heritability of LV mass was 0.23 (P= 0.025) in all subjects, whereas the heritability of LA diameter was not significant (P= 0.12). In model 3, both TDI-derived mitral annular velocities, Ea and Aa, showed moderate heritability (h2= 0.36 and 0.53, respectively), whereas the mitral inflow A peak had weak heritability (h2= 0.25) and the E peak was not heritable (h2= 0.11). Heritability was low for the E/A ratio, and did not reach significance for the Ea/Aa and E/Ea ratios in model 3 (Table 2).
Table 2.
Heritability of left ventricular structure and function
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| h2 ± se | P-value | h2 ± se | P-value | h2 ± se | P-value | |
| LV mass | 0.48 ± 0.11 | <0.001 | 0.25 ± 0.12 | 0.017 | 0.23 ± 0.11 | 0.025 |
| LV internal diameter | 0.62 ± 0.12 | <0.001 | 0.41 ± 0.12 | 0.003 | 0.40 ± 0.15 | 0.004 |
| Interventricular septum | 0.13 ± 0.09 | 0.074 | 0.09 ± 0.09 | 0.182 | 0.09 ± 0.09 | 0.175 |
| Posterior wall | 0.40 ± 0.10 | <0.001 | 0.33 ± 0.09 | <0.001 | 0.30 ± 0.10 | <0.001 |
| Left atrium diameter | 0.24 ± 0.14 | 0.036 | 0.16 ± 0.12 | 0.098 | 0.15 ± 0.13 | 0.120 |
| Aortic root diameter | 0.63 ± 0.10 | <0.001 | 0.50 ± 0.11 | <0.001 | 0.49 ± 0.11 | <0.001 |
| Ejection fraction | 0.47 ± 0.11 | <0.001 | 0.47 ± 0.11 | <0.001 | 0.48 ± 0.11 | <0.001 |
| E peak | 0.17 ± 0.12 | 0.087 | 0.16 ± 0.12 | 0.094 | 0.11 ± 0.12 | 0.170 |
| A peak | 0.35 ± 0.13 | 0.004 | 0.23 ± 0.14 | 0.056 | 0.25 ± 0.13 | 0.031 |
| E/A | 0.38 ± 0.11 | <0.001 | 0.31 ± 0.11 | 0.002 | 0.21 ± 0.10 | 0.016 |
| Ea peak | 0.47 ± 0.12 | <0.001 | 0.36 ± 0.13 | 0.002 | 0.36 ± 0.13 | 0.003 |
| Aa peak | 0.36 ± 0.14 | 0.004 | 0.37 ± 0.14 | 0.005 | 0.53 ± 0.13 | <0.001 |
| Ea/Aa | 0.16 ± 0.14 | 0.126 | 0.13 ± 0.12 | 0.140 | 0.18 ± 0.13 | 0.082 |
| E/Ea | 0.34 ± 0.11 | 0.016 | 0.15 ± 0.15 | 0.158 | 0.26 ± 0.16 | 0.057 |
LV, Left ventricular. E and A, the peak velocities of the transmitral blood flow in early and late diastole. Ea and Aa, the peak velocities of the mitral annulus in early and late diastole. Model 1 was adjusted for sex and age. Model 2 was adjusted for sex, age, body height and weight. Model 3 was adjusted for sex, age, body height and weight, systolic blood pressure, and heart rate. In model 3, measures of LV diastolic function were additionally adjusted for diastolic blood pressure.
Genetic and environmental correlation
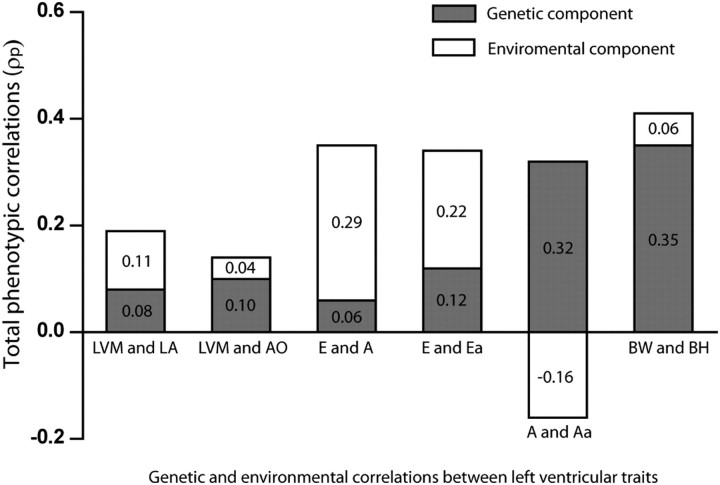
When the total phenotypic correlation (ρP) between two traits reached significance (P< 0.05), we partitioned it into a genetic and an environmental component (Figure 1) with the same adjustments applied as in model 3. We did not study the correlations of LV mass with LV internal diameter and wall thickness, because LV mass is calculated from the latter variables. The genetic (ρG) and environmental (ρE) correlations between different pairs of LV traits appear in Table 3. The genetic correlations were 0.61 for the E vs. Ea peak and 0.90 for the A vs. Aa peak. The corresponding environmental correlations were 0.30 and −0.27, respectively. The genetic correlations were 0.45 for LV mass vs. LA diameter and 0.29 for LV mass vs. AO diameter with environmental correlations of 0.14 (P= 0.003) and 0.07 (P= 0.14), respectively. For comparison, the total phenotypic correlation between body height and body weight was 0.41, and the corresponding genetic and environmental correlations were 0.53 and 0.18, respectively.
Figure 1.
Total phenotypic correlation between pairs of left ventricular traits partitioned into genetic and environmental components. The numbers in the lower and upper parts of the bars indicate the genetic and environmental components calculated as 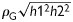 and
and 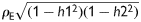 , respectively (see Methods).
, respectively (see Methods).
Table 3.
Genetic and environmental correlations between left ventricular traits
| Trait 1 | Trait 2 | Total phenotypic correlation (ρP) | Genetic correlation (ρG) | Enviromental correlation (ρE) |
|---|---|---|---|---|
| LV mass | Left atrial diameter | 0.19‡ | 0.45‡ | 0.14† |
| Aortic root diameter | 0.14† | 0.29‡ | 0.07 | |
| E peak | A peak | 0.35‡ | 0.34‡ | 0.37‡ |
| Ea peak | 0.34‡ | 0.61‡ | 0.30‡ | |
| A peak | Aa peak | 0.16† | 0.90‡ | −0.27‡ |
| Body weight | Body height | 0.41‡ | 0.53‡ | 0.18‡ |
LV, Left ventricular. E and A, the peak velocities of the transmitral blood flow in early and late diastole. Ea and Aa, the peak velocities of the mitral annulus in early and late diastole. All correlations between cardiac traits were adjusted for sex, age, body height and weight, systolic blood pressure, and heart rate. Correlations involving measures of LV diastolic function were additionally adjusted for diastolic blood pressure. The correlations between weight and height were adjusted for sex and age.
Significance of the correlation coefficients: †P< 0.01; ‡P< 0.001.
In a sensitivity analysis with additional adjustments applied for the 24 h urinary excretion of sodium and aldosterone,29 the genetic correlation was 0.39 (P < 0.001) for LV mass vs. LA diameter and 0.18 (P < 0.001) for LV mass vs. AO diameter. The corresponding environmental correlations were 0.16 (P < 0.001) and 0.11 (P= 0.02), respectively.
Discussion
We investigated the heritability as well as genetic and environmental correlations of LV structural and functional traits in complex pedigrees of a white European population. The multivariable-adjusted heritability of LV mass was 0.23. LV end-diastolic diameter and the AO diameter had a heritability of 0.40 and 0.49, respectively. Ea and Aa showed moderate heritability (h2 = 0.36 and 0.53, respectively), whereas the mitral inflow A peak had weak heritability (h2 = 0.25), and the E peak was not heritable (h2 = 0.11). The genetic correlation of LV mass with LA diameter and AO diameter were moderate, whereas the environmental correlations were weaker. The genetic correlations between the E and Ea peaks and between the A and Aa peaks were high.
In our current report, we identified correlates of LV mass, which were in line with the published literature and which explained 58% of the total variance of LV mass. Body weight was the main determinant of LV mass, explaining on its own 42% of the variance. In the Framingham Heart Study20 as well as in several other studies,7,9,14,18 body weight was the most important single predictor of the echocardiographically measured LV mass. Furthermore, in a study of 341 11-year-old twins,30 92% of the correlation between LV mass and body weight was due to the common genetic effects. Shared common genetic determinants are therefore likely to explain the strong correlation between LV mass and body weight. In our population, LV mass also increased with higher systolic blood pressure, but as in the Framingham Heart Study,20 explained only 1% of the variation in LV mass, perhaps because in both studies subjects on antihypertensive drug treatment were excluded from the analysis.
In our current study, heritability of LV mass was 0.23 in fully adjusted models. This result is comparable with heritability (ranged from 0.15 to 0.32) reported in most of the family studies,14,16,17,19 but lower than those in twin studies.5,8,9 Heritability is a population and situation-specific parameter. Certain population-specific characteristics may influence estimates of heritability obtained by variance component analysis, despite an identical underlying biological mechanism across populations. For example, a genetically homogeneous population will produce a lower estimate than a genetically heterogeneous population, while a population with a greater diversity of environmental factors will often produce a lower heritability than will one with a more homogeneous environment. Our study sample was recruited from a geographically defined area in northern Belgium. Thus, the genetic heterogeneity in our sample was probably lower than in some other studies. Moreover, heritability estimates in twins are usually larger than in population studies due to different estimation methods. Classic twin study estimated heritability by comparing the difference in correlation between monozygotes and dizygotes, while family studies used the intraclass correlation of all relatives within the population. In one study of 25 healthy twins,8 the heritability of LV mass was 0.84, but in this study high-precision imaging by magnetic resonance imaging (MRI) was used for the assessment of LV mass. As in other studies,16,17,20 estimates of the heritability of LV mass in our study also decreased with higher levels of adjustment.
Estimates of the heritability of the mitral inflow E and A peaks were smaller than those for the mitral annular Ea and Aa peaks. A few previous studies5,9,11 also investigated the heritability of mitral inflow E peak, A peak, and the E/A ratio. The mitral E-wave velocity primarily reflects the pressure gradient between the LV and LA during early diastole, which is affected by preload. The E/A ratio is influenced by LV relaxation and filling pressure and several other factors, including heart rate, the PR interval, and the diameter of the mitral annulus.31 In the presence of impaired LV relaxation, LV filling pressure has a minimal effect on TDI-derived mitral annular Ea. Therefore, the TDI-derived Ea wave is considered as a preload-independent index of LV relaxation, and the E/Ea ratio can be used to estimate LV filling pressure.32 According to our knowledge, our paper is the first to investigate the heritability of the TDI-derived Ea and Aa peaks. We found moderate heritability for both the Ea and Aa peaks.
In our study, the genetic correlations between the E and Ea peaks and between the A and Aa peaks were high, whereas the corresponding environmental correlations were weaker. This indicates common genes influencing these hemodynamic traits, which all carry information about LV diastolic function. LV mass also showed moderate genetic correlation with the LA and AO diameters with smaller environmental contributions. These findings justify the search for genes that might explain the genetic covariance between LV structural and functional phenotypes. In a previous study,29 we reported that LV mass independently increased with the 24 h urinary excretion of sodium and aldosterone. Increased mean wall thickness explained the association of LV mass with urinary sodium and increased LV end-diastolic diameter explained the association 24 h urinary aldosterone.29 In our current study, the genetic and environmental correlations of LV mass with LA and the AO diameter remained consistent when we additionally adjusted for these urinary variables.
Our study results have to be interpreted within the context of their potential limitations. First, our sample size was moderate compared with some of other population studies. As in the Framingham Heart Study,20 we excluded subjects on antihypertensive drug treatment. Hypertension clusters within families, but the use of antihypertensive drugs in our sample differed substantially among relatives. By eliminating 160 subjects on antihypertensive drug treatment and subsequently 96 singletons in the remaining treatment-free subjects, we might have diminished the power of our study to demonstrate heritability. However, in analyses including treated subjects (n= 594 from 59 complex families) and adjusted for the use of different classes of antihypertensive drugs, heritability estimates were generally consistent with those reported here and the heritability of LV mass was 0.32 (P= 0.002). Second, LV mass in our study was calculated from the echocardiographically measured LV end-diastolic diameter and wall thickness. This might have resulted in an underestimation of heritability, because of random measurement error. Cardiac MR provides a more precise estimate of LV mass, but this expensive technique cannot be easily deployed in large population studies. On the other hand, in our study, only one experienced observer performed all ultrasound examinations, which avoided variability due to multiple observers. Third, we did not investigate the heritability of LV mass indexed by body weight or body surface area. However, we adjusted our estimates for body height and weight. A previous study18 reported that estimates of the heritability of LV mass were not materially influenced by indexing or the type of indexing.
In summary, our study demonstrated moderate heritability for LV mass as well as the TDI-derived mitral annular Ea and Aa peak. We also found significant genetic correlations between the E and Ea peaks and between the A and Aa peaks. Until now, investigations on candidate genes, such as angiotensin-converting enzyme,33,34 angiotensinogen,35–37 and aldosterone synthase gene38 as well as genome-wide association study showed promising but still inconsistent results for LV mass, probably due to the complex interactions of genetic, environmental, and lifestyle factors on LV phenotypes. Our findings support the currently ongoing research to map and detect genetic variants that might contribute to the variation in LV mass and other LV structural and functional phenotypes.
Supplementary data
Supplementary data are available at European Journal of Echocardiography online.
Funding
Research included in the present study was partially funded by the European Union (grants IC15-CT98-0329-EPOGH, LSHM-CT-2006-037093 InGenious HyperCare, and HEALTH-2007-2.1.1-2 HyperGenes), the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Brussels, Belgium (grants G.0575.06 and G.0734.09), and the Katholieke Universiteit Leuven, Leuven, Belgium (grants OT/04/34 and OT/05/49). M.B. received grants from the Swiss National Science Foundation (PROSPER 3200BO-111361/2), and is supported by the Swiss School of Public Health Plus.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the expert assistance of Sandra Covens and Ya Zhu (Studies Coordinating Centre, Leuven). Some of the results of this paper were obtained by using the program package S.A.G.E., which is supported by a U.S. Public Health Service Resource Grant (RR03655) from the National Center for Research Resources.
Conflict of interest: None declared.
References
- 1.Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–52. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 2.Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension. 2000;35:580–6. doi: 10.1161/01.hyp.35.2.580. [DOI] [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 4.Ghali JK, Liao Y, Simmons B, Castaner A, Cao G, Cooper RS. The prognostic role of left ventricular hypertrophy in patients with or without coronary artery disease. Ann Intern Med. 1992;117:831–6. doi: 10.7326/0003-4819-117-10-831. [DOI] [PubMed] [Google Scholar]
- 5.Bielen E, Fagard R, Amery A. The inheritance of left ventricular structure and function assessed by imaging and Doppler echocardiography. Am Heart J. 1991;121:1743–9. doi: 10.1016/0002-8703(91)90021-9. [DOI] [PubMed] [Google Scholar]
- 6.Adams TD, Yanowitz FG, Fisher AG, Ridges JD, Nelson AG, Hagan AD, et al. Heritability of cardiac size: an echocardiographic and electocardiographic study of monozygotic and dizygotic twins. Circulation. 1985;71:39–44. doi: 10.1161/01.cir.71.1.39. [DOI] [PubMed] [Google Scholar]
- 7.Bielen E, Fagard R, Amery A. Inheritance of heart structure and physical exercise capacity: a study of left ventricular structure and exercise capacity in 7-year-old twins. Eur Heart J. 1990;11:7–16. doi: 10.1093/oxfordjournals.eurheartj.a059595. [DOI] [PubMed] [Google Scholar]
- 8.Busjahn CA, Schulz-Menger J, Abdel-Aty H, Rudolph A, Jordan J, Luft FC, et al. Heritability of left ventricular and papillary muscle heart size: a twin study with cardiac magnetic resonance imaging. Eur Heart J. 2009;30:1643–7. doi: 10.1093/eurheartj/ehp142. [DOI] [PubMed] [Google Scholar]
- 9.Swan L, Birnie DH, Padmanabhan S, Inglis G, Connel JMC, Hillis WS. The genetic determination of left ventricular mass in healthy adults. Eur Heart J. 2003;24:577. doi: 10.1016/s0195-668x(02)00524-9. [DOI] [PubMed] [Google Scholar]
- 10.Arnett DK, Hong Y, Bella JN, Oberman A, Kitzman DW, Hopkins PN, et al. Sibling correlation of left ventricular mass and geometry in hypertensive African Americans and Whites: the HyperGen Study. Am J Hypertens. 2001;14:1226–30. doi: 10.1016/s0895-7061(01)02200-2. [DOI] [PubMed] [Google Scholar]
- 11.Fox ER, Klos KL, Penman AD, Blair GJ, Blossom BD, Arnett D, et al. Heritability and genetic linkage of left ventricular mass, systolic and diastolic funciton in hypertensive African Americans (fro the GENOA study) Am J Hypertens. 2010;23:870–5. doi: 10.1038/ajh.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang W, Arnett DK, Devereux RB, Province MA, Atwood LD, Oberman A, et al. Sibling resemblance for left ventricular structure, contractility, and diastolic filling. Hypertension. 2002;40:233–8. doi: 10.1161/01.hyp.0000028487.62501.12. [DOI] [PubMed] [Google Scholar]
- 13.Assimes TL, Narasimhan B, Seto TB, Yoon S, Curb JD, Olshen RA, et al. Heritability of left ventricular mass in Japanese families living in Hawaii: the SAPPHIRe study. J Hypertens. 2007;25:985–92. doi: 10.1097/HJH.0b013e32809bd740. [DOI] [PubMed] [Google Scholar]
- 14.Garner C, Lecomte E, Visvikis S, Abergel E, Lathrop M, Soubrier F. Genetic and environmental influences on left ventricular mass: a family study. Hypertension. 2000;36:740–6. doi: 10.1161/01.hyp.36.5.740. [DOI] [PubMed] [Google Scholar]
- 15.Palatini P, Krause L, Amerena J, Nesbitt S, Majahalme S, Tikhonoff V, et al. Genetic contribution to the variance in left ventricular mass: the Tecumseh Offspring Study. J Hypertens. 2001;19:1217–22. doi: 10.1097/00004872-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Bella JN, MacCluer JW, Roman MJ, Almasy L, North KE, Best LG, et al. Heritability of left ventricular dimensions and mass in American Indians: the strong heart study. J Hypertens. 2004;22:281–6. doi: 10.1097/00004872-200402000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Chien KL, Hsu HC, Su TC, Chen MF, Lee YT. Heritability and major gene effects on left ventricular mass in the Chinese population: a family study. BMC Cardiovasc Disord. 2006;6:37. doi: 10.1186/1471-2261-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juo SH, Di Tullio MR, Lin H, Rundek T, Boden-Albala B, Homma S, et al. Heritability of left ventricular mass and other morphologic variables in Caribbean Hispanic subjects: the northern Manhattan family study. J Am Coll Cardiol. 2005;46:735–7. doi: 10.1016/j.jacc.2005.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayosi BM, Keavney B, Kardos A, Davies CH, Ratcliffe PJ, Farrall M, et al. Electrocardiographic measures of left ventricular hypertrophy show greater heritability than echocardiographic left ventricular mass. Eur Heart J. 2002;23:1963–71. doi: 10.1053/euhj.2002.3288. [DOI] [PubMed] [Google Scholar]
- 20.Post WS, Larson MG, Myers RH, Galderisi M, Levy D. Heritability of left ventricular mass: the Framingham Heart Study. Hypertension. 1997;30:1025–8. doi: 10.1161/01.hyp.30.5.1025. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Thijs L, Kuznetsova T, Zagato L, Struijker-Boudier H, Bianchi G, et al. Cardiovascular risk in relation to α-adducin Gly460Trp polymorphism and systolic pressure. A prospective population study. Hypertension. 2005;46:527–32. doi: 10.1161/01.HYP.0000174988.81829.72. [DOI] [PubMed] [Google Scholar]
- 22.Kuznetsova T, Manunta P, Casamassima N, Messaggio E, Jin Y, Thijs L, et al. Left ventricular geometry and endogenous ouabain in a Flemish population. J Hypertens. 2009;27:1884–91. doi: 10.1097/HJH.0b013e32832e49a8. [DOI] [PubMed] [Google Scholar]
- 23.Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. A report from the American Society of Echocardiography's Guidelines and Standard Committee and the Task Force on Echocardiography in Clinical Trials. J Am Soc Echocardiogr. 2004;17:1086–119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux RB, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American society of echocardiography committee on standards, subcommittee on quantitation of two-dimensional echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 25.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 26.Kuznetsova T, Herbots L, López B, Jin Y, Richart T, Thijs L, et al. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–12. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- 27.Freeman MS, Mansfield MW, Barrett JH, Grant PJ. Insulin resistance: an atherothrombotic syndrome. Thromb Haemost. 2003;89:161–8. [PubMed] [Google Scholar]
- 28.Kuznetsova T, Citterio L, Herbots L, Delli Carpini S, Thijs L, Casamassima N, et al. Effects of genetic variation in adducin on left ventricular diastolic function as assessed by tissue Doppler imaging in a Flemish population. J Hypertens. 2008;26:1229–36. doi: 10.1097/HJH.0b013e3282f97dcd. [DOI] [PubMed] [Google Scholar]
- 29.Jin Y, Kuznetsova T, Maillard M, Richart T, Thijs L, Bochud M, et al. Independent relations of left ventricular structure with the 24-h urinary excretion of sodium and aldosterone. Hypertension. 2009;54:489–95. doi: 10.1161/HYPERTENSIONAHA.109.130492. [DOI] [PubMed] [Google Scholar]
- 30.Verhaaren HA, Schieken RM, Mosteller M, Hewitt JK, Eaves LJ, Nance WE. Bivariate genetic analysis of left ventricular mass and weight in pubertal twins (The Medical College of Virginia Twin Study) Am J Cardiol. 1991;68:661–8. doi: 10.1016/0002-9149(91)90361-n. [DOI] [PubMed] [Google Scholar]
- 31.Nagueh SF, Appelton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 32.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 33.Kuznetsova T, Staessen JA, Wang JG, Gasowski J, Nikitin Y, Ryabikov A, et al. Antihypertensive treatment modulates the association between the D/I ACE gene polymorphism and left ventricular hypertrophy: a meta-analysis. J Hum Hypertens. 2000;14:447–54. doi: 10.1038/sj.jhh.1001055. [DOI] [PubMed] [Google Scholar]
- 34.Lindpaintner K, Lee M, Larson MC, Rao VS, Pfeffer MA, Ordovas JM, et al. Absence of association or genetic linkage between the angiotensin-converting-enzyme gene and left ventricular mass. N Engl J Med. 1996;334:1023–8. doi: 10.1056/NEJM199604183341604. [DOI] [PubMed] [Google Scholar]
- 35.Kuznetsova T, Staessen JA, Reineke T, Olszanecka A, Ryabikov A, Tikhonoff V, et al. Context-dependency of the relation between left ventricular mass and AGT gene variants. J Hum Hypertens. 2005;19:155–63. doi: 10.1038/sj.jhh.1001793. [DOI] [PubMed] [Google Scholar]
- 36.Sarzani R, Dessì-Fulgheri P, Mazzara D, Catalini R, Cola G, Bersigotti G, et al. Cardiovascular phenotype of young adults and angiotensinogen alleles. J Hypertens. 2001;19:2171–8. doi: 10.1097/00004872-200112000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen-Torvik LJ, North KE, Gu CC, Lewis CE, Wilk JB, Chakravarti A, et al. A population association study of angiotensinogen polymorphisms and haplotypes with left ventricular phenotypes. Hypertension. 2005;46:1294–9. doi: 10.1161/01.HYP.0000192653.17209.84. [DOI] [PubMed] [Google Scholar]
- 38.Sookoian S, Gianotti TF, Pirola CJ. Role of the C-344T aldosterone synthase gene variant in left ventricular mass and left ventricular structure-related phenotypes. Heart. 2008;94:903–10. doi: 10.1136/hrt.2007.119545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.