Abstract
Dietary flavonoids catechin, epicatechin, eriodictyol, and hesperetin were investigated as substrates and inhibitors of human sulfotransferases (hSULTs). Purified recombinant proteins and human intestine cytosol were used as enzyme sources.
hSULT1A1 and hSULT1A3 as well as human intestine cytosol can catalyse the sulfation of the investigated flavonoids.
Sulfation of catechin, epicatechin, eriodictyol, and hesperetin by recombinant hSULTs showed substrate inhibition at high flavonoid concentrations.
Hesperetin and eriodictyol are potent inhibitors of purified hSULT1A1, hSULT1A3, hSULT1E1, and hSULT2A1. Catechin and epicatechin inhibited hSULT1A1 and hSULT1A3, but not hSULT1E1 and hSULT2A1.
The sulfation efficacy and potency of inhibition is related to the C-ring structure of flavonoids. These results suggest that dietary flavonoids may regulate human SULT activity and, therefore, affect the regulation of hormones and neurotransmitters, detoxification of drugs, and the bioactivation of pro-carcinogens and pro-mutagens.
Keywords: Sulfotransferase, flavonoids, sulfation, inhibition, kinetics
Introduction
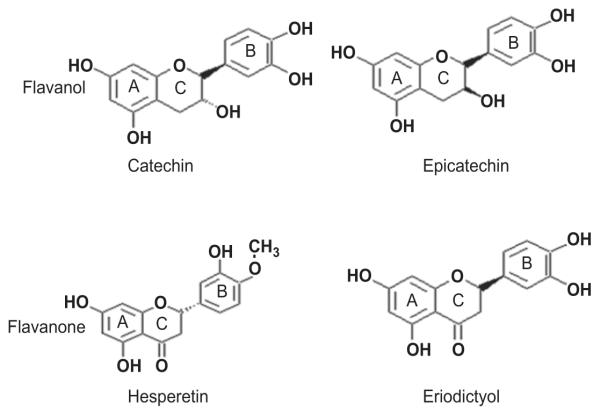
Flavonoids are widely distributed in plants, including vegetables, fruits, medicinal herbs, and in beverages such as tea, and wine (Harnly et al. 2006; Justesen et al. 1998; Khokhar & Magnusdottir 2002; Yang et al. 2006). Because of their variety and their relatively low toxicity compared with other active plant compounds, many animals, including humans, ingest large quantities of flavonoids in their diet. Flavonoids have been referred to as nature’s biological response modifiers because of strong experimental evidence of their inherent ability to modify the body’s reaction to allergens, viruses, and carcinogens. They show anti-allergic (Park et al. 2005), anti-inflammatory (Selmi et al. 2006), antimicrobial (Veluri et al. 2004), antioxidant (Torres & Bobet 2001; Cho 2006), and anti-cancer activity (Frydoonfar et al. 2003; Nair et al. 2004; Lin et al. 2006; Fink et al. 2007) as well as cardioprotective effects (Fisher & Hollenberg 2005; Schroeter et al. 2006). To date, more than 5000 naturally occurring flavonoids have been characterized from various plants. They have been classified according to their chemical structure. Six main subgroups of flavonoids exist: flavone, isoflavone, flavonol, flavanone, flavanol, and anthocyanin. Catechin, epicatechin, hesperetin, and eriodictyol (Figure 1) are naturally occurring flavonoids representing two different subgroups of flavonoids: flavanol and flavanone. The flavanols catechin and epicatechin are the most common flavonoids found in green tea, chocolate, red wine, grape seeds, hawthorn, motherwort, and other herbs (Torres & Bobet 2001; Mateus et al. 2002; Fisher et al. 2003). The flavanones hesperetin and eriodictyol are present in high amounts in citrus fruits such as orange, lemon, lime, and grapefruit (Justesen et al. 1998; Kim et al. 2001; Gardana et al. 2007). The flavanols commonly occur as aglycones whereas the flavanones occur almost exclusively as a selection of glycosides (Kanaze et al. 2007). Glycosides can be hydrolysed by microorganisms in the colon (Hollman & Katan 1999). Both aglycone and glycoside forms can be biologically active (Kren & Martinkova 2001). In recent years, flavanol-rich foods have been reported to exert cardiovascular health benefits (Heptinstall et al. 2006; Schroeter et al. 2006), inhibit angiotensin-converting enzyme activity, reduce blood pressure (Actis-Goretta et al. 2006), scavenge free radicals (Steffen et al. 2005; Zhu et al. 2005), and induce nitric oxide-dependent vasodilation in humans (Fisher et al. 2003). In addition, flavanols modulate platelet function (Pearson et al. 2002) and help to prevent oxidative damage in the blood (Terao 1999). Flavanols also show cancer prevention activity (Henning et al. 2003; Pezzato et al. 2004; Sehm et al. 2005). Citrus flavanone has a variety of biological and pharmacological activities including cholesterol-lowering activity (Borradaile et al. 1999), anti-influenza virus activity (Kim et al. 2001), antioxidant effects (Miyake et al. 2003), passive cutaneous anaphylaxis-inhibitory activity (Park et al. 2005), and protective effects against cardiovascular diseases and cancer (Silberberg et al. 2006).
Figure 1.
Chemical structures of flavonoids.
Cytosolic sulfotransferases (SULTs) are phase II drug-metabolizing enzymes that catalyse the sulfation of many hormones, neurotransmitters, drugs, and xenobiotic compounds. Human phenol-preferring sulfotransferase (hSULT1A1) catalyses the sulfate conjugation of phenolic xenobiotics (Falany et al. 1994). Human monoamine-preferring sulfotransferase (hSULT1A3) catalyses the sulfate conjugation of phenolic monoamines (neurotransmitters such as dopamine, norepinephrine, and serotonin) and phenolic drugs (Lu et al. 2005). Human oestrogen SULT (hSULT1E1) plays a major role in oestrogen sulfation (Her et al. 1995), and human hydroxysteroid SULT (hSULT2A1) is important for dehydroepiandrosterone and other hydroxysteroid regulation (Otterness et al. 1992). Flavonoids have been reported to serve as potential chemopreventive agents in sulfation-induced carcinogenesis (Ghazali & Waring 1999; Fink et al. 2007). Indeed, several flavonoids inhibit SULT activity. The flavones apigenin and chrysin (Eaton et al. 1996); the flavonols quercetin, myricetin, and kaempferol (Walle et al. 1995; Eaton et al. 1996; De Santi et al. 2002; Mesia-Vela & Kauffman 2003); and the isoflavones genistein and diadzein (Eaton et al. 1996; Mesia-Vela & Kauffman 2003) are potent inhibitors of hSULT1A1. The flavone baicalein is a potent inhibitor of hSULT1A3 (Harris et al. 2004). Furthermore, the isoflavones genistein and diadzein and the flavonol quercetin inhibit hSULT1E1 (Harris et al. 2004; Ohkimoto et al. 2004). Although many studies show that flavonoids inhibit SULTs, most previous studies to date have only examined certain flavonoid subgroups (flavone, isoflavone, and flavonol). Scarce information is available on the effects of the flavanone and flavanol subgroups. The flavanols catechin and epicatechin have been reported to inhibit hSULT1A1 present in human platelet cytosol as well as hSULT1A3 and hSULT1E1 in liver cytosol (Harris et al. 2004).
This paper investigated the sulfation of the flavanones hesperetin and eriodictyol and the flavanols catechin and epicatechin by human intestine cytosol SULTs and by recombinant hSULT1A1 and hSULT1A3. The inhibition of SULTs in human intestine cytosol as well as the inhibition of recombinant hSULT1A1, hSULT1A3, hSULT1E1, and hSULT2A1 by these four flavonoids was also studied.
Material and methods
Materials
[2,4,6,7-3H(N)]oestradiol ([3H]E2; 72 Ci mmol−1) and [1,2,6,7,-3H(N)]dehydroepiandrosterone ([3H]DHEA; 60 Ci mmol−1) were purchased from NEN (Boston, MA, USA). Hesperetin and (−)epicatechin were from MP Biochemicals, LLC (Irvine, CA, USA); eriodictyol was from Sigma Chemical Co. (St. Louis, MO, USA); and (+)catechin was from ALEXIS Biochemicals (San Diego, CA, USA). Dopamine, 3′-phosphoadenosine 5′-phosphosulfate (PAPS), 4-nitrophenylsulfate (pNPS), 2-napthol, and [14C]2-naphthol were purchased from Sigma Chemical Co. Human intestine cytosols were collected earlier at the University of Arkansas for Medical Sciences (Chen et al. 2003). Cytosols were prepared as previously described (Radominska-Pyrek et al. 1987; Radominska-Pandya et al. 1998).
Recombinant human SULTs
Recombinant human phenol-sulfating phenol sulfotransferase (hSULT1A1), monoamine-sulfating phenol sulfotransferase (hSULT1A3), dehydroepiandrosterone sulfotransferase (hSULT2A1), and oestrogen sulfotransferase (hSULT1E1) were expressed using the pMAL-c2 expression system (New England Biolabs) as previously described (Chen et al. 2000; Chen & Chen 2003). All human SULT proteins were expressed in Escherichia coli, and the recombinant proteins were purified on an amylase affinity column (New England Biolabs) (Chen et al. 2000; Chen & Chen 2003).
Sulfation of flavonoids by hSULT1A1, hSULT1A3, and human intestine cytosol
Sulfation of flavonoids by hSULT1A1, hSULT1A3, and human intestine cytosol was determined in a reaction mixture containing 50 mM sodium phosphate buffer (pH 6.2), 5 mM pNPS, 20 μM PAPS, and flavonoids ranging in concentration from 1 μM to 1000 μM. hSULT1A1 protein (3 μg), hSULT1A3 protein (3 μg), and human intestine cytosol (from a 59-year-old male) (50 μg) were used as enzyme sources in a total reaction volume of 250 μl of reaction mixture. After 30 min of incubation at 37°C in a water shaker bath, the reaction was stopped by adding 250 μl of 0.25 M Tris (pH 8.7). The reaction mixtures were read at 401 nm with a spectrophotometer. Under the experimental conditions, the product concentrations were linear to reaction time. Specific activity (SA) was expressed as nanomoles per minute per milligram of protein. All incubations were carried out in duplicate and were repeated at least twice.
Enzyme assays
Two different enzyme assay methods were used in the present investigation.
pNPS assay
hSULT1A3 activity was determined as described previously (Chen et al. 1999, 2000). Briefly, sulfation activity was determined in a reaction mixture containing 50 mM Tris buffer (pH 6.2), 5 mM pNPS, 20 μM PAPS, 0.05 mM dopamine, and flavonoids (dissolved in DMSO, final concentration 1% v/v). The concentration of flavonoids ranged from 0.01 μM to 1000 μM. Recombinant hSULT1A3 protein (7 μg) was used as the enzyme source in a total reaction volume of 250 μl of reaction mixture. After 30 min of incubation at 37°C in a water shaker bath, the reaction was stopped by adding 250 μl of 0.25 M Tris (pH 8.7). Reaction mixtures were read at 401 nm with a spectrophotometer. SA was expressed as nanomoles per minute per milligram of protein. The data shown in the figures represent averages of at least three determinations.
Radioactive assay
hSULT1A1 activity and 2-naphthtol sulfation by human intestinal cytosol were determined by a radioactive assay method described previously (Chen et al. 2002). Briefly, sulfation activity was determined in a reaction mixture containing 50 mM Tris buffer (pH 6.2); 20 μM PAPS; [14C]2-naphthol (4.7 mCi mmol−1; 0.1 mM final concentration), which was used as a substrate; and flavonoids (dissolved in DMSO, final concentration 1% v/v). The concentration of flavonoids ranged from 0.01 μM to 1000 μM. Recombinant hSULT1A1 protein (3 μg) and human intestine cytosol (from a 15-year-old female) (100 μg) were used as enzyme sources in a total reaction volume of 250 μl of reaction mixture. To determine hSULT2A1 activity and DHEA sulfation in human intestine cytosol, [3H ]DHEA (diluted to 0.4 Ci mmol−1; 2 μM final concentration) was used as a substrate. Recombinant hSULT2A1 protein (3 μg) and human intestine cytosol (from a 15-year-old male) (200 μg) were used as enzyme sources. For the assay of hSULT1E1 activity and oestradiol sulfation in human intestine cytosol, [3H]E2 (diluted to 1.0 Ci mmol−1; 0.15 μM final concentration) was used as a substrate. Recombinant hSULT1E1 protein (3 μg) and human intestine cytosol (from a 17-year-old male) (100 μg) were used as enzyme sources. After 30 min of incubation at 37°C in a water shaker bath, the reaction was stopped by adding 250 μl of 0.25 M Tris (pH 8.7). Extraction was performed twice by adding 0.5 ml of water-saturated chloroform each time. After the final extraction, 100 μl of aqueous phase was used for scintillation counting. The data shown in the figures represent averages of at least three determinations of each time-duplicated experiment.
Data analysis
Results are expressed as means ± standard deviation (SD). Kinetic parameters of flavonoid sulfation were estimated using SigmaPlot 2004 software (version 9.01; Systat Software, Inc., San Jose, CA, USA). Michaelis–Menten (equation 1) and substrate inhibition (equation 2) equations were used to fit the data to calculate Km, Vmax, and Ki; equation (3) was used to calculate IC50:
| (1) |
| (2) |
| (3) |
where V is the reaction rate; Vmax is the maximum velocity; Km is the Michaelis constant; S is the concentration of substrate; and Ki is the inhibition constant. The ratio Vmax/Km represents the enzymatic efficacy. Vi is the total activity; V0 is the remaining activity, and I is the concentration of flavonoids.
Results
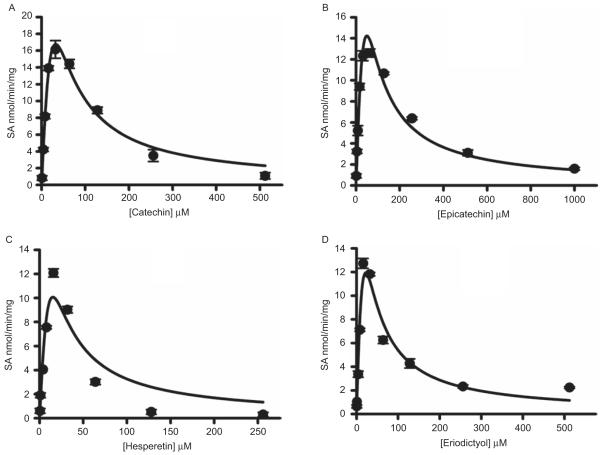
Sulfation of hesperetin, eriodictyol, (+)catechin, and (−)epicatechin by human intestine cytosol
Results for the sulfation of hesperetin, eriodictyol, (+) catechin, and (−)epicatechin by human intestine cytosol is shown in Figure 2. When the concentration of hesperetin exceeded 128 μM and that of eriodictyol exceeded 32 μM, sulfation gradually decreased, indicating substrate inhibition. The sulfation of (+)catechin and (−)epicatechin did not exhibit substrate inhibition within the concentration ranges used. When the apparent enzyme kinetic parameters were calculated using the Michaelis–Menten equation (equation (1); see the Materials and methods section), the Vmax, Km, and Vmax/Km values are shown in Table 1.
Figure 2.
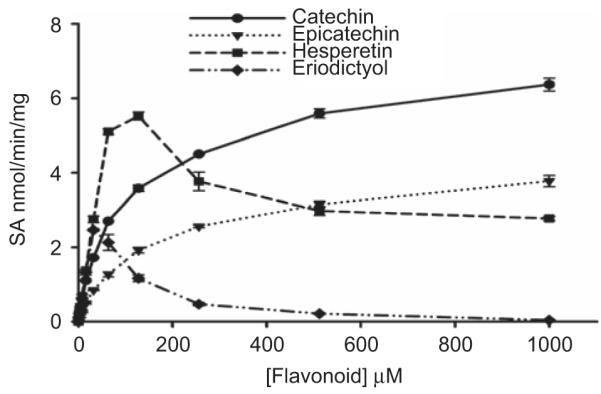
Catechin, epicatechin, hesperetin, and eriodictyol sulfation by human intestine cytosol. The pNPS assay was used to assess sulfation activity. Data shown are the means ± standard deviation (SD) of three determinations.
Table 1.
Kinetic parameters for flavonoid sulfation catalysed by sulfotransferases (SULTs) in human intestine cytosola.
| Flavonoid |
Vmax (nmol mg−1 min−1)b |
Km (μM)b |
Vmax/Km (ml mg−1 min−1)b |
|---|---|---|---|
| Catechin | 6.7 ± 0.02 | 104 ± 7.3 | 0.065 ± 0.002 |
| Epicatechin | 4.2 ± 0.2 | 149 ± 9.2 | 0.028 ± 0.001 |
| Hesperetin | 8.9 ± 0.3 | 66 ± 3.3 | 0.134 ± 0.004 |
| Eriodictyol | 19.4 ± 2.4 | 220 ± 28 | 0.088 ± 0.001 |
Notes: Data are means ± standard deviation (SD) of three determinations.
Kinetic parameters were estimated using the Michaelis–Menten equation (see equation (1) in the Materials and methods section) and the following concentrations of flavonoids: 1–1000 μM catechin; 1–1000 μM epicatechin; 1–128 μM hesperetin; and 1–32 μM eriodictyol.
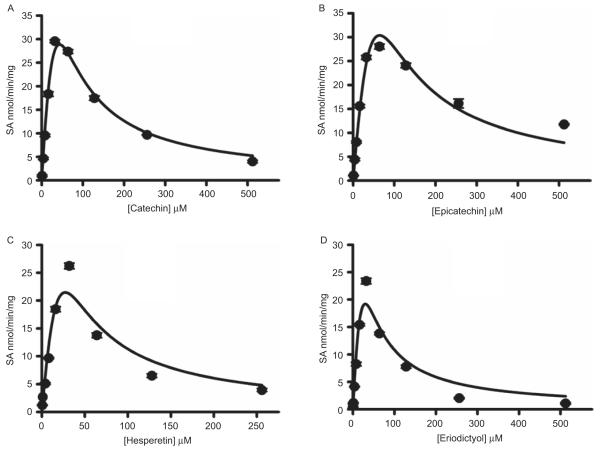
Kinetics of hesperetin, eriodictyol, (+)catechin, and (−)epicatechin sulfation by recombinant hSULT1A1 and hSULT1A3
Results for the sulfation of hesperetin, eriodictyol, (+)catechin, and (−)epicatechin by recombinant hSULT1A1 is shown in Figure 3. When catechin and epicatechin concentrations were greater than 32 μM or when hesperetin and eriodictyol concentrations were greater than 16 μM, the formation of flavonoid sulfate gradually decreased, indicating substrate inhibition. When the apparent enzyme kinetic parameters were estimated by fitting the data to the substrate inhibition equation (equation 2), the Vmax, Km, Vmax/Km, and Ki values are shown in Table 2.
Figure 3.
Kinetics of catechin (A), epicatechin (B), hesperetin (C), and eriodictyol (D) sulfation catalysed by recombinant human SULT1A1. The pNPS assay was used to assess sulfation activity. Data shown are the means ± standard deviation (SD) of three determinations. The solid line represents curve fitting to the substrate inhibition equation (2) (see the Materials and methods section).
Table 2.
Kinetic parameters for flavonoid sulfation catalysed by hSULT1A1.
| Flavonoid |
Vmax (nmol mg−1 min−1) |
Km (μM) |
Vmax/Km (ml mg−1 min−1) |
Ki (μM) |
|---|---|---|---|---|
| Catechin | 187 ± 9.9 | 165 ± 8.1 | 1.14 ± 0.01 | 6.37 ± 0.22 |
| Epicatechin | 188 ± 53 | 301 ± 89.0 | 0.62 ± 0.01 | 8.7 ± 3.07 |
| Hesperetin | 92.7 ± 2.3 | 63.0 ± 0.6 | 1.47 ± 0.02 | 3.7 ± 0.11 |
| Eriodictyol | 98.6 ± 2.5 | 80.8 ± 3.3 | 1.22 ± 0.03 | 6.1 ± 0.14 |
Notes: Data are the means ± standard deviation (SD) of three determinations.
Kinetic parameters were estimated using equation (2) (see the Materials and methods section).
As shown in Figure 4, the kinetics of flavonoid sulfation by hSULT1A3 is similar to hSULT1A1. When flavonoid concentrations were greater than 32 μM, the formation of flavonoid sulfate gradually decreased, indicating substrate inhibition. When the apparent enzyme kinetic parameters were estimated by fitting the data to the substrate inhibition equation, the Vmax, Km, Vmax/Km, and Ki values are shown in Table 3.
Figure 4.
Kinetics of catechin (A), epicatechin (B), hesperetin (C), and eriodictyol (D) sulfation catalysed by recombinant human SULT1A3. The pNPS assay was used to assess sulfation activity. Data shown are the means ± standard deviation (SD) of three determinations. The solid line represents curve fitting to the substrate inhibition equation (2) (see the Materials and methods section).
Table 3.
Kinetic parameters for flavonoid sulfation catalysed by hSULT1A3a.
| Flavonoid |
Vmax (nmol mg−1 min−1) |
Km (μM) |
Vmax/Km (ml mg−1 min−1) |
Ki (μM) |
|---|---|---|---|---|
| Catechin | 342 ± 8.2 | 236 ± 5.6 | 1.45 ± 0.02 | 8.0 ± 0.29 |
| Epicatechin | 442 ± 9.7 | 437 ± 10.1 | 1.01 ± 1.02 | 9.5 ± 0.21 |
| Hesperetin | 303 ± 5.8 | 179 ± 3.7 | 1.69 ± 0.02 | 4.2 ± 0.06 |
| Eriodictyol | 269 ± 5.2 | 193 ± 3.3 | 1.39 ± 0.03 | 4.6 ± 0.02 |
Notes: Data are the means ± standard deviation (SD) of three determinations.
Kinetic parameters were estimated using equation (2) (see the Materials and methods section).
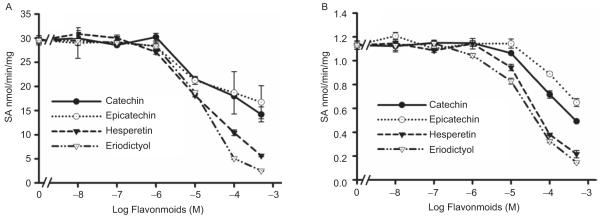
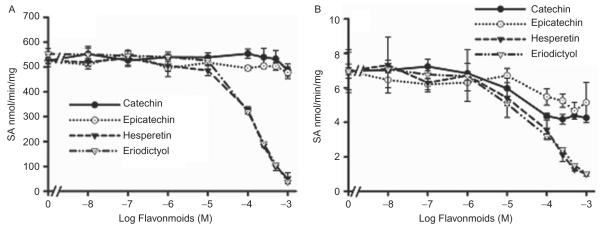
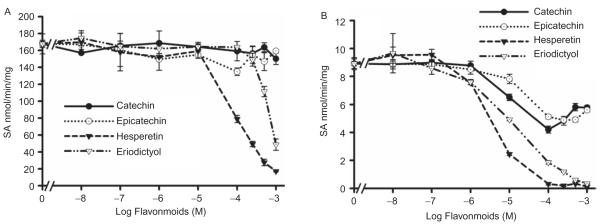
Effect of flavonoids on recombinant hSULT1A1, hSULT1A3, hSULT2A1, and hSULT1E1 enzymatic activities
All of the investigated flavonoids inhibited 2-naphthol sulfation by recombinant hSULT1A1 (Figure 5A) and dopamine sulfation by recombinant hSULT1A3 (Figure 6). The IC50 values are shown in Table 4. DHEA sulfation by recombinant hSULT2A1 was inhibited by hesperetin, eriodictyol (Figure 7A); the IC50 values are shown in Table 4. (+)Catechin and (−)epicatechin did not significantly inhibit hSULT2A1-catalysed DHEA sulfation. Hesperetin and eriodictyol inhibited recombinant hSULT1E1-catalysed oestradiol sulfation (Figure 8A and Table 4). Although (+)catechin and (−)epicatechin did not significantly inhibit hSULT1E1-catalysed oestradiol sulfation, they inhibited hSULT1A1-catalysed oestradiol sulfation (Table 4).
Figure 5.
Effect of catechin, epicatechin, hesperetin, and eriodictyol on 2-naphthol sulfation catalysed by recombinant hSULT1A1 (A) and human intestine cytosol (B). The substrate was 100 μM 2-naphthol. Results are means ± standard deviation (SD) of three determinations.
Figure 6.
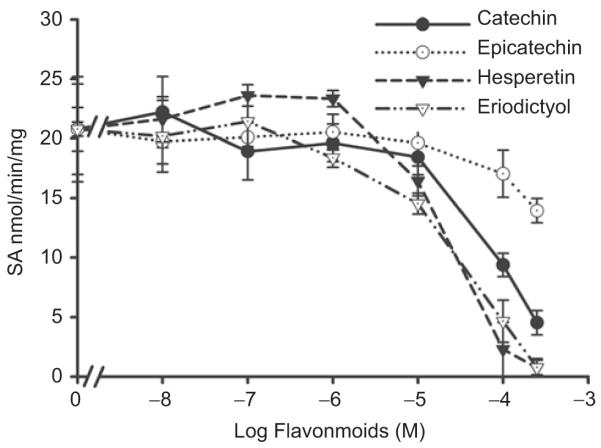
Effect of catechin, epicatechin, hesperetin, and eriodictyol on dopamine sulfation catalysed by recombinant hSULT1A3. The substrate was 50 μM dopamine. Results are means ± standard deviation (SD) of three determinations.
Table 4.
Inhibition constants (IC50) of dietary flavonoid inhibition of recombinant and human intestine cytosolic SULTs.
| Flavonoid | 2-Naphthol sulfation |
Dopamine sulfation by hSULT1A3 |
Oestradiol sulfation |
DHEA sulfation |
||||
|---|---|---|---|---|---|---|---|---|
| hSULT1A1 | Cytosol | hSULT1E1 | hSULT1A1 | Cytosol | hSULT2A1 | Cytosol | ||
| Flavanones | ||||||||
| Hesperetin | 23.4 ± 5.75 | 32.2 ± 3.6 | 20.8 ± 8.1 | 90.4 ± 11.5 | n.a. | 3.6 ± 0.4 | 152 ± 14.9 | 91.0 ± 9.5 |
| Eriodictyol | 15.2 ± 0.9 | 28.9 ± 4.1 | 14.7 ± 8.9 | 677 ± 120.3 | n.a. | 11.8 ± 2.0 | 139 ± 8.8 | 66.1 ± 20.2 |
| Flavonols | ||||||||
| Catechin | 8.4 ± 3.3 | 67.1 ± 6.3 | 82.5 ± 31.5 | n.a. | 2.6 ± 0.4 | 10.1 ± 1.7 | n.a. | 13.6 ± 2.6 |
| Epicatechin | 42.5 ± 8.5 | 157 ± 37.6 | 117 ± 12.9 | n.a. | 6.1 ± 1.0 | 22.7 ± 5.6 | n.a. | 88.4 ± 46.0 |
Notes: Substrates used were as follows: 100 μM 2-naphthol, 50 μM dopamine, 2 μM DHEA, and 0.15 μM oestradiol. IC50 (μM) was calculated using SigmaPlot 9.01. Results are means ± standard deviation (SD) of three determinations.
n.a., Not applicable.
Figure 7.
Effect of catechin, epicatechin, hesperetin, and eriodictyol on DHEA sulfation catalysed by recombinant hSULT2A1(A) and human intestine cytosol (B). The substrate was 2 μM DHEA. Results are the means ± standard deviation (SD) of three determinations.
Figure 8.
Effect of catechin, epicatechin, hesperetin, and eriodictyol on oestradiol sulfation catalysed by recombinant hSULT1E1 (A) and human intestine cytosol (B). The substrate was 0.15 μM oestradiol. Results are means ± standard deviation (SD) of three determinations.
Effect of flavonoids on sulfotransferase activities in human intestine cytosol
All of the investigated flavonoids inhibited 2-naphthol sulfation (SULT1A1 and SULT1A3) by human intestine cytosol (Figure 5B and Table 4). DHEA sulfation by human intestine cytosol was inhibited by catechin, epicatechin, hesperetin, and eriodictyol (Figure 7B and Table 4). Oestradiol sulfation by human intestine cytosol was inhibited by catechin, epicatechin, hesperetin, and eriodictyol (Figure 8B and Table 4).
Discussion
The flavanols catechin and epicatechin are the quantitatively major found flavonoids in tea, red wine, grape seeds, and herbs (Torres & Bobet 2001; Khokhar & Magnusdottir 2002; Fisher et al. 2003; Henning et al. 2003). The flavanones hesperetin and eriodictyol are present in high amounts in citrus fruits such as orange, lemon, lime, and grapefruit (Kim et al. 2001; Scholz et al. 2006). These flavonoids have been reported potentially to prevent certain diseases such as coronary heart disease, stroke, and cancer (Fisher et al. 2003; Fink et al. 2007).
Sulfate conjugation by cytosolic sulfotransferases (SULTs) is an important biotransformation reaction in the metabolism of drugs, hormones, neurotransmitters, and xenobiotic compounds (Aust et al. 2005a, 2005b). hSULT1A1 is a simple phenol-preferring SULT, and hSULT1A3 is a monoamine-preferring SULT (Falany et al. 1994; Lu et al. 2005). hSULT1E1 plays a major role in oestrogen sulfation, and hSULT2A1 is important for dehydroepiandrosterone regulation (Otterness et al. 1992; Her et al. 1995). Sulfation of flavonoids by human cytosolic SULTs has been described in previous studies (Vaidyanathan & Walle 2002). Dietary flavonoids have been suggested to affect the bioavailability of endogenous hormones by competing as substrates and/or inhibitors of human SULTs (Walle et al. 1995; Vietri et al. 2002; O’Leary et al. 2003; Waring et al. 2008). Furthermore, sulfation is also an important step in the bioactivation of pro-mutagens and pro-carcinogens (Arlt et al. 2002; Meinl et al. 2002; Aust et al. 2005a).
In the current study, we characterized the sulfation of catechin, epicatechin, hesperetin, and eriodictyol by recombinant hSULT1A1 and hSULT1A3 as well as by human intestine cytosol SULTs. All of the four flavonoids investigated can act as substrates. The purified monoamine-preferring sulfotransferase hSULT1A3 exhibited significantly higher Vmax (nmol mg−1 min−1) estimates for all the flavonoids investigated compared with the purified phenol-preferring sulfotransferase hSULT1A1 (catechin: 342 versus 187; epicatechin: 442 versus 188; hesperetin: 303 versus 92.7; and eriodictyol: 269 versus 98.6) (Tables 2 and 3). The sulfation of all four flavonoids by hSULT1A1 and hSULT1A3 showed substrate inhibition kinetics with flavonoid concentrations greater than 32 μM (Figures 3 and 4). Comparison of the flavanols catechin and epicatechin with the flavanones hesperetin and eriodictyol revealed that the Ki values of catechin and epicatechin sulfation by both hSULT1A1 and hSULT1A3 were greater than those of hesperetin and eriodictyol (Tables 2 and 3). With human intestine cytosol, sulfation of hesperetin and eriodictyol exhibited substrate inhibition kinetics with hesperetin and eriodictyol concentrations greater than 128 and 32 μM, respectively. However, sulfation of catechin and epicatechin failed to show substrate inhibition at 1–1000 μM concentrations (Figure 2).
The Vmax/Km ratio represents catalytic efficacy of an enzyme. The estimated Vmax/Km values of hesperetin and eriodictyol sulfation by human intestine cytosol SULTs were two to six times greater than those of catechin and epicatechin (Table 1). In addition, the estimated Vmax values of hesperetin and eriodictyol sulfation by recombinant hSULT1A3 were greater than those of catechin and epicatechin (Table 3), whereas the estimated Vmax values of hesperetin and eriodictyol sulfation by recombinant hSULT1A1 were less than those of catechin and epicatechin (Table 2). Flavanones (Figure 1) have a keto group at position 4 of the C ring, but do not have a hydroxyl group at position 3 of the C ring. By contrast, flavanols have a hydroxyl group at position 3 of the C ring, but do not have a keto group at position 4 of the C ring. The results of the kinetics analyses above suggest that the sulfation efficiency of flavonoids is related to the C-ring structure.
Many studies have reported that flavonoids inhibit SULTs (Gibb et al. 1987; Walle et al. 1995; Eaton et al. 1996; Marchetti et al. 2001; De Santi et al. 2002; Mesia-Vela & Kauffman 2003; Pacifici 2004), and previous studies to date have only examined the flavonoid subgroups flavone, isoflavone, and flavonol. Available information on the subgroups flavanone and flavanol is still scarce. In the present study, we characterized the potential inhibitory action of the flavanols catechin and epicatechin as well as the flavanones hesperetin and eriodictyol on SULTs. All four flavonoids inhibited the sulfation of 2-naphthol (Figure 5B), oestradiol (Figure 8B), and DHEA (Figure 7B) by SULTs present in human intestine cytosol with IC50 values ranging from 3.6 to 157 μM (Table 4). Furthermore, recombinant hSULT1A1, hSULT1A3, hSULT2A1, and hSULT1E1 activities were inhibited by the flavanones hesperetin and eriodictyol (Figures 5A, 6, 7A, and 8A). However, the flavanols catechin and epicatechin inhibited hSULT1A1 and hSULT1A3 activities (Figures 5A and 6), but not hSULT2A1 and hSULT1E1 activities (Figures 7A and 8A). In addition, the hesperetin- and eriodictyol-mediated inhibition of dopamine sulfation by hSULT1A3 as well as 2-naphthol sulfation by hSULT1A1 had lower IC50 values than the catechin- and epicatechin-mediated inhibition of dopamine sulfation by hSULT1A3 and 2-naphthol sulfation by hSULT1A1 (Table 4). These results suggest that the flavanones hesperetin and eriodictyol are more potent inhibitors of human SULTs than the flavanols catechin and epicatechin. Interestingly, the estimated Ki values of the hesperetin and eriodictyol sulfation by SULTs were lower than those of catechin and epicatechin sulfation, and the sulfation efficacy of hesperetin and eriodictyol were higher than that of catechin and epicatechin. These results suggest that inhibition potency of flavonoids is linked to sulfation efficacy, such that flavonoids with high sulfation efficacy are more potential inhibitors.
The sulfation of DHEA and oestradiol by human intestine cytosol SULTs was inhibited by catechin and epicatechin at 1–100 μM concentrations (Figures 7B and 8B). When the concentrations of catechin and epicatechin were greater than 100 μM, oestradiol and DHEA sulfation did not decrease further. The results show that oestradiol sulfation by recombinant hSULT1E1 and DHEA sulfation by recombinant hSULT2A1 were not inhibited by catechin and epicatechin in the concentration range of 1–1000 μM (Figures 7A and 8A). The reason for this is likely related to the presence of other SULT isoforms that are capable of catalysing oestradiol and DHEA sulfation. We hypothesize that the reason why oestradiol and DHEA sulfation did not decrease further at catechin and epicatechin concentrations greater than 100 μM is because of the presence of hSULT1E1 and hSULT2A1 in human intestine cytosol. These two cytosolic human SULTs may contribute to oestradiol and DHEA sulfation but are not inhibited by catechin and epicatechin at higher substrate concentrations. To confirm this hypothesis, we investigated the inhibition potency of catechin and epicatechin on oestradiol sulfation by recombinant hSULT1A1. The results indicate that catechin and epicatechin potently inhibit oestradiol sulfation by hSULT1A1 but not by hSULT1E1 (Table 4).
In conclusion, catechin, epicatechin, hesperetin, and eriodictyol can act as substrates of recombinant hSULT1A1 and hSULT1A3 as well as substrates of human intestine cytosol SULTs. hSULT1A3 displayed greater sulfation activity than hSULT1A1. The sulfation efficacy of the flavanones hesperetin and eriodictyol are greater than that of the flavanols catechin and epicatechin. Hesperetin and eriodictyol are potent inhibitors of human intestine cytosol SULTs as well as of recombinant hSULT1A1, hSULT1A3, hSULT1E1, and hSULT2A1. Catechin and epicatechin are potent inhibitors of human intestine cytosol SULTs but not of recombinant hSULT1E1 and hSULT2A1. Flavonoids with high sulfation efficacy are more potent inhibitors. The sulfation efficacy and potency of inhibition is related to the flavonoids’ C-ring structure.
SULT1A1, SULT1A3, SULT2A1, and SULT1E1 are known to be highly expressed in human intestinal tract (Chen et al. 2003). The gastrointestinal tract is a major target tissue for dietary mutagens and carcinogens. A large number of dietary pro-mutagens and pro-carcinogens are known to be bioactivated by SULTs, and the sulfation of those xenobiotics can result in the pathogenesis of gastrointestinal tumors. Examples of chemicals involved in sulfation for generation of DNA- and protein-adducting species include estragole, safrole, benzylic alcohols of polycyclic aromatic hydrocarbons, hydroxyarylamines and arylhydroxamic acids (Meerman & Van de Poll 1994; Miller 1994; Chou et al. 1995; Wakazono et al. 1998). All the investigated flavonoids in this study can act as inhibitors of SULTs present in human intestine. This suggests that these flavonoids may act as natural chemopreventive agents. These flavonoids may also regulate the biological activities of hydroxysteroids. Various epidemiological studies have reported a relatively lower incidence of cardiovascular disease in premenopausal women than in menopause women, and oestrogen has been proposed as cardioprotective agent, especially in women (Stampfer et al. 1991). SULT1E1 and SULT1A1 play an important role in regulating the biological activity of oestrogen. The inhibition of SULT1E1 and SULT1A1 by flavonoids may raise the oestrogen biological activity in cardiovascular tissue, which suggests that flavonoids may play a preventive role for cardiovascular disease.
Flavonoids are rich in various diets. Humans consume considerable amount of flavonoids daily. Human faecal water content of various flavonoids can reach up to millimolar range with very high individual variations (Jenner et al. 2005). The concentrations of flavonoids used in this investigation are all below 1 mM.
Flavonoids are polyphenols. Therefore, they are mainly metabolized by phase II drug-metabolizing enzymes. As describe above, sulfation is one of the major metabolic pathways for flavonoids. The other major conjugation reaction is glucuronidation (Terao 1999; Vaidyanathan & Walle 2002) catalysed by UDP-glucuronosyltransferases (UGTs). The major functional groups for UGTs are carboxyl group and hydroxyl group, while the major functional group for SULTs is hydroxyl group. SULTs are unable to sulfate a carboxyl group. Both UGTs and SULTs have broad substrate specificity, and they share almost all the xenobiotic hydroxyl substrates. SULTs usually have high affinity to substrates (very low Km), while UGTs have high capacity (high Vmax and high Km). The relative contributions of glucuronidation and sulfation to flavonoids metabolism will depend on the in vivo flavonoid concentrations. At lower concentrations, sulfation should dominate, while at higher concentrations, glucuronidation will be the major conjugation reaction.
Flavonoids are important bioactive food components. They contribute to human health. SULTs are important drug metabolizing enzymes. They also regulate the biological activities of various hormones and biological signalling molecules. It is significant to understand how flavonoids are metabolized by SULTs and how flavonoids affect the biological activities of human SULTs. These results may contribute to the understanding of the biological roles of flavonoids and their potential roles for human health.
Acknowledgements
Declaration of interest: This work was supported in part by the NIH (Grant Number GM078606) to G. Chen; the American Cancer Society (Grant Number RSG-07-028-01-CNE to G. Chen; the US Department of Agriculture (USDA) (Grant Number 2006-35200-17137 to G. Chen; and the Oklahoma Center for the Advancement of Science and Technology (OCAST) (Grant Number HR05-015 to G. Chen).
References
- Actis-Goretta L, Ottaviani JI, Fraga CG. Inhibition of angiotensin converting enzyme activity by flavanol-rich foods. J Agric Food Chem. 2006;54(1):229–234. doi: 10.1021/jf052263o. [DOI] [PubMed] [Google Scholar]
- Arlt VM, Glatt H, Muckel E, Pabel U, Sorg BL, Schmeiser HH, Phillips DH. Metabolic activation of the environmental contaminant 3-nitrobenzanthrone by human acetyltransferases and sulfotransferase. Carcinogenesis. 2002;23(11):1937–1945. doi: 10.1093/carcin/23.11.1937. [DOI] [PubMed] [Google Scholar]
- Aust S, Jaeger W, Klimpfinger M, Mayer K, Baravalle G, Ekmekcioglu C, Thalhammer T. Biotransformation of melatonin in human breast cancer cell lines: role of sulfotransferase 1A1. J Pineal Res. 2005a;39(3):276–282. doi: 10.1111/j.1600-079X.2005.00246.x. [DOI] [PubMed] [Google Scholar]
- Aust S, Obrist P, Klimpfinger M, Tucek G, Jager W, Thalhammer T. Altered expression of the hormone- and xenobiotic-metabolizing sulfotransferase enzymes 1A2 and 1C1 in malignant breast tissue. Int J Oncol. 2005b;26(4):1079–1085. [PubMed] [Google Scholar]
- Borradaile NM, Carroll KK, Kurowska EM. Regulation of HepG2 cell apolipoprotein B metabolism by the citrus flavanones hesperetin and naringenin. Lipids. 1999;34(6):591–598. doi: 10.1007/s11745-999-0403-7. [DOI] [PubMed] [Google Scholar]
- Chen CL, Yu G, Venkatachalam TK, Uckun FM. Metabolism of stavudine-5′-[p-bromophenyl methoxyalaninyl phosphate], stampidine, in mice, dogs, and cats. Drug Metab Dispos. 2002;30(12):1523–1531. doi: 10.1124/dmd.30.12.1523. [DOI] [PubMed] [Google Scholar]
- Chen G, Battaglia E, Senay C, Falany CN, Radominska-Pandya A. Photoaffinity labeling probe for the substrate binding site of human phenol sulfotransferase (SULT1A1): 7-azido-4-methylcoumarin. Protein Sci. 1999;8(10):2151–2157. doi: 10.1110/ps.8.10.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Chen X. Arginine residues in the active site of human phenol sulfotransferase (SULT1A1) J Biol Chem. 2003;278(38):36358–36364. doi: 10.1074/jbc.M306045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Rabjohn PA, York JL, Wooldridge C, Zhang D, Falany CN, Radominska-Pandya A. Carboxyl residues in the active site of human phenol sulfotransferase (SULT1A1) Biochemistry. 2000;39(51):16000–16007. doi: 10.1021/bi0021479. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhang D, Jing N, Yin S, Falany CN, Radominska-Pandya A. Human gastrointestinal sulfotransferases: identification and distribution. Toxicol Appl Pharmacol. 2003;187(3):186–197. doi: 10.1016/s0041-008x(02)00073-x. [DOI] [PubMed] [Google Scholar]
- Cho J. Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch Pharm Res. 2006;29(8):699–706. doi: 10.1007/BF02968255. [DOI] [PubMed] [Google Scholar]
- Chou HC, Lang NP, Kadlubar FF. Metabolic activation of N-hydroxy arylamines and N-hydroxy heterocyclic amines by human sulfotransferase(s) Cancer Res. 1995;55(3):525–529. [PubMed] [Google Scholar]
- De Santi C, Pietrabissa A, Mosca F, Rane A, Pacifici GM. Inhibition of phenol sulfotransferase (SULT1A1) by quercetin in human adult and foetal livers. Xenobiotica. 2002;32(5):363–368. doi: 10.1080/00498250110119108. [DOI] [PubMed] [Google Scholar]
- Eaton EA, Walle UK, Lewis AJ, Hudson T, Wilson AA, Walle T. Flavonoids, potent inhibitors of the human P-form phenolsulfotransferase. Potential role in drug metabolism and chemoprevention. Drug Metab Dispos. 1996;24(2):232–237. [PubMed] [Google Scholar]
- Falany CN, Zhuang W, Falany JL. Characterization of expressed human phenol-sulfating phenol sulfotransferase: effect of mutating cys70 on activity and thermostability. Chem Biol Interact. 1994;92(1–3):57–66. doi: 10.1016/0009-2797(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Fink BN, Steck SE, Wolff MS, Britton JA, Kabat GC, Schroeder JC, Teitelbaum SL, Neugut AI, Gammon MD. Dietary flavonoid intake and breast cancer risk among women on Long Island. Am J Epidemiol. 2007;165(5):514–523. doi: 10.1093/aje/kwk033. [DOI] [PubMed] [Google Scholar]
- Fisher ND, Hollenberg NK. Flavanols for cardiovascular health: the science behind the sweetness. J Hypertens. 2005;23(8):1453–1459. doi: 10.1097/01.hjh.0000174605.34027.9d. [DOI] [PubMed] [Google Scholar]
- Fisher ND, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens. 2003;21(12):2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- Frydoonfar HR, McGrath DR, Spigelman AD. The variable effect on proliferation of a colon cancer cell line by the citrus fruit flavonoid Naringenin. Colorectal Dis. 2003;5(2):149–152. doi: 10.1046/j.1463-1318.2003.00444.x. [DOI] [PubMed] [Google Scholar]
- Gardana C, Guarnieri S, Riso P, Simonetti P, Porrini M. Flavanone plasma pharmacokinetics from blood orange juice in human subjects. Br J Nutr. 2007;98(1):165–172. doi: 10.1017/S0007114507699358. [DOI] [PubMed] [Google Scholar]
- Ghazali RA, Waring RH. The effects of flavonoids on human phenolsulphotransferases: potential in drug metabolism and chemoprevention. Life Sci. 1999;65(16):1625–1632. doi: 10.1016/s0024-3205(99)00423-3. [DOI] [PubMed] [Google Scholar]
- Gibb C, Glover V, Sandler M. In vitro inhibition of phenolsulphotransferase by food and drink constituents. Biochem Pharmacol. 1987;36(14):2325–2330. doi: 10.1016/0006-2952(87)90598-3. [DOI] [PubMed] [Google Scholar]
- Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, Gebhardt S. Flavonoid content of U.S. fruits, vegetables, and nuts. J Agric Food Chem. 2006;54(26):9966–9977. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- Harris RM, Wood DM, Bottomley L, Blagg S, Owen K, Hughes PJ, Waring RH, Kirk CJ. Phytoestrogens are potent inhibitors of estrogen sulfation: implications for breast cancer risk and treatment. J Clin Endocrinol Metab. 2004;89(4):1779–1787. doi: 10.1210/jc.2003-031631. [DOI] [PubMed] [Google Scholar]
- Henning SM, Fajardo-Lira C, Lee HW, Youssefian AA, Go VL, Heber D. Catechin content of 18 teas and a green tea extract supplement correlates with the antioxidant capacity. Nutr Cancer. 2003;45(2):226–235. doi: 10.1207/S15327914NC4502_13. [DOI] [PubMed] [Google Scholar]
- Heptinstall S, May J, Fox S, Kwik-Uribe C, Zhao L. Cocoa flavanols and platelet and leukocyte function: recent in vitro and ex vivo studies in healthy adults. J Cardiovasc Pharmacol. 2006;47(Suppl. 2):S197–205. doi: 10.1097/00005344-200606001-00015. discussion S6–9. [DOI] [PubMed] [Google Scholar]
- Her C, Aksoy IA, Kimura S, Brandriff BF, Wasmuth JJ, Weinshilboum RM. Human estrogen sulfotransferase gene (STE): cloning, structure, and chromosomal localization. Genomics. 1995;29(1):16–23. doi: 10.1006/geno.1995.1210. [DOI] [PubMed] [Google Scholar]
- Hollman PC, Katan MB. Health effects and bioavailability of dietary flavonols. Free Radic Res. 1999;31(Suppl.):S75–S80. doi: 10.1080/10715769900301351. [DOI] [PubMed] [Google Scholar]
- Jenner AM, Rafter J, Halliwell B. Human fecal water content of phenolics: the extent of colonic exposure to aromatic compounds. Free Radic Biol Med. 2005;38(6):763–772. doi: 10.1016/j.freeradbiomed.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Justesen U, Knuthsen P, Leth T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photodiode array and mass spectrometric detection. J Chromatogr A. 1998;799(1–2):101–110. doi: 10.1016/s0021-9673(97)01061-3. [DOI] [PubMed] [Google Scholar]
- Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr. 2007;61(4):472–477. doi: 10.1038/sj.ejcn.1602543. [DOI] [PubMed] [Google Scholar]
- Khokhar S, Magnusdottir SG. Total phenol, catechin, and caffeine contents of teas commonly consumed in the United kingdom. J Agric Food Chem. 2002;50(3):565–570. doi: 10.1021/jf010153l. [DOI] [PubMed] [Google Scholar]
- Kim HK, Jeon WK, Ko BS. Flavanone glycosides from Citrus junos and their anti-influenza virus activity. Planta Med. 2001;67(6):548–549. doi: 10.1055/s-2001-16484. [DOI] [PubMed] [Google Scholar]
- Kren V, Martinkova L. Glycosides in medicine: ‘the role of glycosidic residue in biological activity’. Curr Med Chem. 2001;8(11):1303–1328. doi: 10.2174/0929867013372193. [DOI] [PubMed] [Google Scholar]
- Lin J, Zhang SM, Wu K, Willett WC, Fuchs CS, Giovannucci E. Flavonoid intake and colorectal cancer risk in men and women. Am J Epidemiol. 2006;164(7):644–651. doi: 10.1093/aje/kwj296. [DOI] [PubMed] [Google Scholar]
- Lu JH, Li HT, Liu MC, Zhang JP, Li M, An XM, Chang WR. Crystal structure of human sulfotransferase SULT1A3 in complex with dopamine and 3′-phosphoadenosine 5′-phosphate. Biochem Biophys Res Commun. 2005;335(2):417–423. doi: 10.1016/j.bbrc.2005.07.091. [DOI] [PubMed] [Google Scholar]
- Marchetti F, De Santi C, Vietri M, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Differential inhibition of human liver and duodenum sulphotransferase activities by quercetin, a flavonoid present in vegetables, fruit and wine. Xenobiotica. 2001;31(12):841–847. doi: 10.1080/00498250110069159. [DOI] [PubMed] [Google Scholar]
- Mateus N, Silva AM, Santos-Buelga C, Rivas-Gonzalo JC, de Freitas V. Identification of anthocyanin-flavanol pigments in red wines by NMR and mass spectrometry. J Agric Food Chem. 2002;50(7):2110–2116. doi: 10.1021/jf0111561. [DOI] [PubMed] [Google Scholar]
- Meerman JH, Van de Poll ML. Metabolic activation routes of arylamines and their genotoxic effects. Environ Health Perspect. 1994;102(Suppl. 6):153–159. doi: 10.1289/ehp.94102s6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinl W, Meerman JH, Glatt H. Differential activation of promutagens by alloenzymes of human sulfotransferase 1A2 expressed in Salmonella typhimurium. Pharmacogenetics. 2002;12(9):677–689. doi: 10.1097/00008571-200212000-00002. [DOI] [PubMed] [Google Scholar]
- Mesia-Vela S, Kauffman FC. Inhibition of rat liver sulfotransferases SULT1A1 and SULT2A1 and glucuronosyltransferase by dietary flavonoids. Xenobiotica. 2003;33(12):1211–1220. doi: 10.1080/00498250310001615762. [DOI] [PubMed] [Google Scholar]
- Miller JA. Sulfonation in chemical carcinogenesis – history and present status. Chem Biol Interact. 1994;92(1–3):329–341. doi: 10.1016/0009-2797(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Minato K, Fukumoto S, Yamamoto K, Oya-Ito T, Kawakishi S, Osawa T. New potent antioxidative hydroxyflavanones produced with Aspergillus saitoi from flavanone glycoside in citrus fruit. Biosci Biotechnol Biochem. 2003;67(7):1443–1450. doi: 10.1271/bbb.67.1443. [DOI] [PubMed] [Google Scholar]
- Nair HK, Rao KV, Aalinkeel R, Mahajan S, Chawda R, Schwartz SA. Inhibition of prostate cancer cell colony formation by the flavonoid quercetin correlates with modulation of specific regulatory genes. Clin Diagn Lab Immunol. 2004;11(1):63–69. doi: 10.1128/CDLI.11.1.63-69.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkimoto K, Liu MY, Suiko M, Sakakibara Y, Liu MC. Characterization of a zebrafish estrogen-sulfating cytosolic sulfotransferase: inhibitory effects and mechanism of action of phytoestrogens. Chem Biol Interact. 2004;147(1):1–7. doi: 10.1016/j.cbi.2003.09.001. [DOI] [PubMed] [Google Scholar]
- O’Leary KA, Day AJ, Needs PW, Mellon FA, O’Brien NM, Williamson G. Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human beta-glucuronidase, sulfotransferase, catechol-O-methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolism. Biochem Pharmacol. 2003;65(3):479–491. doi: 10.1016/s0006-2952(02)01510-1. [DOI] [PubMed] [Google Scholar]
- Otterness DM, Wieben ED, Wood TC, Watson WG, Madden BJ, McCormick DJ, Weinshilboum RM. Human liver dehydroepiandrosterone sulfotransferase: molecular cloning and expression of cDNA. Mol Pharmacol. 1992;41(5):865–872. [PubMed] [Google Scholar]
- Pacifici GM. Inhibition of human liver and duodenum sulfotransferases by drugs and dietary chemicals: a review of the literature. Int J Clin Pharmacol Ther. 2004;42(9):488–495. doi: 10.5414/cpp42488. [DOI] [PubMed] [Google Scholar]
- Park SH, Park EK, Kim DH. Passive cutaneous anaphylaxis-inhibitory activity of flavanones from Citrus unshiu and Poncirus trifoliata. Planta Med. 2005;71(1):24–27. doi: 10.1055/s-2005-837746. [DOI] [PubMed] [Google Scholar]
- Pearson DA, Paglieroni TG, Rein D, Wun T, Schramm DD, Wang JF, Holt RR, Gosselin R, Schmitz HH, Keen CL. The effects of flavanol-rich cocoa and aspirin on ex vivo platelet function. Thromb Res. 2002;106(4–5):191–197. doi: 10.1016/s0049-3848(02)00128-7. [DOI] [PubMed] [Google Scholar]
- Pezzato E, Sartor L, Dell’Aica I, Dittadi R, Gion M, Belluco C, Lise M, Garbisa S. Prostate carcinoma and green tea: PSA-triggered basement membrane degradation and MMP-2 activation are inhibited by (−)epigallocatechin-3-gallate. Int J Cancer. 2004;112(5):787–792. doi: 10.1002/ijc.20460. [DOI] [PubMed] [Google Scholar]
- Radominska-Pandya A, Little JM, Pandya JT, Tephly TR, King CD, Barone GW, Raufman JP. UDP-glucuronosyltransferases in human intestinal mucosa. Biochim Biophys Acta. 1998;1394(2–3):199–208. doi: 10.1016/s0005-2760(98)00115-5. [DOI] [PubMed] [Google Scholar]
- Radominska-Pyrek A, Zimniak P, Irshaid YM, Lester R, Tephly TR, St Pyrek J. Glucuronidation of 6 alpha-hydroxy bile acids by human liver microsomes. J Clin Invest. 1987;80(1):234–241. doi: 10.1172/JCI113053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz EP, Zitron E, Kiesecker C, Thomas D, Kathofer S, Kreuzer J, Bauer A, Katus HA, Remppis A, Karle CA, Greten J. Orange flavonoid hesperetin modulates cardiac hERG potassium channel via binding to amino acid F656. Nutr Metab Cardiovasc Dis. 2006 doi: 10.1016/j.numecd.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA. 2006;103(4):1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehm J, Polster J, Pfaffl MW. Effects of varied EGCG and (+)-catechin concentrations on proinflammatory cytokines mrna expression in cona-stimulated primary white blood cell cultures. J Agric Food Chem. 2005;53(17):6907–6911. doi: 10.1021/jf0503107. [DOI] [PubMed] [Google Scholar]
- Selmi C, Mao TK, Keen CL, Schmitz HH, Gershwin M Eric. The anti-inflammatory properties of cocoa flavanols. J Cardiovasc Pharmacol. 2006;47(Suppl. 2):S163–S171. doi: 10.1097/00005344-200606001-00010. discussion S72–S76. [DOI] [PubMed] [Google Scholar]
- Silberberg M, Gil-Izquierdo A, Combaret L, Remesy C, Scalbert A, Morand C. Flavanone metabolism in healthy and tumor-bearing rats. Biomed Pharmacother. 2006;60(9):529–535. doi: 10.1016/j.biopha.2006.07.083. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen therapy and cardiovascular disease. Ten-year follow-up from the nurses’ health study. N Engl J Med. 1991;325(11):756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- Steffen Y, Schewe T, Sies H. Epicatechin protects endothelial cells against oxidized LDL and maintains NO synthase. Biochem Biophys Res Commun. 2005;331(4):1277–1283. doi: 10.1016/j.bbrc.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Terao J. Dietary flavonoids as antioxidants in vivo: conjugated metabolites of (−)-epicatechin and quercetin participate in antioxidative defense in blood plasma. J Med Invest. 1999;46(3–4):159–168. [PubMed] [Google Scholar]
- Torres JL, Bobet R. New flavanol derivatives from grape (Vitis vinifera) byproducts. Antioxidant aminoethylthio-flavan-3-ol conjugates from a polymeric waste fraction used as a source of flavanols. J Agric Food Chem. 2001;49(10):4627–4634. doi: 10.1021/jf010368v. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan JB, Walle T. Glucuronidation and sulfation of the tea flavonoid (−)-epicatechin by the human and rat enzymes. Drug Metab Dispos. 2002;30(8):897–903. doi: 10.1124/dmd.30.8.897. [DOI] [PubMed] [Google Scholar]
- Veluri R, Weir TL, Bais HP, Stermitz FR, Vivanco JM. Phytotoxic and antimicrobial activities of catechin derivatives. J Agric Food Chem. 2004;52(5):1077–1082. doi: 10.1021/jf030653+. [DOI] [PubMed] [Google Scholar]
- Vietri M, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. 7-OH-flavone is sulfated in the human liver and duodenum, whereas 5-OH-flavone and 3-OH-flavone are potent inhibitors of SULT1A1 activity and 7-OH-flavone sulfation rate. Xenobiotica. 2002;32(7):563–571. doi: 10.1080/00498250210130582. [DOI] [PubMed] [Google Scholar]
- Wakazono H, Gardner I, Eliasson E, Coughtrie MW, Kenna JG, Caldwell J. Immunochemical identification of hepatic protein adducts derived from estragole. Chem Res Toxicol. 1998;11(8):863–872. doi: 10.1021/tx9702188. [DOI] [PubMed] [Google Scholar]
- Walle T, Eaton EA, Walle UK. Quercetin, a potent and specific inhibitor of the human P-form phenosulfotransferase. Biochem Pharmacol. 1995;50(5):731–734. doi: 10.1016/0006-2952(95)00190-b. [DOI] [PubMed] [Google Scholar]
- Waring RH, Ayers S, Gescher AJ, Glatt HR, Meinl W, Jarratt P, Kirk CJ, Pettitt T, Rea D, Harris RM. Phytoestrogens and xenoestrogens: the contribution of diet and environment to endocrine disruption. J Steroid Biochem Mol Biol. 2008;108(3–5):213–220. doi: 10.1016/j.jsbmb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Yang SH, Tao J, Liu XF, Guo DA, Zheng JH. Effects of carbon source and nitrogen source on callus growth and flavonoid content in Glycyrrhiza uralensis. Zhongguo Zhong Yao Za Zhi. 2006;31(22):1857–1859. [PubMed] [Google Scholar]
- Zhu QY, Schramm DD, Gross HB, Holt RR, Kim SH, Yamaguchi T, Kwik-Uribe CL, Keen CL. Influence of cocoa flavanols and procyanidins on free radical-induced human erythrocyte hemolysis. Clin Dev Immunol. 2005;12(1):27–34. doi: 10.1080/17402520512331329514. [DOI] [PMC free article] [PubMed] [Google Scholar]