Abstract
Heat sensitivity is a sensory modality that plays a critical role in close-range host-seeking behaviors of adult female Anopheles gambiae, the principal Afrotropical vector for human malaria. An essential step in this activity is the ability to discriminate and respond to increases in environmental temperature gradients through the process of peripheral thermoreception. Here, we report on the characterization of the anopheline homolog of the transient receptor potential (TRP) A1/ANKTM1 channel that is consistent with its role as a heat-sensor in host-seeking adult female mosquitoes. We identify a set of distal antennal sensory structures that specifically respond to temperature gradients and express AgTRPA1. Functional characterization of AgTRPA1 in Xenopus oocytes supports its role in the molecular transduction of temperature gradients in An. gambiae, providing a basis for targeting mosquito heat responses as a means toward reducing malaria transmission.
Keywords: coeloconic sensilla, temperature receptor, TRP channel
Introduction
The malaria vector Anopheles gambiae and other blood-feeding mosquitoes rely on temperature cues in addition to olfactory stimuli for host location (Bowen, 1991; Takken & Knols, 1999). Heat is emitted by mammalian hosts, serves as a universal attractant to many mosquito species (Bowen, 1991; Takken & Knols, 1999) and synergizes with host odor to increase the efficiency of host-seeking behaviors (Laarman, 1958; Schreck et al., 1990; Kline & Lemire, 1995). Furthermore, recent behavioral studies have indicated that heat alone has no effect on the landing response of female An. gambiae, but significantly synergizes the attraction to the host odor (J. Spitzen and W. Takken, unpublished observations).
Early antennal ablation studies suggested that the antenna might harbor the thermoreceptive neurons that underlie the heat-evoked behaviors of mosquitoes (Ismail, 1962). In addition, ultrastructural studies have characterized antennal small sensilla coeloconic (SC) and sensilla ampullacea as probable sites of temperature detection (McIver, 1973; Boo & McIver, 1975; McIver & Siemicki, 1976). In accord with these efforts, electrophysiological studies in the yellow-fever vector mosquito, Aedes aegypti, identified an antagonistic pair of thermoreceptive neurons within the small SC on the antennal tip (Davis & Sokolove, 1975; Gingl et al., 2005). Here, one thermoreceptive neuron is warm-sensitive and increases its spike frequency to temperature rises; the other is cold-sensitive and increases its spike frequency to temperature falls (Davis & Sokolove, 1975; Gingl et al., 2005).
Despite some knowledge of the physiology of antennal thermoreceptive neurons, little is known about genes that are involved in peripheral temperature detection in mosquitoes. In Drosophila, a family of 13 transient receptor potential (TRP) genes (Montell, 2005) encode six-transmembrane nonselective cation channels, several of which have been identified as central nervous system (CNS) thermoreceptors (Tracey et al., 2003; Lee et al., 2005; Rosenzweig et al., 2005; Hamada et al., 2008). Of these, dTRPA1 is activated by high temperatures, with a threshold of ~27°C, and regulates thermotaxis (Viswanath et al., 2003; Rosenzweig et al., 2005; Hamada et al., 2008). Furthermore, dTRPA1 has recently been shown to be activated indirectly through pathways coupled to phospholipase C to discriminate between the optimal temperature of 18°C and slightly higher temperatures (19–24°C) (Kwon et al., 2008). Not surprisingly, additional studies in Drosophila have revealed distinct TRP channels that are required for avoidance of cold (Rosenzweig et al., 2008).
Here we examine the molecular basis for peripheral heat sensitivity in adult female An. gambiae. We confirm that a pair of small SCs on the distal tip of female An. gambiae antennae contains neurons that specifically respond to a temperature rise with high sensitivity. We have screened the An. gambiae homologs of Drosophila TRPA channels for antennal expression and have identified AgTRPA1 as a consequence of its specific localization to the distal antennal small SCs. Lastly, we have functionally characterized AgTRPA1 heterologously expressed in Xenopus oocytes to demonstrate that it encodes a functional thermoreceptor. In these studies, AgTRPA1 was activated by temperature increases from 25 to ~37°C, consistent with the hypothesis that An. gambiae relies on antennally expressed AgTRPA1 to detect increasing temperature gradients derived from host body heat during crucial close-range, host-seeking behaviors.
Materials and methods
Insects
Anopheles gambiae sensu stricto Giles, originating from Suakoko, Liberia, were reared as described previously (Fox et al., 2001; Qiu et al., 2006a). Non-blood-fed, 5- to 7-day-old females were used for all experiments. All animal experiments followed the guidelines set by the Vanderbilt Institutional Animal Care and Use Committee (IACUC) to minimize the number of animals used. Xenopus laevis were from eNASCO (Modesto, CA, USA). Before surgery, animals were placed in a solution containing 2 g ethyl-3-amino-benzoate methanesulfonate salt from Sigma (in 1 L H2O) for 20–30 min until completely anesthetized.
In vivo single sensillum recordings
Extracellular recordings of neuronal activity in female An. gambiae were made as described (Qiu et al., 2006b) using an USB-IDAC analog-to-digital conversion interface with Autospike software for sampling, visualization and analysis (Syntech, Kirchzarten, Germany). Recordings were made from the paired small SCs at the distal tip of the 13th antennal segment. The recording electrode (tungsten, tip c. 1 μm) was placed into the pit of the sensillum and pierced through the cuticle of the peg located inside the pit.
An increase of air temperature was generated to study whether warm-sensitive neurons are located in these small SCs. A charcoal-filtered air stream was pumped out of the compensatory air pulse outlet of a stimulus controller (CS-55, Syntech) at 1.5 L/min and passed through a Pasteur pipette that was maintained at room temperature (c. 25°C). At discrete time intervals, the air stream was switched to the normal air pulse outlet of the stimulus controller and led through a Pasteur pipette heated by direct contact with hot water (85 ± 3°C). Each air stream was connected to one arm of a Y-shaped connector, the common outlet (inner diameter 2 mm) of which was positioned c. 5 mm from the tip of the mosquito antenna. Warmed air was led over the antenna during a period of 10 s and, subsequently, the air stream was switched back to the airflow at room temperature. This sequence was repeated twice. The temperature of the air stream increased from approximately 25–37°C and was constantly monitored at a distance of c. 1 mm from the sensilla using a type-K thermocouple and TC-08 data logger (Pico Technology, St Neots, UK).
Anterograde dye filling of distal antennal small SC neurons
Anterograde dye filling from the distal pair of antennal small SC structures was performed by slivering the sensilla with either electrodes or fine scissors, followed by immediate immersion in 2% Texas red or Lucifer yellow in PBS solution (w/v) in the glass electrode for 4 h at 4°C. At that point the electrodes were removed and the antennae were amputated from the mosquito head. Whole mounts of the antenna were washed in 0.1% PBST for 1–2 h and mounted on a glass slide using Vectashield (Vector Laboratories, Burlingame, CA, USA). Whole mounts of the stained mosquito antenna were observed by using an LSM 510 confocal microscope (Zeiss, Thornwood, NY, USA).
Reverse transcriptase PCR (RT-PCR)
Mosquitoes were cold anesthetized and 100 antennae were dissected by hand on a chilled table. RNA preparation and cDNA synthesis were carried out as described previously (Kwon et al., 2006; Lu et al., 2007). Negative control samples without reverse transcriptase were included in each cDNA synthesis and subsequent PCR analysis. PCR cDNA template levels were monitored for integrity and amount using the ubiquitously expressed An. gambiae ribosomal protein S7 gene (rps7) (Salazar et al., 1993) as an internal control. PCR was performed using a DNA Engine Dyad (GMI, Ramesy, MN, USA) under the following conditions: 94°C for 2 min; 32–35 cycles of 94°C for 15 s, 60°C for 20 s and 72°C for 20 s; and 72°C for 5 min. Primer pairs that span introns were used to distinguish cDNA amplicons from those amplified from remaining genomic DNA. The complete set of AgTRP primers can be accessed at http://www.cas.vanderbilt.edu/zwiebel/primers3.htm. PCR amplification products were run on a 1.5% agarose gel and verified by DNA sequencing.
In situ hybridization and immunolabeling
Riboprobes for in situ hybridization comprising approximately 900 bp of AgTRPA1 coding sequence were prepared as follows. AgTRPA1 sequences were amplified from An. gambiae antenna cDNA samples utilizing the following PCR primers: AgtrpA1-F 5′-CTATTCGGCGGCTTCAATAAC-3′ and AgTrpA1r 5′-TCATTTGCCAATAGATTTGTTGAAGC-3′. PCR products were ligated to pCRII-Topo (Invitrogen, Carlsbad, CA, USA), and digoxigenin-labeled RNA probes were generated for sense and antisense utilizing SP6 and T7 RNA polymerase, respectively. Detailed procedures for probe preparation and in situ hybridization on An. gambiae olfactory tissues were described previously (Kwon et al., 2006; Lu et al., 2007). Cell nuclei were labeled using YOYO-1 (Invitrogen) and anti-horseradish peroxidase (hrp) immuno-labeling was used to mark neuronal axon and dendrites (Pitts et al., 2004). Antennal sections were mounted in Vectashield (Vector Laboratories) and visualized using an LSM510 confocal microscope (Carl Zeiss).
AgTRPA1 expression in X. laevis oocytes and two-electrode, voltage-clamp electrophysiological recording
Full-length coding sequences of AgTRPA1 were PCR amplified from female An. gambiae antennal cDNA under the following conditions: 94°C for 2 min; 32 cycles of 94°C for 30 s, 58°C for 30 s and 72°C for 3 min 30 s; and 72°C for 10 min. The PCR product was first cloned into pENTR/D-TOPO (Invitrogen) and then sub-cloned into pSP64DV by means of the Gateway LR reaction (Lu et al., 2007). Complementary RNA (cRNA) was synthesized from linearized vectors with mMESSAGE mMACHINE (Ambion).
Mature healthy oocytes (stage V–VII) were treated with 2 mg/mL collagenase S-1 in washing buffer [96 mm NaCl, 2 mm KCl, 5 mm MgCl2 and 5 mm HEPES (pH 7.6)] for 1–2 h at room temperature. Oocytes were later microinjected with 50 nL AgTRPA1 cRNA. After injection, oocytes were incubated for 5–7 days at 18°C in 1 × Ringer’s solution [96 mm NaCl, 2 mm KCl, 5 mm MgCl2, 0.8 mm CaCl2 and 5 mm HEPES (pH 7.6)] supplemented with 5% dialysed horse serum, 50 mg/mL tetracycline, 100 mg/mL streptomycin and 550 mg/mL sodium pyruvate. Whole-cell currents were recorded from the injected Xenopus oocytes with a two-electrode voltage clamp. Temperature control was achieved with a combination of an HE-104R thermal stage and an HCC-100A temperature controller (Dagan Instruments, Minneapolis, MN, USA) and constant perfusion of cooled/heated solution. Currents were recorded using an OC-725C oocyte clamp (Warner Instruments, Hamden, CT, USA), and pCLAMP8.2 software suite (Axon Instruments, Sunnyvale, CA, USA).
Results
Physiological responses to temperature rise in female antennae
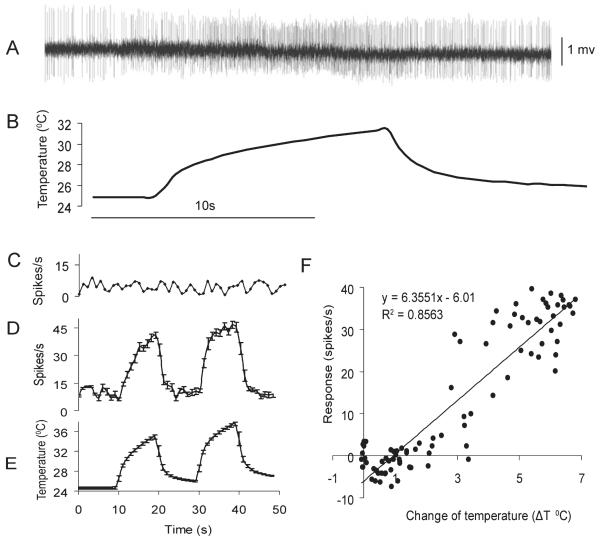
As is the case for several other mosquito species, each antenna of An. gambiae adult females bears approximately seven small SC structures (Fig. 1A) (McIver, 1982; Pitts & Zwiebel, 2006). A set of paired small SCs are located on the distal tip of the 13th segment, three are located on the distal edge of the first segment and one each on segments 12 and 13 (Fig. 1). Extracellular recordings were made from the distal-most small SC pair and identified a specific neuron that displayed both excitatory responses to swift increases of temperature and the largest spike amplitude of the 20 sensilla from which successful recordings were obtained (Fig. 2A). The impulse frequency of the heat-sensitive small SC neuron increased and decreased instantly with the corresponding temperature rise and fall, respectively (Fig. 2D and E). In contrast, the impulse frequency of a control olfactory receptor neuron (ORN) recorded extracellularly from a nearby antennal trichoid sensillum remained unchanged regardless of the temperature rise or fall (Fig. 2C). Furthermore, the change in impulse frequency relative to the spontaneous frequency at room temperature showed a linear correlation with the change in temperature (ΔT) (F-test, P < 0.001, Fig. 2F). The differential sensitivity, represented as the difference in impulse frequency per degree centigrade of change in temperature, was 6.36 spikes/s. Although application of multiple chemical odorants known to elicit antennal ORN activity (Qiu et al., 2006b) failed to evoke any alteration in the activity of the heat-sensitive small SC (data not shown), stimulation with high humidity (100% dH2O-saturated air) elicited significant increases in impulse frequency of a neuron with large spike amplitude (see Supporting Information Fig. S4).
Fig. 1.
The ultrastructure of small coeloconic sensilla. (A) An. gambiae antenna showing the positions of small coeloconic sensilla (white dots) according to Pitts & Zwiebel (2006). (B) Scanning electron micrograph showing the small coeloconic sensilla on the 13th antennal segment. Small coeloconic sensilla are indicated by arrowheads. (C, D) Small coeloconic sensilla on the tip of the antenna.
Fig. 2.
Single-sensillum electrophysiological recordings from small coeloconic sensilla. (A) Extracellular recording from a small coeloconic sensillum at the tip of the antenna (segment 13) of female An. gambiae showing the excitatory response of a neuron to an increase in air temperature. (B) Time course of air temperature applied during the recording shown in A. Time scale of A and B are the same and indicated by the bar under B. (C) The impulse frequency of a control olfactory receptor neuron in an antennal trichoid sensillum remains unchanged in response to temperature alterations. (D) Mean impulse frequency (spikes/s ± SEM) of the heat-sensitive neuron in response to two increase/decrease cycles of air temperature (n = 7). (E) Time course of air temperature (mean ± SEM) applied during the experiment shown in C (n = 7). (F) Linear regression of the response intensity of the heat-sensitive neuron to changes in air temperature, ΔT. For the first stimulation cycle of temperature, ΔT = temperature at time x minus mean room temperature; for the second stimulation cycle, ΔT = temperature at time x minus average temperature during the last 3 s before the onset of the temperature rise. Neuron response = impulse frequency at time x minus impulse frequency at room temperature. Data in D–F originate from seven of the 20 distal small coeloconic sensilla from which recordings were taken. Although responses to temperature were observed in all 20 sensilla, either due to low signal-to-noise ratios or different stimuli intensity and duration used, data from only seven sensilla were summarized.
Expression of AgTRPA1 in adult female antennae
We next investigated whether TRPA channels, which had been directly linked to heat reception in Drosophila, play significant roles in this peripheral temperature detection in An. gambiae. With the notable exception of painless (Tracey et al., 2003), three of the four Drosophila TRPA channels (Viswanath et al., 2003) have close homologs in the genome of An. gambiae. In the present study, putative AgTRPA genes were first screened for antennal expression using standard RT-PCR protocols. These semi-quantitative studies indicated that AgTRPA1 is robustly expressed in antennae, while AgTRPA2 is expressed at much lower levels and AgTRPA3 is not expressed at all (Fig. S1A). Additional RT-PCR studies were used to examine the expression pattern of AgTRPA1 in sensory tissues of adult female An. gambiae mosquitoes. The resulting data indicated that AgTRPA1 is highly expressed in the female antenna as well as at lower levels in the head, maxillary palp and proboscis relative to constitutively expressed rps7 controls (Fig. S1B). In contrast, dTRPA1 expression is restricted to the proboscis and a set of warmth-activated anterior cell (AC) neurons in the Drosophila CNS (Hamada et al., 2008).
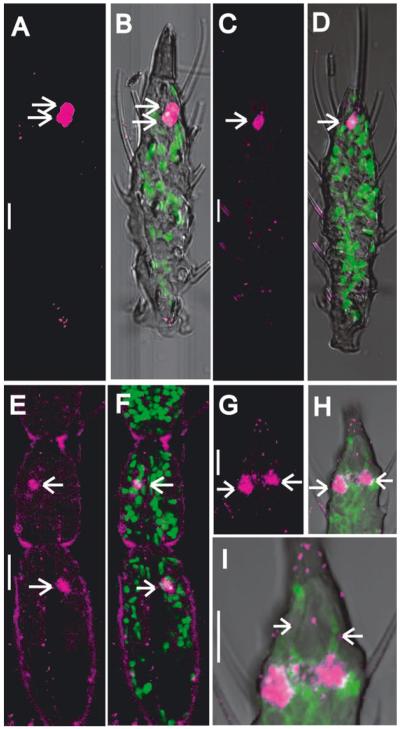
The antennal expression of AgTRPA1 was confirmed and elaborated upon with fluorescence in situ hybridization (FISH) coupled with immunolabeling. These data indicated that AgTRPA1 expression is restricted to a very limited number of antennal neurons. Indeed, in paraffin sections, we consistently observed only one neuron in the first and second antennal segments and typically two neurons in the distal-most region of the 13th segment, which were labeled by antisense AgTRPA1 riboprobes (Fig. 3). The 10 mid-segments (from the third to the 12th antennal segments) were entirely devoid of AgTRPA1 signals. Furthermore, the position of each AgTRPA1-positive neuron cell body correlated with the small SC localization reported previously in morphological ultrastructural studies (Ismail, 1964; Pitts & Zwiebel, 2006). Lastly, anterograde dye-filling studies (Fig. S2) indicated AgTRPA1 labeling in the 13th antennal segment closely matched the position of the cell bodies associated with paired small SCs on the antennal tip, which we have shown to harbor thermoreceptive neurons in An. gambiae and which have previously been implicated in heat sensitivity in Ae. aegypti (Davis & Sokolove, 1975; Gingl et al., 2005).
Fig. 3.
Expression of the AgTRPA1 gene in the antenna. Panels A–B and C–D are single and double (merged) channel visualizations of replicate lateral optical sections through the distal-most (13th) antennal segment, respectively. Panels E–I are single and double (merged) channel visualizations of medial sections through the first and second (E, F) and 13th (G–I) antennal segments. A, C, E and G display Cy-3 (red-see colours in the on-line graphic) immunofluorescence indicative of AgTRPA1-positive cells (arrows). In the distal most (13th) antennal segment there are two AgTRPA1-positive cells (arrows, A–D and G–H, respectively). There is one AgTRPA1-positive cell in the first and second antennal segments (arrows, E and F, respectively). Panels B, D, F, H and I represent merged images where the green immunofluorescence in B, D and F display cell nuclei labeled using YOYO-1 (Invitrogen) and in H and I display anti-HRP immunolabeling to mark neuronal axons and dendrites. Panel I is a high-magnification Z-series image stack illustrating that the two AgTRPA1-positive neurons extend their dendrites into the tip of the antenna (arrowheads, anti-HRP labeling of neuronal dendrites). The scale bar represents 10 μm.
AgTRPA1 is activated by increasing temperatures
To investigate further its activation by increasing temperatures, AgTRPA1 was heterologously expressed in Xenopus oocytes and functionally characterized using two-electrode, voltage-clamp physiology. A temperature increase from room temperature (22°C) to ~37°C consistently elicited a large inward current of over 500 nA in Xenopus oocytes injected with cRNA encoding AgTRPA1, and no significant adaptation was observed when the stimulus was repeated (Fig. 4B). The thermal response continuously increased along with the temperature from 25 to ~37°C (Fig. 4C), reaching a maximal response at ~40°C (Fig. S5). Importantly, control oocytes injected with buffer alone failed to manifest any response to temperature increases from 22 to ~37°C (Figs 4A and S5). Additional controls included oocytes coexpressing AgOR28 and AgOR7 which have previously been shown to support odorant-induced currents in response to 6-methyl-5-hepten-2-one and other chemical stimuli (Lu et al., 2007). In this case AgOR28/AgOR7 oocytes failed to respond to a temperature shift from 25 to ~37°C (Fig. S3). These results correlate with the response properties of the warm-sensitive neuron in our in vivo single-sensillum recording (SSR) analyses and are consistent with similar independent studies (Hamada et al., 2008). Taken together, these data support the hypothesis that AgTRPA1 is responsible for peripheral thermoreceptive responses as a consequence of its expression in small SC neurons.
Fig. 4.
AgTRPA1 is activated by increasing temperature. (A) Response of control oocytes injected with buffer alone to heat stimuli. Inset: higher resolution of the inter-stimulus interval to demonstrate the viability of the control oocyte. (B) Inward currents of the oocytes expressing AgTRPA1 to heat stimuli. (C) The relationship between temperature and response of oocytes expressing AgTRPA1 (n = 5; error bars indicate SEM).
Discussion
We have characterized the electrophysiological response to changes in ambient air temperature of distal small SC-associated neurons on the antennae of adult female An. gambiae. Using in vivo SSR analyses, we have confirmed the presence of thermoreceptive neurons within small SC structures that specifically respond with excitation to an increase in air temperature. In Ae. aegypti, a heat-excited neuron was also found in a similarly located small SC (peg in-pit sensilla) at the antennal tip segment (Gingl et al., 2005). It is noteworthy that the differential sensitivity we detected in this study for An. gambiae was nearly twice that reported previously for Ae. aegypti. We have tested 11 compounds and one synthetic mixture representing known ligands of various antennal ORNs of female An. gambiae (Qiu et al., 2006b) and none elicited responses by neurons innervating SC. Interestingly, an increase in humidity in the air stream did elicit an excitatory response by one of the neurons in the SCs (Fig. S4). It is possible that the heat-sensitive neuron is also responding to the change in humidity, as both heat and humidity stimuli evoked similar spike amplitudes. Bimodal thermo-/hygroreceptor neurons have indeed been observed in other insects (Loftus & Corbiere-Tichane, 1981; Alter & Loftus, 1985).
To begin to address the molecular basis for peripheral thermoreception functionally, we mapped a member of the TRP gene family of related channel proteins in An. gambiae, AgTRPA1, to neurons associated with distal thermoreceptive antennal small SCs. In addition to significant homology to Drosophila dTRPA1 and other TRP channel homologs, AgTRPA1 conferred responses to a temperature increase from room temperature to ~40°C when functionally expressed in Xenopus oocytes. This agrees well with a previous study (Hamada et al., 2008) and indicates that AgTRPA1, like dTRPA1, functions as a warm-sensitive thermoreceptor. AgTRPA1 has close homologs in several other mosquito species, including Ae. aegypti and Culex pipiens (Hamada et al., 2008), suggesting that it is likely to function as a peripheral heat sensor in those mosquito species as well.
Taken together, these data support the hypothesis that AgTRPA1 is expressed in the antenna where it functions as a peripheral thermoreceptor in An. gambiae. This is in contrast to the situation in Drosophila, in which dTRPA1 is thought to function as an internal heat detector in the brain (Rosenzweig et al., 2005; Hamada et al., 2008); and although peripheral dTRPA1 expression is noted in the proboscis, ablation studies have failed to support a role for this expression in warmth-avoidance paradigms (Hamada et al., 2008). The expression of a peripheral thermoreceptor in the major olfactory organ of An. gambiae provides a basis for the observation that temperature, an important sensory cue in host location, is a bona fide antennal stimulus. Indeed, temperature sensitivity may be encoded along with chemosensory information, and the two stimulus modalities may be integrated through post-synaptic events in the antennal lobe and higher brain centers (Zeiner & Tichy, 2000). Consistent with this view, behavioral studies have demonstrated that elevated source temperatures of ~32–37°C synergize to increase the attraction to host-derived odorants in host-seeking An. gambiae females (J. Spitzen and W. Takken, unpublished observations). Indeed, it will be of interest to elucidate the mechanism by which the two types of sensory inputs are coupled and transformed into an ultimate sensory percept of the host.
In previous physiological studies in Ae. aegypti, it has been demonstrated that antennal small SCs respond far more sensitively to convective than to radiant heat (Davis & Sokolove, 1975; Gingl et al., 2005). It is likely that heat, emitted from a host’s breath and body, is incorporated into host odor blends, resulting in an odor/heat plume that is detected by the mosquito antenna. As heat is a universal stimulus for mosquitoes and most other blood-feeding insects, the identification of a peripheral mosquito thermoreceptor provides a potential target for the design of useful insect repellents. Such an advance would facilitate the ongoing fight against malaria by reducing the vectorial capacity of An. gambiae mosquitoes.
Supplementary Material
Acknowledgements
We thank Z. Li, L. Sun, F. van Aggelen, A.J. Gidding and L. Koopman for mosquito rearing and H. Smid for technical support. Dr J. Harbinson is acknowledged for providing the thermocouple and data logger. Members of the Takken and Zwiebel laboratories are acknowledged for discussions and comments on the manuscript and the editorial assistance of Dr A. M. McAinsh of Lektorin Services. This work was funded in part by Wageningen University, Vanderbilt University, and grants from the National Institutes of Health (AI56492, to L.J.Z.), the Bill and Melinda Gates Foundation (to W.T.) and the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (GCGH#121 to L.J.Z.).
Abbreviations
- AgTRPA1
Anopheles gambiae transient receptor potential A1
- CNS
central nervous system
- FISH
fluorescent in situ hybridization
- ORN
olfactory receptor neuron
- SC
sensilla coeloconic
- SSR
single-sensillum recording
- TRP
transient receptor potential
Footnotes
Supporting Information Additional supporting information may be found in the online version of this article:
Please note: As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset by Wiley-Blackwell. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Alter H, Loftus R. Ultrastructure and function of insect thermo- and hygroreceptors. Annu. Rev. Entomol. 1985;30:273–295. [Google Scholar]
- Boo KS, McIver SB. Fine structure of sunken thick-walled pegs (sensilla ampullacea and coeloconica) on the antennae of mosquitoes. Can. J. Zool. 1975;53:262–266. doi: 10.1139/z75-033. [DOI] [PubMed] [Google Scholar]
- Bowen MF. The sensory physiology of host-seeking behavior in mosquitoes. Annu. Rev. Entomol. 1991;36:139–158. doi: 10.1146/annurev.en.36.010191.001035. [DOI] [PubMed] [Google Scholar]
- Davis EE, Sokolove PG. Temperature responses of antennal receptors of the mosquito Aedes aegypti. J. Comp. Physiol. [A] 1975;96:223–236. [Google Scholar]
- Fox AN, Pitts RJ, Robertson HM, Carlson JR, Zwiebel LJ. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proc. Natl Acad. Sci. USA. 2001;98:14693–14697. doi: 10.1073/pnas.261432998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingl E, Hinterwirth A, Tichy H. Sensory representation of temperature in mosquito warm and cold cells. J. Neurophysiol. 2005;94:176–185. doi: 10.1152/jn.01164.2004. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail IAH. Sense organs in the antennae of Anopheles maculipennis atroparvus (V.Thiel) and their possible function in relation to the attraction of female mosquito to man. Acta Trop. 1962;19:1–58. [Google Scholar]
- Ismail IAH. Comparative study of sense organs in the antennae of Culicine and Anopheline female mosquitoes. Acta Trop. 1964;21:115–168. [PubMed] [Google Scholar]
- Kline DL, Lemire GF. Field evaluation of heat as an added attractant to traps baited with carbon dioxide and octenol for Aedes taeniorhynchus. J. Am. Mosq. Control Assoc. 1995;11:454–456. [PubMed] [Google Scholar]
- Kwon HW, Lu T, Rutzler M, Zwiebel LJ. Olfactory responses in a gustatory organ of the malaria vector mosquito Anopheles gambiae. Proc. Natl Acad. Sci. USA. 2006;103:13526–13531. doi: 10.1073/pnas.0601107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat. Neurosci. 2008;11:871–873. doi: 10.1038/nn.2170. [DOI] [PubMed] [Google Scholar]
- Laarman JJ. The host-seeking behaviour of anopheline mosquitoes. Trop. Geogr. Med. 1958;10:293–305. [PubMed] [Google Scholar]
- Lee Y, Lee Y, Lee J, Bang S, Hyun S, Kang J, Hong ST, Bae E, Kaang BK, Kim J. Pyrexia is a new thermal transient receptor potential channel endowing tolerance to high temperatures in Drosophila melanogaster. Nat. Genet. 2005;37:305–310. doi: 10.1038/ng1513. [DOI] [PubMed] [Google Scholar]
- Loftus R, Corbiere-Tichane G. Antennal warm and cold receptors of the cave beetle, Speophyes lucidulus Delar., in sensilla with a lamellated dendrite. J. Comp. Physiol. 1981;143:443–452. [Google Scholar]
- Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, Pitts RJ, van Loon JJA, Takken W, Carlson JR, Zwiebel LJ. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol. 2007;17:1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver SB. Fine structure of antennal sensilla coeloconica of culicine mosquitoes. Tissue Cell. 1973;5:105–112. doi: 10.1016/s0040-8166(73)80009-6. [DOI] [PubMed] [Google Scholar]
- McIver SB. Sensilla of mosquitoes (Diptera: Culicidae) J. Med. Entomol. 1982;19:489–535. doi: 10.1093/jmedent/19.5.489. [DOI] [PubMed] [Google Scholar]
- McIver S, Siemicki R. Fine structure of the antennal tip of the crabhole mosquito, Deinocerites cancer Theobald (Diptera: Culicidae) Int. J. Insect Morphol. Embryol. 1976;5:319–334. [Google Scholar]
- Montell C. Drosophila TRP channels. Pflugers Arch. 2005;451:19–28. doi: 10.1007/s00424-005-1426-2. [DOI] [PubMed] [Google Scholar]
- Pitts RJ, Zwiebel LJ. Antennal sensilla of two female anopheline sibling species with differing host ranges. Malar. J. 2006;5:26. doi: 10.1186/1475-2875-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Fox AN, Zwiebel LJ. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc. Natl Acad. Sci. USA. 2004;101:5058–5063. doi: 10.1073/pnas.0308146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YT, Smallegange RC, Van Loon JJ, Ter Braak CJ, Takken W. Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae s. s. Med. Vet. Entomol. 2006a;20:280–287. doi: 10.1111/j.1365-2915.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- Qiu YT, van Loon JJ, Takken W, Meijerink J, Smid HM. Olfactory coding in antennal neurons of the malaria mosquito, Anopheles gambiae. Chem. Senses. 2006b;31:845–863. doi: 10.1093/chemse/bjl027. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig M, Kang K, Garrity PA. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 2008;105:14668–14673. doi: 10.1073/pnas.0805041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar CE, Mills-Hamm D, Kumar V, Collins FH. Sequence of a cDNA from the mosquito Anopheles gambiae encoding a homologue of human ribosomal protein S7. Nucleic Acids Res. 1993;21:4147. doi: 10.1093/nar/21.17.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck CE, Kline DL, Carlson DA. Mosquito attraction to substances from the skin of different humans. J. Am. Mosq. Control Assoc. 1990;6:406–410. [PubMed] [Google Scholar]
- Takken W, Knols BG. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu. Rev. Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- Zeiner R, Tichy H. Integration of temperature and olfactory information in cockroach antennal lobe glomeruli. J. Comp. Physiol. [A] 2000;186:717–727. doi: 10.1007/s003590000125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.