Abstract
Restricted repetitive behaviors (stereotypy, compulsions, rituals) are diagnostic for autism spectrum disorder and common in related neurodevelopmental disorders. Despite their prevalence in clinical populations, underlying mechanisms associated with the development of these behaviors remain poorly understood. We examined the role of the indirect basal ganglia pathway in the development of stereotypy using deer mice. We measured neuronal metabolic activity in the subthalamic nucleus (STN) and other relevant brain regions using cytochrome oxidase (CO) histochemistry at three developmental time-points. Although no differences were observed in STN across development, significant differences were found when mice were grouped by developmental trajectory. At 6 weeks post-weaning, significantly lower CO activity in STN was found in those trajectory groups that developed high levels of repetitive behavior versus the trajectory group that did not, suggesting the development of stereotypy is associated with decreased indirect basal ganglia pathway activity. In addition, we tested the hypothesis that preferential activation of striatal striosomes relative to surrounding matrix would be associated with the development of stereotypy. No differences in the relative activation of these striatal compartments were observed across development or among trajectory groups. Our results point to dynamic changes in the indirect pathway associated with the development of repetitive behavior and extends our prior work linking reduced indirect pathway activation to stereotypy in adult deer mice.
Keywords: autism, stereotypy, subthalamic nucleus, neurodevelopmental disorders, deer mice, animal models
Introduction
Restricted, repetitive behaviors (e.g., motor stereotypy, compulsions, restricted interests) constitute one of three diagnostic domains of autism spectrum disorder (Lewis and Bodfish, 1998 for review). They are also common in related neurodevelopmental disorders (e.g., Rett’s syndrome, intellectual and developmental disability) as well as several neuropsychiatric and neurological disorders (e.g., obsessive-compulsive disorder, Tourette syndrome, frontotemporal dementia). Despite their clinical importance, the specific neurobiological mechanisms associated with the abnormal development and expression of these behaviors are largely unidentified. Several neuroimaging studies in autism and related disorders have identified structural and functional alterations in the basal ganglia that are linked to repetitive behaviors (e.g., Hollander et al., 2005; O'Sullivan et al., 1997; Peterson et al., 1998; Sears et al., 1999). Moreover, experimental manipulations in animals have provided more direct evidence for the importance of cortico-basal ganglia circuitry in mediating these abnormal behaviors (e.g., Baup et al., 2008; Grabli et al., 2004; Graybiel, 2000; Klavir et al., 2009; Welch et al., 2007; Winter et al., 2008a, 2008b).
Our laboratory has employed deer mice (Peromyscus maniculatus), which develop motor stereotypies (repetitive jumping and/or backward somersaulting) as a consequence of being reared in a standard laboratory environment. These behaviors occur at a high rate, appear relatively early in development, persist across much of the life of the animal and display considerable heterogeneity in individual levels of expression (Tanimura et al., 2010b). We have previously shown that alterations of basal ganglia circuitry are linked to the expression of these repetitive behaviors in adult deer mice (Presti et al., 2004; Presti and Lewis, 2005; Turner et al., 2002).
The main input structure of the basal ganglia, the striatum, consists of medium spiny GABAergic neurons, the large majority of which receive projections from sensory-motor and associative areas of cortex, and, in turn, give rise to the direct and indirect pathways. These pathways contribute to the final output signal of basal ganglia structures to the thalamus and cortex. The direct pathway projects to the globus pallidus interna (GPi) and substantia nigra (SN), whereas indirect pathway cells project to GPe, which relays to GPi and SN through the subthalamic nucleus (STN). The direct and indirect pathways play complementary roles in movement facilitation and movement inhibition, respectively. We have demonstrated that decreased indirect basal ganglia pathway activity was linked to the increased expression of repetitive behaviors in deer mice. Moreover, these behaviors were attenuated by selective activation of striatal indirect pathway neurons via co-administration of adenosine A2A and A1 receptor agonists (Tanimura et al., 2010a).
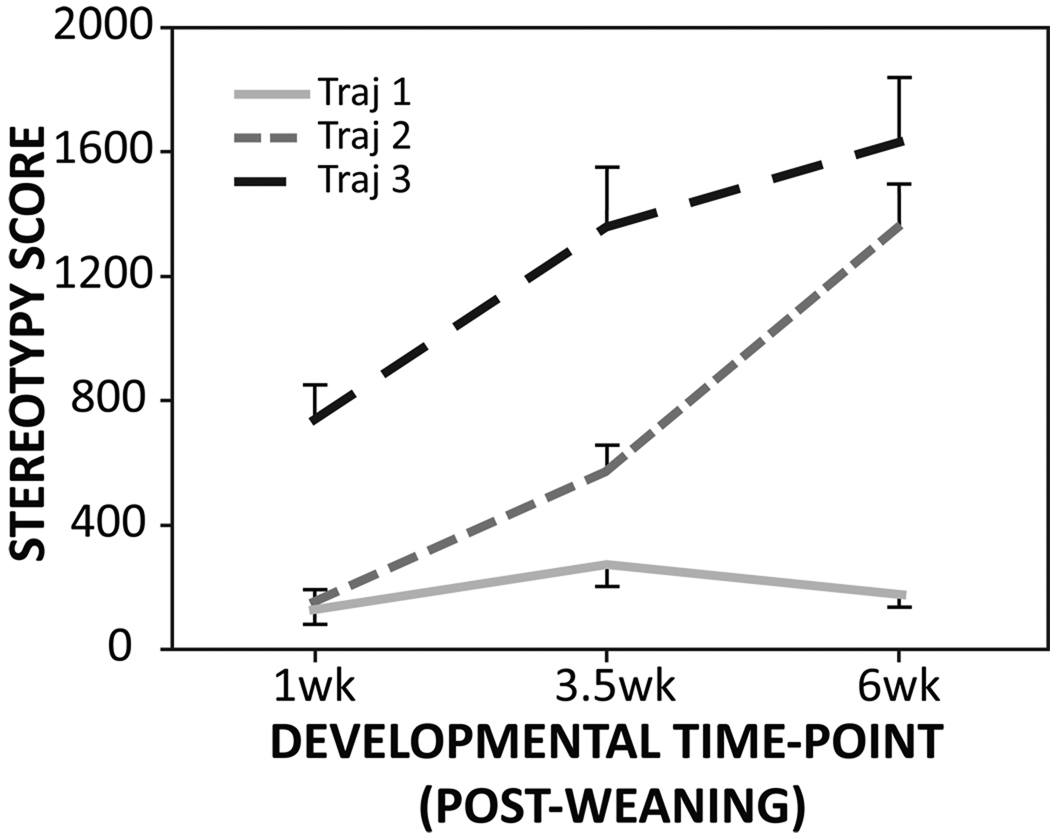
In the present study we sought to assess the role of alterations in indirect pathway activity in the development of repetitive motor behavior. This assessment builds on our recent application of group-based trajectory modeling (PROC Traj; Jones et al., 2001; Jones and Nagin, 2007) of repetitive behavior, which demonstrated three distinct developmental trajectory groups (Tanimura et al., 2010b). This analysis involved weekly assessments starting at 1 week post-weaning of a large number of mice and established that asymptotic levels of repetitive behavior were reached at approximately 6 weeks post-weaning. Importantly, three qualitatively different trajectory groups were identified: Traj 1 mice (11%) maintained low rates of stereotypy throughout development; Traj 2 mice (41%) moved from low to high rates of stereotypy across development, and Traj 3 mice (48%) already showed high rates of stereotypy at 1 week post-weaning with little developmental change.
Based on these results, we selected three developmental time-points (1, 3.5, and 6 weeks post-weaning) for assessment of neuronal activation. In addition, we compared indirect pathway activity among the three trajectory groups at 6 weeks post-weaning. As in Tanimura et al. (2010a), activity of the indirect pathway was indexed by neuronal metabolic capacity of STN using cytochrome oxidase (CO) histochemistry. We predicted that the CO activity in STN would decrease from 1 week to 6 weeks post-weaning. In addition, we predicted that the three distinct developmental trajectory groups would exhibit differential patterns of CO activity in STN.
Development of repetitive behavior may also involve differential activation of striatal compartments (striosomes and matrix) across time. In contrast to the matrix, which gives rise to the direct and indirect basal ganglia pathways, striosomes, which comprise approximately 15% of striatal medium spiny neurons, receive input preferentially from limbic cortical areas (e.g., orbitofrontal cortex, anterior cingulate/posterior medial PFC) and, in turn, project to SN pars compacta (SNpc) (Eblen and Graybiel, 1995).
Canales and Graybiel (2000) reported that chronic intermittent administration of psychostimulants induced preferential activation of striosomes over the extrastriosomal matrix, an outcome highly correlated with the development of drug-induced repetitive behavior. In addition, some evidence suggests that striosomes may mediate reinforcement (White and Hiroi, 1998), whereas the matrix may mediate naturalistic species-typical behaviors (Brown et al., 2002), and disruptions of balanced activity between these compartments may be associated with expression of compulsive, repetitive behaviors. Moreover structural and functional changes of striosomes were recently found in patients with Huntington’s chorea (Tippett et al., 2007) and in animal models of movement disorder-related neurological diseases (Crittenden et al., 2009; Lawhorn et al., 2008; Sato et al., 2008). Because high rates of stereotyped behaviors in deer mice likely compete with naturalistic behaviors, we hypothesized that a relative increase of striosomal activity largely due to decreased matrix activity would be linked to the development of stereotypy. Thus in addition to STN, we assessed CO activity of striosomes and surrounding matrix.
Methods
Subjects
All deer mice (Peromyscus maniculatus) were obtained from the breeding colony maintained in our laboratory, and kept on a 16:8-h light/dark cycle with lights off at 10:00 AM. Rodent chow and water were available ad libitum. The room was maintained at 20–25°C and 50–70% humidity. Mice sharing the same weaning date were group-caged (5–6 mice/cage) at weaning (PND21) in standard rodent cages (48 × 27 × 15 cm) and they remained in the same cage group throughout the experiment. All procedures were performed in accordance with the guidelines set forth in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Florida Institutional Animal Care and Use Committee.
Stereotypy Assessment
Deer mice were tested for stereotypy at 1, 3.5, and 6 weeks post-weaning using a modified automated photocell detection apparatus (Columbus Instruments). Each of these three sessions consisted of the eight hours of the dark cycle. Mice were individually placed in testing cages (22 × 28 × 25 cm) made of Plexiglas and habituated for one hour prior to the beginning of the dark cycle. Food and water were provided.
Measurement of stereotypy was done through the use of photobeams positioned (13.5cm above the floor) which were interrupted by the vertical motion of jumping and somersaulting but not by rearing. Each photobeam interruption (individual stereotyped response) was recorded automatically as a stereotypy count and these counts were expressed as the number of counts per hour. In addition, all sessions were digitally video-recorded for identification of behavioral topographies and to insure accuracy of the automated counters. Frequency counts generated by an observer viewing random video samples confirmed the accuracy of the automated counts.
Following behavioral testing at each of three developmental time-points, a randomly selected sample of animals was killed and their brains were collected for histochemical assay (n=13, n=14, and n=25 for the 1, 3.5, and 6 week post-weaning time points respectively) (Figure 1). For the animals selected at 6 weeks post-weaning, trajectory group membership (Traj 1, 2, or 3) was determined (n=8, n=12, n=5 respectively) based on their stereotypy scores (counts per hour) at those three time-points (Figure 2) using cluster analysis. This analysis confirmed the three trajectory groups.
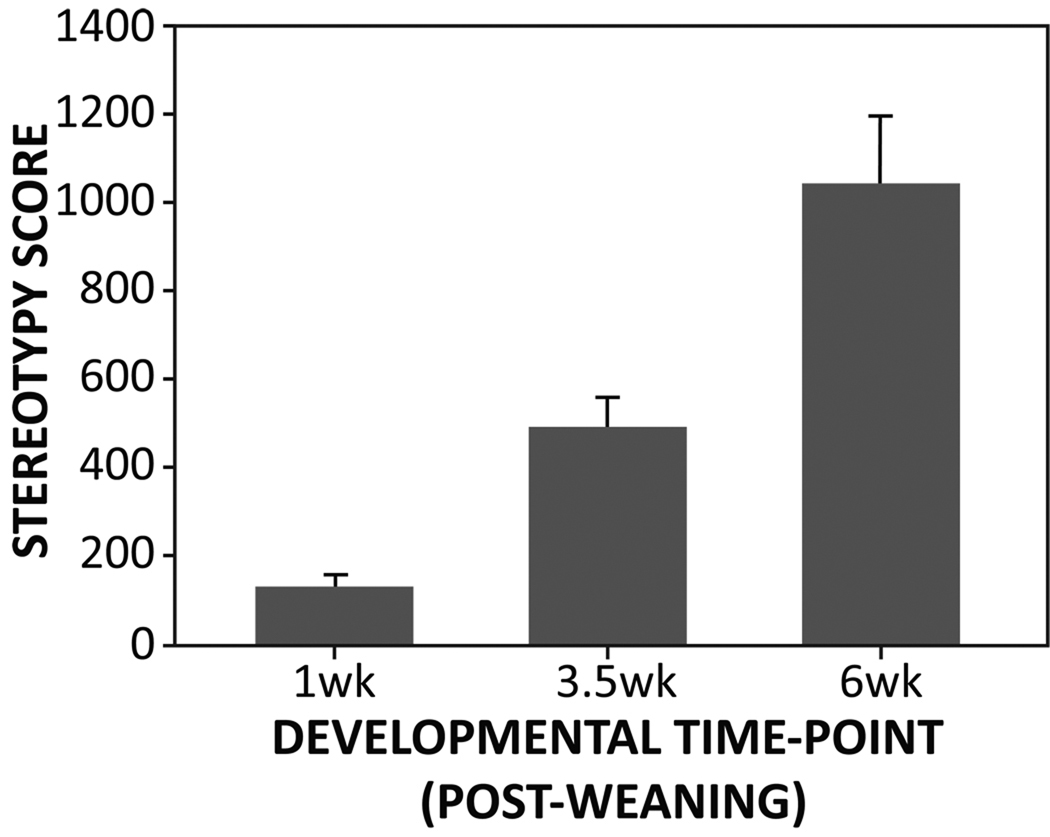
Figure 1.
The development of stereotypy assessed at three developmental time-points. Each bar represents separate sets of animals sacrificed at the respective time-points (mean + SEM).
Figure 2.
The three developmental trajectories of animals whose brains were harvested at 6 weeks post-weaning.
CO histochemistry and MOR1 immunohistochemistry
The CO spectrophotometric analysis and staining assay was carried out according to the Gonzalez-Lima protocol (1998) as described previously (Tanimura et al., 2010a). Briefly, however, sections were cut sagittally at 20µm at −20°C starting approximately 2.7 mm lateral to the midline and collected every 100µm for both hemispheres. Standards were cut at five thicknesses (20, 30, 40, 50, and 60µm) and assayed with other brain sections. The CO staining was quantified by densitometric analysis using ImagePro (Media Cybernetics), and standards were used to convert optical density values into enzymatic activity values (µmol/min/g). Multiple optical density readings were made per animal for STN as well as for other areas comprising cortico-basal ganglia circuitry (motor cortex, striatum, SN pars reticulata (SNpr), SNpc) and negative controls (hippocampus, somatosensory cortex) with number of sections varying by size of brain region. Dorsal and ventral aspects of the striatum were defined by horizontal bisection into approximately equivalent halves, whereas medial versus lateral aspects were defined by approximately <2.0mm or >2.0mm from the midline respectively. Under our experimental conditions, the staining intensity was highly correlated to the thickness of the standard sections (r =0.95).
This method has been used to identify sustained alterations in neuronal activity (Hevner and Wong-Riley, 1989; Sakata et al., 2005) as CO activity reflects oxidative metabolic capacity of neurons. Stereotyped behaviors in deer mice are likely due to chronic, experience-dependent neurobiological changes (Tanimura et al., 2010a,b; Turner et al., 2002). Thus this method was chosen over other approaches such as 2-DG autoradiography or c-Fos immunohistochemistry as these methods are more suited to rapid onset, transient effects.
The brain sections adjacent to CO-stained sections were used to identify striosomes by labeling the µ opioid receptor (MOR1). For this assay, tissue was fixed on slides with 4% paraformaldehyde, rinsed 3× with 0.01M PBS, incubated with 3% hydrogen peroxide, rinsed 3× with PBS, incubated with 5% normal goat serum for 30min, and incubated with MOR1 antibody (Abcam) at a dilution of 1:1500 in PBS with 0.2% TritonX-100 and 0.1% sodium azide overnight at 4°C. The sections were rinsed 3× with PBS, incubated with biotinylated secondary antibody (goat anti-rabbit, Millipore) at a dilution of 1:300 in PBS with TritonX-100 for 2hrs, rinsed 3× with PBS, incubated with avidin-biotin-peroxidase complex (Sigma) at a dilution of 1:300 in PBS with TritonX-100 for 2hrs, and rinsed 3× with PBS. The sections were visualized by 3,3-diaminobenzidene (Sigma) with nickel intensification, dehydrated in successive ethanol, cleaned in xylene, and coverslipped.
Image analysis
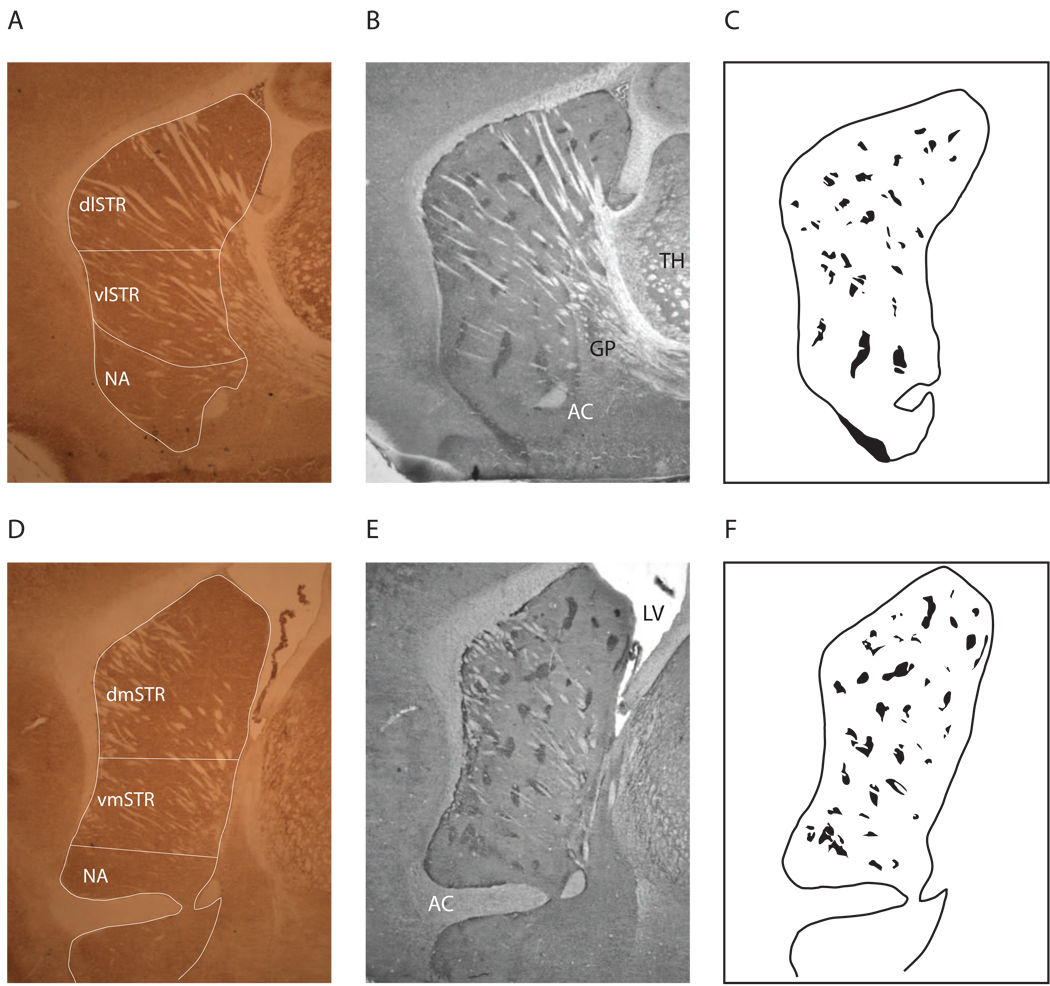
CO activity was quantified by densitometric analysis using ImagePro (Media Cybernetics), and standards were used to convert optical density values into enzymatic activity values (µmol/min/g). This measure of neuronal capacity was used to assess differences in STN and other brain regions (e.g., striatum, nucleus accumbens, motor cortex, hippocampus). The optical density readings for striosome and matrix (sampled in the area adjacent to striosome, excluding passing fibers) in the striatum were made by superimposing the MOR1-stained sections onto the CO-stained sections (Figure 3). Multiple optical density readings were made per animal. Striosomal volume was also calculated using ImagePro and an index of striosomal to matrix preferential (ISMP; Canales and Graybiel, 2000) activation was calculated by dividing CO activity in striosomes by CO activity in matrix. Striosomal regions were outlined and striosomal volumes were automatically calculated using ImagePro.
Figure 3.
CO-stained sections (A,D), MOR1-stained adjacent sections (B,E), and identified striosomes (C,F). MOR1-stained sections were superimposed onto the adjacent CO-stained sections to measure striosome and matrix optical density. dlSTR: dorsolateral striatum; dmSTR: dorsomedial striatum; vlSTR: ventrolateral striatum; vmSTR: ventromedial striatum; AC: anterior commissure; GP: globus pallidus; LV: lateral ventricle; TH: thalamus.
Statistical analysis
Differences in CO activity across the three developmental time-points were assessed by one-way ANCOVA, using assay as the covariate, with differences in STN and ISMP being the primary comparisons of interest. Additional differences in other brain regions were also examined but as these were more exploratory and given the relatively small sample sizes, corrections for multiple comparisons were not employed. In addition, at 6 weeks post-weaning, differences in CO activity among the three trajectory groups were also assessed by one-way ANCOVA with assay as the covariate. The association between the frequency of stereotypies and CO activity was analyzed by Pearson correlation. The striosomal volume across development and among the trajectories was also assessed by ANOVA. All tests were two-tailed and effects were considered significant when p<0.05.
Results
Stereotypy
Average stereotypy scores from animals killed at each developmental time-point are depicted in Figure 1. As previously shown (Tanimura et al., 2010b), rates of stereotypy were generally low at 1 week post-weaning and increased gradually over development (F(2,49)=13.6, p<0.001). Post-hoc pair-wise comparisons indicated that stereotypy scores at each of the three developmental periods all differed significantly from each other (p<0.001). In addition, animals killed at 6 weeks post-weaning were grouped into three developmental trajectories (Traj 1, 2, and 3) based on their stereotypy scores from all three time-points (1, 3.5, and 6 weeks post-weaning). As reflected in Figure 2, a significant difference across trajectory groups was found (F(2,22)=27.0, p<0.001) with post-hoc comparisons showing that all three trajectory groups differed significantly from each other (p<0.001).
CO activity across development
CO enzymatic activity at each developmental time-point is summarized in Table 1. Contrary to our hypothesis, no significant difference was found in STN across development (F(2,44)=2.6, p=0.07) despite the increase in stereotypy scores. Significant differences across development were found for the ventromedial striatum (F(2,44)=3.4, p=0.04) and SNpc (F(2,44)=8.6, p=0.001). In the case of the ventromedial striatum, CO activity at 1 week post-weaning was significantly lower than at 3.5 or 6 weeks post-weaning. In the case of SNpc, there was a decrease in CO activity across development with significant differences found between 1 and 6 weeks post-weaning and 3.5 and 6 weeks post-weaning. The difference between 1 and 3.5 weeks post-weaning failed to reach significance (p=0.07).
Table 1.
CO activity (µmol/min/g) of STN and the other brain areas in deer mice at three developmental time-points. Values expressed are group means with SEM in parentheses.
| 1wk | 3.5wk | 6wk | P-value | |
|---|---|---|---|---|
| Subthalamic Nucleus | 66.9 (1.09) | 66.3 (0.88) | 64.0 (0.96) | 0.07 |
| Motor Cortex | 49.2 (0.79) | 50.3 (0.69) | 49.1 (0.55) | 0.69 |
| Somatosensory Cortex | 49.6 (0.63) | 51.2 (0.64) | 50.3 (0.59) | 0.32 |
| Hippocampus | 40.2 (0.45) | 41.4 (0.57) | 40.6 (0.52) | 0.40 |
| Dorsolateral Striatum | 47.5 (1.09) | 49.2 (1.21) | 48.9 (0.71) | 0.12 |
| Dorsomedial Striatum | 48.0 (0.74) | 49.9 (0.74) | 49.9 (0.56) | 0.08 |
| Ventrolateral Striatum | 48.8 (1.60) | 50.3 (1.08) | 49.1 (0.68) | 0.37 |
| Ventromedial Striatum | 47.0 (0.80) | 49.2 (0.80) | 48.9 (0.55) | 0.04 |
| Nucleus Accumbens | 49.6 (0.99) | 52.8 (0.97) | 51.6 (0.73) | 0.10 |
| Substantia Nigra pars Compacta | 47.2 (0.84) | 45.0 (1.38) | 44.5 (0.79) | 0.001 |
| Substantia Nigra pars Reticulata | 50.7 (0.90) | 49.9 (1.49) | 49.6 (0.96) | 0.07 |
To evaluate the relationship between stereotypy and CO activity at each time-point more closely, animals killed at 1 and 3.5 weeks post-weaning were divided into low- and high-stereotypy groups using a median split based on their stereotypy score. This secondary analysis was done as we thought the median split might discriminate trajectory group 3 (already high at 1 week post-weaning) from trajectory groups 1 and 2 (low at 1 week post-weaning) (see Figure 2). There was no difference, however, between these groups in STN CO activity, an outcome found in other brain regions as well. In addition, for animals killed at 3.5 weeks post-weaning, CO activity in STN was also analyzed based on the median split in the change in stereotypy counts from 1 to 3.5 weeks post-weaning. We thought that this secondary analysis might have discriminated Traj 2 mice (higher change scores expected) from the Traj 1 and 3 groups (lower change scores expected). Despite the prediction that lower CO activity in STN would be associated with the greater change in stereotypy score, no such association was found by either group comparison or correlation.
CO activity in trajectory groups
Animals killed at 6 weeks post-weaning were grouped into three developmental trajectories (Traj 1, 2, and 3). The CO enzymatic activity of these trajectory groups is summarized in Table 2. A significant difference across trajectory groups was found in STN with Traj 2 and 3 mice showing lower CO activity in STN compared to Traj 1 mice (F(2,22)=13.3, p<0.001). Statistical differences were also found among the trajectory groups in the nucleus accumbens (NA) (F(2,22)=4.8, p=0.02), dorsolateral striatum (F(2,22)=3.5, p=0.05), ventrolateral striatum (F(2,22)=4.4, p=0.03), and SNpc (F(2,22)=7.6, p=0.004). In all these areas, Traj 1 mice showed higher CO activity compared to Traj 2 and 3 mice. Moreover, stereotypy scores at 6 weeks post-weaning were negatively correlated with STN (r=−0.68, p<0.001), NA (r=−0.50, p=0.01), SNpc (r=−0.68, p<0.001), and SNpr (r=−0.54, p<0.01) (Table 3).
Table 2.
CO activity (µmol/min/g) of STN and the other brain areas in three developmental trajectories at 6 weeks post-weaning. Values expressed are group means with SEM in parentheses.
| Traj 1 | Traj 2 | Traj 3 | P-value | |
|---|---|---|---|---|
| Subthalamic Nucleus | 69.1 (1.21) | 61.5 (0.99) | 61.7 (0.85) | <0.001 |
| Motor Cortex | 50.5 (1.20) | 48.2 (0.67) | 48.8 (0.85) | 0.25 |
| Somatosensory Cortex | 51.8 (1.22) | 48.9 (0.67) | 51.3 (1.08) | 0.13 |
| Hippocampus | 40.6 (1.37) | 40.8 (0.61) | 40.1 (0.50) | 0.86 |
| Dorsolateral Striatum | 50.9 (1.30) | 47.4 (0.65) | 49.8 (2.12) | 0.05 |
| Dorsomedial Striatum | 51.4 (1.13) | 49.2 (0.73) | 48.8 (0.96) | 0.26 |
| Ventrolateral Striatum | 51.3 (1.19) | 47.7 (0.70) | 49.4 (1.95) | 0.03 |
| Ventromedial Striatum | 51.3 (1.19) | 48.0 (0.66) | 47.9 (0.88) | 0.09 |
| Nucleus Accumbens | 54.9 (1.51) | 50.3 (0.69) | 49.7 (0.62) | 0.02 |
| Substantia Nigra pars Compacta | 47.6 (1.21) | 43.9 (1.01) | 40.8 (0.47) | 0.004 |
| Substantia Nigra pars Reticulata | 52.0 (1.46) | 49.8 (1.42) | 45.4 (1.14) | 0.06 |
Table 3.
Correlation between stereotypy score and CO activity (µmol/min/g) in three developmental trajectories at 6 weeks post-weaning.
| r | P-value | |
|---|---|---|
| Subthalamic Nucleus | −0.67 | <0.001 |
| Motor Cortex | −0.18 | 0.40 |
| Somatosensory Cortex | −0.12 | 0.56 |
| Hippocampus | 0.13 | 0.53 |
| Dorsolateral Striatum | −0.19 | 0.37 |
| Dorsomedial Striatum | −0.13 | 0.47 |
| Ventrolateral Striatum | −0.23 | 0.28 |
| Ventromedial Striatum | −0.22 | 0.28 |
| Nucleus Accumbens | −0.50 | 0.01 |
| Substantia Nigra pars Compacta | −0.68 | <0.001 |
| Substantia Nigra pars Reticulata | −0.54 | <0.01 |
Striosome-matrix CO activity
There were significant developmental effects on CO enzymatic activity such that metabolic activity in the matrix increased as animals developed (F(2,36)=3.7, p=0.04), whereas no statistically significant developmental change was observed in striosomes (F(2,36)=2.4, p=0.10) (Table 4). Importantly, there was no significant difference across development in the ratio or index of striosome to matrix predominance (ISMP) (F(2,36)=0.68, p=0.51).
Table 4.
CO activity (µmol/min/g) of striosome and matrix in deer mice at three developmental time-points (mean ± SEM in parentheses).
| 1wk | 3.5wk | 6wk | P-value | |
|---|---|---|---|---|
| Striosome | 51.8 (1.61) | 53.0 (0.99) | 54.7 (0.64) | 0.10 |
| Matrix | 50.6 (2.45) | 52.4 (1.01) | 53.3 (0.72) | 0.04 |
| ISMP | 0.9837 (0.0157) | 0.9744 (0.0070) | 0.9706 (0.0456) | 0.51 |
At 6 weeks post-weaning, there was no difference among the trajectory groups in CO activity in striosome (F(2,21)=0.6, p=0.54), matrix (F(2,21)=1.0, p=0.41), or ISMP (F(2,21)=2.3, p=0.13) (Table 5). High- versus low-stereotypy mice based on the median split of their stereotypy scores were also not statistically different on these CO measures at either 1 or 3.5 weeks post-weaning.
Table 5.
CO activity (µmol/min/g) of striosome and matrix in three developmental trajectories at 6 weeks post-weaning (mean ± SEM in parentheses).
| Traj 1 | Traj 2 | Traj 3 | P-value | |
|---|---|---|---|---|
| Striosome | 55.0 (1.09) | 53.9 (0.68) | 55.7 (2.19) | 0.54 |
| Matrix | 56.0 (1.15) | 55.8 (0.74) | 58.1 (2.22) | 0.41 |
| ISMP | 0.9828 (0.0063) | 0.9672 (0.0074) | 0.9584 (0.0046) | 0.13 |
There was a general increase in striosomal volume over development (F(2,31)=15.7, p<0.001), although no association with trajectory was found at 6 weeks post-weaning (F(2,39)=0.2, p=0.78). The relative striosome volume and the overall striatal volume were also not associated with stereotypy at any of the three time-points.
Discussion
The present experiments tested the hypothesis based on Tanimura et al. (2010a) that the development of spontaneous stereotypy in deer mice is linked to a gradual decrease in the activity of the indirect basal ganglia pathway. CO activity in STN, our primary dependent measure, did not differ significantly across development, although a decrease was observed at the last developmental time point. This was also the case with the significant differences found in the SNpc. If reliable, this may suggest that neuronal activation changes in the indirect pathway may lag rather than lead stereotypy.
When CO activity in STN at 6 weeks post-weaning was analyzed by trajectory group, the difference was apparent; animals in the Traj 2 and 3 groups showed significantly lower levels of CO activity in STN compared to animals in the Traj 1 group. This finding suggests that the development of stereotypy is linked to decreased indirect pathway activity but only in those animals that develop high rates of such behavior. Consistent with our previous finding in adult mice, other regions such as NA and SN showed higher CO activity in Traj 1 animals as compared to Traj 2 and 3 animals at 6 weeks post-weaning. The decreased activity in SNpc and SNpr, although the latter failed to reach statistical significance (p=0.06) is expected due to the monosynaptic glutamatergic connections from STN to these areas (Chergui et al., 1994; Hammond et al., 1978; Kita and Kitai, 1987; Shimo and Wichmann, 2009).
It is noteworthy that trajectory group differences were found in NA and the ventrolateral striatum. The ventral striatum in non-human primates is heavily innervated from the prefrontal cortices such as medial prefrontal cortex and orbitofrontal cortex (Calzavara et al., 2007; Haber et al., 2006). In rats, tracer studies showed that cortico-striatal projections originating in medial prefrontal cortex terminate in NA (e.g., Berendse et al., 1992; Gabbott et al., 2005), whereas fibers originating from the motor cortex innervate the dorsolateral striatum (e.g., Christensen et al., 1999; Ebrahimi et al., 1992; Gorelova and Yang, 1997). Abnormalities of these prefrontal cortical regions have been implicated in compulsive behaviors as well as impaired cognitive functions (e.g., cognitive flexibility, set-shifting, planning) in humans and animals (e.g., Chudasama et al., 2003; Evans et al., 2004; Joel et al., 2005). These results complement our previous finding that high rates of motor stereotypy in deer mice were associated with cognitive inflexibility in a reversal learning task (Tanimura et al., 2008) and lower ventromedial striatal CO activity in high-stereotypy mice, (Tanimura et al., 2010a) and suggest that spontaneously emitted motor stereotypy in this animal model may develop due to alterations of cortico-basal ganglia circuitry involving the prefrontal cortices.
Aggregation of developmental changes and trajectory group differences in CO activity as well as the correlations of CO activity with behavior points to several key brain regions in the mediation of stereotypy. These include STN, NA, SNpc, and SNpr. The pattern of results in these areas reflects decreased neuronal activation across development (coincident with increasing repetitive behavior), higher neuronal activation in mice that do not develop high levels of repetitive behavior, and strong negative associations between stereotypy levels and neuronal metabolic activation. In our previous work (Tanimura et al., 2010a), decreased CO activity in STN, SNpc, and SNpr were also associated with stereotypy. Taken together, these results plus our previous pharmacological findings indicate an important role for decreased indirect pathway activity in the development of stereotypy.
The second part of the present study examined the association between the striosomal pathway and the development and expression of stereotypy in deer mice. Our hypotheses came from the finding by Canales and Graybiel (2000) that preferential activation of striosomes versus matrix was observed following chronic administration of psychostimulants (apomorphine, amphetamine, and cocaine), which induced repetitive behaviors in animals. The stereotypy seen in deer mice is linked to chronic exposure to a restricted environment with associated brain changes (Presti and Lewis, 2005; Turner et al., 2002). Thus, we assessed whether stereotypy in deer mice shares a common underlying mechanism with stereotypy typical of the psychostimulant sensitization model. In addition, environmental restriction, which limits animals’ expression of many species-typical behaviors, suggests that matrix activity may also be decreased (e.g., Brown et al., 2002). Contrary to our hypothesis, the present study showed that the striosomal/matrix metabolic activity, volume, and cell counts (data not shown) were not associated with stereotypy in deer mice. Thus, it is unlikely that a preferential increase in striosomal activation is linked to development of repetitive behaviors in deer mice. To complement this finding, intermittent amphetamine administration (sensitization) in young and adult deer mice did not exacerbate the development and expression of stereotypy, despite being behaviorally sensitized as conventionally assessed (Tanimura et al., 2009). Although we did not directly assess neuronal activation (e.g., c-fos) within striosome vs. matrix as a result of amphetamine sensitization, these results support our present finding that the differential activation of striosome and matrix does not play a significant role in the development of spontaneous stereotypy.
Lastly, the present experiment utilized the novel approach of assessing differences in measures of neurobiological activity as a function of developmental trajectory. The group-based trajectory modeling described in Tanimura et al. (2010b) allowed us to categorize animals into three groups based on their longitudinal behavioral data and assess the neurochemical changes over development. This approach proved satisfactory in evaluating mice at 6 weeks post-weaning whereas developmental trajectories of animals killed at 1 and 3.5 weeks post-weaning could not be satisfactorily determined using the trajectory modeling approach. More frequent behavioral assessments earlier in development would potentially have permitted earlier categorization of animals by trajectory group (e.g., at 3.5 weeks post-weaning). This would allow us in future studies to refine our ability to test the relative importance of the indirect basal ganglia versus striosomal pathways in the mediation of the development and expression of repetitive behavior
Acknowledgement
This work was supported by NIH grant MH080055.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baup N, Grabli D, Karachi C, Mounayar S, Francois C, Yelnik J, Feger J, Tremblay L. High-frequency stimulation of the anterior subthalamic nucleus reduces stereotyped behaviors in primates. J. Neurosci. 2008;28:8785–8788. doi: 10.1523/JNEUROSCI.2384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Brown LL, Feldman SM, Smith DM, Cavanaugh JR, Ackermann RF, Graybiel AM. Differential metabolic activity in the striosome and matrix compartments of the rat striatum during natural behaviors. J. Neurosci. 2002;22:305–314. doi: 10.1523/JNEUROSCI.22-01-00305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzavara R, Mailly P, Haber SN. Relationship between the corticostriatal terminals from areas 9 and 46, and those from area 8A, dorsal and rostral premotor cortex and area 24c: an anatomical substrate for cognition to action. Eur. J. Neurosci. 2007;26:2005–2024. doi: 10.1111/j.1460-9568.2007.05825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales JJ, Graybiel AM. A measure of striatal function predicts motor stereotypy. Nat. Nurosci. 2000;3:377–383. doi: 10.1038/73949. [DOI] [PubMed] [Google Scholar]
- Chergui K, Akaoka H, Charlety PJ, Saunier CF, Buda M, Chouvet G. Subthalamic nucleus modulates burst firing of nigral dopamine neurones via NMDA receptors. Neuroreport. 1994;5:1185–1188. doi: 10.1097/00001756-199406020-00006. [DOI] [PubMed] [Google Scholar]
- Christensen J, Sorensen JC, Ostergaard K, Zimmer J. Early postnatal development of the rat corticostriatal pathway: an anterograde axonal tracing study using biocytin pellets. Anat. Embryol. 1999;200:73–80. doi: 10.1007/s004290050261. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav. Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Crittenden JR, Dunn DE, Merali FI, Woodman B, Yim M, Borkowska AE, Frosch MP, Bates GP, Housman DE, Lo DC, Graybiel AM. CalDAG-GEFI down-regulation in the striatum as a neuroprotective change in Huntington's disease. Hum. Mol. Genet. 2009;19(9):1756–1765. doi: 10.1093/hmg/ddq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eblen F, Graybiel AM. Highly restricted origin of prefrontal cortical inputs to striosomes in the macaque monkey. J. Neurosci. 1995;15(9):5999–6013. doi: 10.1523/JNEUROSCI.15-09-05999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi A, Pochet R, Roger M. Topographical organization of the projections from physiologically identified areas of the motor cortex to the striatum in the rat. Neurosci. Res. 1992;14:39–60. doi: 10.1016/s0168-0102(05)80005-7. [DOI] [PubMed] [Google Scholar]
- Evans DW, Lewis MD, Iobst E. The role of the orbitofrontal cortex in normally developing compulsive-like behaviors and obsessive-compulsive disorder. Brain Cognition. 2004;55:220–234. doi: 10.1016/S0278-2626(03)00274-4. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J. Comp. Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima, Jones . Cytochrome oxidase in neuronal metabolism and Alzheimer's disease. New York and London: Plenum Press; 1998. [Google Scholar]
- Gorelova N, Yang CR. The course of neural projection from the prefrontal cortex to the nucleus accumbens in the rat. Neuroscience. 1997;76:689–706. doi: 10.1016/s0306-4522(96)00380-6. [DOI] [PubMed] [Google Scholar]
- Grabli D, McCairn K, Hirsch EC, Agid Y, Feger J, Francois C, Tremblay L. Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain. 2004;127:2039–2054. doi: 10.1093/brain/awh220. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–R511. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. J. Neurosci. 2006;26:8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Deniau JM, Rizk A, Feger J. Electrophysiological demonstration of an excitatory subthalamonigral pathway in the rat. Brain Res. 1978;151:235–244. doi: 10.1016/0006-8993(78)90881-8. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Wong-Riley MT. Brain cytochrome oxidase: purification, antibody production, and immunohistochemical/histochemical correlations in the CNS. J. Neurosci. 1989;9:3884–3898. doi: 10.1523/JNEUROSCI.09-11-03884.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, Wasserman S, Soorya L, Buchsbaum M. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol. Psychiat. 2005;58:226–232. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Joel D, Doljansky J, Schiller D. 'Compulsive' lever pressing in rats is enhanced following lesions to the orbital cortex, but not to the basolateral nucleus of the amygdala or to the dorsal medial prefrontal cortex. Eur. J. Neurosci. 2005;21:2252–2262. doi: 10.1111/j.1460-9568.2005.04042.x. [DOI] [PubMed] [Google Scholar]
- Jones B, Nagin D. Advances in group-based trajectory modeling and a SAS procedure for estimating them. Sociological Methods and Research. 2007;35(4):542–571. [Google Scholar]
- Jones B, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods and Research. 2001;29:374–393. [Google Scholar]
- Kita H, Kitai ST. Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. J. Comp. Neurol. 1987;260:435–452. doi: 10.1002/cne.902600309. [DOI] [PubMed] [Google Scholar]
- Klavir O, Flash S, Winter C, Joel D. High frequency stimulation and pharmacological inactivation of the subthalamic nucleus reduces 'compulsive' lever-pressing in rats. Exp. Neurol. 2009;215:101–109. doi: 10.1016/j.expneurol.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Lawhorn C, Smith DM, Brown LL. Striosome-matrix pathology and motor deficits in the YAC128 mouse model of Huntington's disease. Neurobiol. Dis. 2008;32:471–478. doi: 10.1016/j.nbd.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Bodfish JW. Repetitive behavior diosrders in autism. Ment. Retard. Dev. Dis. 1998;4:80–89. [Google Scholar]
- O'Sullivan RL, Rauch SL, Breiter HC, Grachev ID, Baer L, Kennedy DN, Keuthen NJ, Savage CR, Manzo PA, Caviness VS, Jenike MA. Reduced basal ganglia volumes in trichotillomania measured via morphometric magnetic resonance imaging. Biol. Psychiat. 1997;42:39–45. doi: 10.1016/S0006-3223(96)00297-1. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, Leckman JF, Gore JC. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch. Gen. Psychiat. 1998;55:326–333. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Presti MF, Gibney BC, Lewis MH. Effects of intrastriatal administration of selective dopaminergic ligands on spontaneous stereotypy in mice. Physiol. Behav. 2004;80:433–439. doi: 10.1016/j.physbeh.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Presti MF, Lewis MH. Striatal opioid peptide content in an animal model of spontaneous stereotypic behavior. Behav. Brain Res. 2005;157:363–368. doi: 10.1016/j.bbr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Sakata JT, Crews D, Gonzalez-Lima F. Behavioral correlates of differences in neural metabolic capacity. Brain Res. Rev. 2005;48:1–15. doi: 10.1016/j.brainresrev.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Sato K, Sumi-Ichinose C, Kaji R, Ikemoto K, Nomura T, Nagatsu I, Ichinose H, Ito M, Sako W, Nagahiro S, Graybiel AM, Goto S. Differential involvement of striosome and matrix dopamine systems in a transgenic model of dopa-responsive dystonia. P. Natl. Acad. Sci. U.S.A. 2008;105:12551–12556. doi: 10.1073/pnas.0806065105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Prog. Neuro-Psychoph. 1999;23:613–624. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Shimo Y, Wichmann T. Neuronal activity in the subthalamic nucleus modulates the release of dopamine in the monkey striatum. Eur. J. Neurosci. 2009;29:104–113. doi: 10.1111/j.1460-9568.2008.06565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura Y, Ogoegbunam FC, Lewis MH. Amphetamine-induced sensitization and spontaneous stereotypy in deer mice. Pharmacol. Biochem. Be. 2009;92:670–675. doi: 10.1016/j.pbb.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura Y, Vaziri S, Lewis MH. Indirect basal ganglia pathway mediation of repetitive behavior: attenuation by adenosine receptor agonists. Behav. Brain Res. 2010a;210:116–122. doi: 10.1016/j.bbr.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura Y, Yang MC, Lewis MH. Procedural learning and cognitive flexibility in a mouse model of restricted, repetitive behaviour. Behav. Brain Res. 2008;189:250–256. doi: 10.1016/j.bbr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Tanimura Y, Yang MC, Ottens AK, Lewis MH. Development and temporal organization of repetitive behavior in an animal model. Dev. Psychobio. 2010b;52:813–824. doi: 10.1002/dev.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippett LJ, Waldvogel HJ, Thomas SJ, Hogg VM, van Roon-Mom W, Synek BJ, Graybiel AM, Faull RL. Striosomes and mood dysfunction in Huntington's disease. Brain. 2007;130:206–221. doi: 10.1093/brain/awl243. [DOI] [PubMed] [Google Scholar]
- Turner CA, Yang MC, Lewis MH. Environmental enrichment: effects on stereotyped behavior and regional neuronal metabolic activity. Brain Res. 2002;938:15–21. doi: 10.1016/s0006-8993(02)02472-1. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM, Hiroi N. Preferential localization of self-stimulation sites in striosomes/patches in the rat striatum. P. Natl. Acad. Sci. U.S.A. 1998;95:6486–6491. doi: 10.1073/pnas.95.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter C, Flash S, Klavir O, Klein J, Sohr R, Joel D. The role of the subthalamic nucleus in 'compulsive' behavior in rats. Eur. J. Neurosci. 2008a;27:1902–1911. doi: 10.1111/j.1460-9568.2008.06148.x. [DOI] [PubMed] [Google Scholar]
- Winter C, Lemke C, Sohr R, Meissner W, Harnack D, Juckel G, Morgenstern R, Kupsch A. High frequency stimulation of the subthalamic nucleus modulates neurotransmission in limbic brain regions of the rat. Exp. Brain Res. 2008b;185:497–507. doi: 10.1007/s00221-007-1171-1. [DOI] [PubMed] [Google Scholar]