Abstract
According to the cancer stem cell hypothesis tumors are maintained by a cancer stem cell population which is able to initiate and maintain tumors. Tumor-initiating stem cells display stem or progenitor cell properties such as self-renewal and capacity to re-establish tumors that recapitulate the tumor of origin. In this paper, we discuss data relative to the presence of cancer stem cells in human renal carcinoma and their possible origin from normal resident stem cells. The cancer stem cells identified in human renal carcinomas are not derived from the normal CD133+ progenitors of the kidney, but rather from a more undifferentiated population that retains a mesenchymal phenotype. This population is able to self-renewal, clonogenicity, and in vivo tumor initiation. Moreover, they retain pluripotent differentiation capability, as they can generate not only the epithelial component of the tumor, but also tumor endothelial cells. This suggests that renal cancer stem cells may contribute to the intratumor vasculogenesis.
1. Introduction
Emerging evidence showed that the capacity of a tumor to grow and propagate resides in a small population of tumor low proliferating cells, termed cancer stem cells (CSCs) or tumor-initiating cells [1]: the cancer stem cell hypothesis suggests that tumors are maintained by a CSC population which is chemo- and radioresistant and therefore can persist after treatment, leading to tumor recurrence [2, 3].
A tumor-initiating population able to sustain and maintain the tumor growth was identified in the majority of solid tumors [4–14]. These stem-like cells are identified by their ability to give rise to new serially transplantable tumors when xenografted in small number into immunodeficient mice, and to display stem or progenitor cell properties. In particular, they are characterized by self-renewal and capacity to re-establish tumor that recapitulates the tumor of origin, thanks to the ability to undergo asymmetric division leading to the generation of a progeny that can differentiate to produce tumor cells with heterogeneous phenotype [3].
Renal carcinoma is a common form of urologic tumor, representing 3% of total human malignancies, with a high metastatic index at the diagnosis and a high rate of relapse because of the resistance to radiations and chemotherapies [15]. Due to the ability of CSCs to recapitulate the tumor, strategies of targeting CSCs should be taken into account. Recent encouraging data in other tumors have provided proof of principle that selective targeting of CSCs is possible [16–18].
In this paper, we will present the existing data of the literature on the role of stem cells in human renal carcinoma. Normal stem cells may participate to renal carcinomas, sustaining tumor growth and vascularization. In addition, tumor stem cells with tumor-initiating ability have been described (Table 1), suggesting the relevance of CSCs in generation, growth, and recurrence of renal carcinomas. Finally, evidences will be presented on the ability of renal CSCs to differentiate into endothelial cells and to participate to tumor vasculogenesis.
Table 1.
Different populations of stem cells identified in human renal carcinomas and role in tumor growth. N. D.: not done.
| Identification method | Role in carcinogenesis | References |
|---|---|---|
| Expression of CD105 | Tumor initiation Endothelial differentiation |
Bussolati et al. [36] |
| Expression of CD133 | Nontumor initiation Promotion of tumor growth and vascularization |
Bruno et al. [33] |
| Side population | N.D. | Addla et al. [37] Huges et al. [38] |
| Sphere formation | Tumor initiation Radio- and chemoresistance |
Zhong et al. [39] |
2. Cancer Stem Cell Hypothesis for Tumor Origin
The concept of a less differentiated, pluripotent cell forming cancer relies on common properties and phenotype of tumor stem cells and embryonic or adult normal stem cells. This could be due to acquirement of stem cell properties by fully differentiated proliferating cells following accumulation of critical mutations or alternatively to the origin of CSCs from mutated normal stem cells. Several evidences support both possibilities [19, 20]. It has been shown that the epithelial-to mesenchymal transition of tumors enhances the number of CSCs [21], supporting the notion that the dedifferentiation process may favor the stem-related phenotype of tumors. Indeed, recent studies showing that activation of critical embryonic genes or oncogenes may induce pluripotency in differentiated cells [22] further support this possibility.
Other evidences support the notion that tumorigenesis may originate from mutation of normal adult tissue resident stem cells or from deriving progenitor cells that have acquired mutations that enable them to escape from niche control [23]. Alternatively, deregulation of extrinsic factors within the niche might lead to uncontrolled proliferation of stem cells and tumorigenesis. This concept is supported first by the fact that stem cells have, or can, reacquire the self-renewal properties needed for maintaining and expanding stem cell numbers [24]. Second, the stem cell pool is anchored to the niche and is maintained through the entire life and therefore may have the time to acquire mutations [25]. This is particularly pertinent to cancers of continually renewing cell populations, such as those of the intestine, stomach, and skin [26, 27]. Under this light, it can be proposed that cancers may represent a maturation arrest of stem cells. Depending on the differentiative state of the mutated cell, from stem to progenitor to differentiated cells, CSCs may display different stem properties and malignancy.
3. Normal Renal Stem Cells
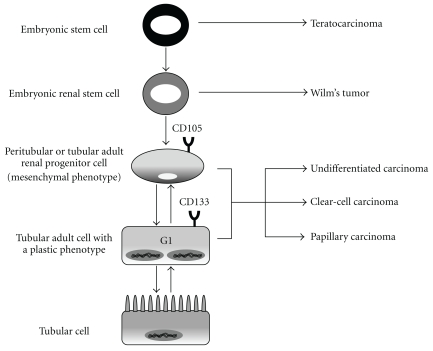
According to the hierarchical lineage view of tumors [20], renal tumors may arise from the embryonic stem cell compartment, giving rise to the Wilm's tumor, as well as from the stem cell pool of the adult kidney or from the deriving progenitors, giving rise to the different histologic types of carcinomas (undifferentiated, clear-cell, papillary carcinomas) (Figure 1). Embryonic stem cells have been described in the embryonic kidney until relatively late in gestation in the nephrogenic zone, from which they have been isolated and characterized [28]. Comparative analysis of embryonic stem cells and of the pediatric renal malignancy Wilm's tumor showed that Wilm's tumor resulted from a differentiation arrest of embryonic progenitors committed to the nephrogenic lineage [29]. These cells were present in the tumor both as undifferentiated blastema and as renal differentiated elements.
Figure 1.
Scheme representative of the possible stem cell origin of renal CSCs. Mutations/epigenetic alterations of the renal embryonic stem cells may originate the pediatric malignancy Wilm's tumor. The different histologic types of renal carcinomas of the adult may derive from mutations occurring in the stem cell compartment of the adult kidney. The lack of CD133+ marker in renal CSCs may support the idea of an origin from a yet unidentified mesenchymal population, which is ahead of CD133+ renal progenitors in the differentiation linage. Alternatively, a process of de-differentiation of renal progenitors or mature cells could be involved.
In the human adult kidney, CD133+ renal progenitor cells have been characterized by our group in the renal cortex from the tubule/interstitium [30]. In addition, multipotent CD133+/CD24+ stem cells have been isolated from the Bowman's capsule [31]. A genomic characterization of multipotent CD133+/CD24+ renal progenitor cells from glomeruli and tubules of adult human kidney revealed no significant differences in the gene expression patterns suggesting that tubular and glomerular renal progenitor cells represent a genetically homogeneous population [32]. Indeed, the presence of cytokeratin expression by the CD133+/CD24+ cells suggests an epithelial commitment that characterizes these cells as epithelial progenitors.
4. CD133+/CD34− Cells and Renal Carcinomas
Following the stem cell view of tumor generation, CD133 has been investigated as a marker for identification of CSCs in renal carcinomas. A small population of CD133+/CD34− cells (less than 1% of the total cells) was found in human renal carcinomas [33]. These cells, that represent a progenitor cell population in the normal kidney [30], showed the same mesenchymal phenotype and differentiative ability of their normal counterpart. They expressed CD73, CD44, CD29, the developmental renal marker PAX-2, and the mesenchymal marker vimentin and were able to differentiate both in endothelial and epithelial cells in vitro and in vivo [33]. When injected subcutaneously within Matrigel in immunodeficient mice, CD133+/CD34− stem cells were not able to form tumors suggesting that they did not represent the tumor-initiating cell population [33], at variance of CD133+ cells derived from brain, prostate and pulmonary tumors [5, 10, 13, 34]. The observation that CD133+/CD34− cells cultured in presence of the tumor supernatant differentiated in endothelial cells led to the idea that the tumor microenvironment could be involved in the endothelial commitment of these renal progenitors. Indeed, when cotransplanted with renal tumor cells, at a 1 : 100 ratio, CD133+/CD34− cells significantly enhanced tumor engraftment, growth, and vascularization, suggesting that these progenitors may produce a growth factor environment favoring tumor growth and vascularization [33]. Recently, D'Alterio et al. evaluated the expression of CD133 on samples of human renal clear cell carcinoma, showing that CD133 expression did not correlate to clinical pathological features or affected patients prognosis [35].
5. CD105+ Renal Cancer Stem Cells
We recently identified a population of CD105+ tumor-initiating cells in renal human carcinomas [36]. The mesenchymal origin of these cells is in accordance with the mesenchymal origin of the kidney and with the mesenchymal phenotype of stem cells found in normal rodent kidney as well as in the human embryo [40–42]. By magnetic cell sorting of specimens of human renal carcinomas, our laboratory isolated a subpopulation of cell expressing the mesenchymal marker CD105, representing less than 10% of the tumor mass. The CD105+ population induced tumors in SCID mice with 100% incidence, whereas the CD105− population induced tumors with only 10% incidence. CD105+ cells expressed markers characteristic of mesenchymal stem cells, such as CD44, CD90, CD146, CD73, CD29, and vimentin. Characterization of the phenotype of CD105+ clones revealed several stem cells properties, such as clonogenic ability, sphereformation in presence of nonadhesive cell culture medium, expression of stem cells markers (Nanog, Oct4, Musashi, Nestin, and the embryonic marker Pax2), lack of differentiative epithelial markers, in vitro differentiation on both endothelial and epithelial cell types [36]. In addition, in vivo they generated serially transplantable carcinomas containing a small population of undifferentiated CD105+ tumorigenic cells and differentiated CD105− tumor epithelial cells. Only the CD105+ population was able to propagate the tumor [36]. These data suggest that the CD105+ cells represent a tumor-initiating cell population that may originate from resident renal stem cells with mesenchymal characteristics. The lack of CD133 and CD24 expression, which are present in adult and embryonic renal progenitors [30, 31, 43] by tumor-initiating CD105+ clones, suggests that they are not derived from the CD133+ population. Moreover, CD133+ progenitors previously isolated from renal tumors did not express CD105 and were not tumorigenic [33].
6. Sphere-Derived CSCs
Generation of spheres is considered a useful culture system to select for CSCs. Cells growing as tumor spheres in serum-free medium supplemented with EGF and bFGF were isolated from the renal cell carcinoma cell line SK-RC-42. This sphere-forming population showed the ability of self-renewing in vitro and in vivo, higher mRNA expression levels of several “stemness” genes, stronger tumorigenicity and resistance to chemotherapeutic agents and irradiation compared with the monolayer adherent cells [39].
7. Side Population in Renal Carcinomas
According to the fact that the isolation of CSCs using specific markers can be very difficult, Addla et al. identified a side population (SP) from both normal and malignant renal epithelial cells [37], using the Hoechst 33342 dye efflux assay developed by Goodell et al. in the hematopoietic system [44]. The SP of normal kidney expresses the same markers identified by Bussolati et al. [30]. In addition, the authors found a differential expression of CD133 marker between the side population of normal and malignant kidney, being CD133 only expressed by the normal SP. According to these findings, the loss of CD133 might be a very early event in stem cells differentiation and possibly in malignant transformation [37]. The characterization of SP cells with high brilliance synchrotron-FITR spectroscopy revealed the presence of different subpopulations of cells, with different cellular biochemistry [38], but actually there is no evidence of a differential role of these subpopulations in carcinogenesis, neither any specific marker has been identified.
8. Renal CSCs and Endothelial Differentiation
The stemness and the origin of CSCs from tissue stem/progenitor cells may support the capacity of CSCs to differentiate in cell types present in the tumor other than the epithelial tumor cells. In renal carcinomas CD105+ CSCs were shown to be bipotent, as they were able to differentiate into tumor epithelial and endothelial cells both in vitro and in vivo [36]. The evidence of an in vivo differentiation of stem cells into endothelial cells was provided by the observation that at least a fraction of the vessels present in the transplanted tumors originated from tumor stem cells were of human origin. Clonal studies showing endothelial differentiation of single-cell-derived spheres or clonal cell lines derived from renal CSCs confirmed the multipotency of renal CSCs. This endothelial differentiation has been also shown for CSCs derived from glioblastomas [45–48], hepatocellular carcinomas [49], ovarian [50] and breast cancer [51], suggesting that this is rather a general phenomenon applicable to stem cells in different tumors, possibly due to the maintenance of stem properties in cancer stem cells. Of interest, a mechanism involved in the endothelial differentiation of tumor stem cells was reported to be hypoxia [51]. This concept is of particular relevance in tumor antiangiogenic therapy, as therapy-induced hypoxia may promote alternative strategies to support tumor vascularization and induce a switch from normal to cancer stem-dependent vascularization. Anti-angiogenic therapies currently implied in the treatment of renal carcinoma [52] should therefore be aimed to target not only normal, but also tumor-derived endothelial cells.
9. Conclusions
Altogether the data of the literature indicate that renal carcinomas possess a population of CSCs with mesenchymal properties (Table 1). These CSCs have a high tumorigenic ability and are resistant to chemo- and radiotherapy [36, 39]. In addition, the differentiation of CSCs into endothelial cells and the possible role of infiltrating normal stem cells, such as CD133+ resident cells and MSCs, suggest a complex relationship between tumor and its microenvironment, that should be taken into account for the design of future therapies.
Concerning the stem cell origin of renal CSCs, the data are still discordant. The lack of tumorigenicity of CD133+ cells [33] is in contrast with the idea that, also in the kidney, carcinomas originate from renal progenitors expressing CD133. A possible explanation is the origin of renal carcinomas from a more undifferentiated stem cell compartment (Figure 1). Alternatively, it could be postulated that a true stem cell compartment is lacking in the renal tubuli. In particular, the plastic phenotype of tubular cells, evidenced by the finding that they are in the G1 phase of the cell cycle [53], can suggest that tubular cells may modulate their phenotype toward stemness in response to environmental stimuli. According to this idea, being the kidney a low regenerating organ, a different mechanism could occur in respect to highly proliferating organs such as the gut implying the de-differentiation of mature cells.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annual Review of Medicine. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 3.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells—perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Research. 2006;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Research. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 6.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 8.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Research. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 10.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Research. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 11.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Research. 2005;65(8):3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 12.Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Research. 2005;65(20):9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 13.Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death and Differentiation. 2008;15(3):504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 14.Bertolini G, Roz L, Perego P, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(38):16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lane BR, Kattan MW. Predicting outcomes in renal cell carcinoma. Current Opinion in Urology. 2005;15(5):289–297. doi: 10.1097/01.mou.0000178336.94991.17. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz ÖH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441(7092):475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 17.Smith KM, Datti A, Fujitani M, et al. Selective targeting of neuroblastoma tumour-initiating cells by compounds identified in stem cell-based small molecule screens. EMBO Molecular Medicine. 2010;2(9):371–384. doi: 10.1002/emmm.201000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sehl ME, Sinsheimer JS, Zhou H, Lange KL. Differential destruction of stem cells: Implications for targeted cancer stem cell therapy. Cancer Research. 2009;69(24):9481–9489. doi: 10.1158/0008-5472.CAN-09-2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJA. The origin of the cancer stem cell: current controversies and new insights. Nature Reviews Cancer. 2005;5(11):899–904. doi: 10.1038/nrc1740. [DOI] [PubMed] [Google Scholar]
- 20.Sell S. On the stem cell origin of cancer. American Journal of Pathology. 2010;176(6):2584–2594. doi: 10.2353/ajpath.2010.091064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okita K, Yamanaka S. Induction of pluripotency by defined factors. Experimental Cell Research. 2010;316(16):2565–2570. doi: 10.1016/j.yexcr.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Wodarz A, Gonzalez C. Connecting cancer to the asymmetric division of stem cells. Cell. 2006;124(6):1121–1123. doi: 10.1016/j.cell.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 24.LaBarge MA. The difficulty of targeting cancer stem cell niches. Clinical Cancer Research. 2010;16(12):3121–3129. doi: 10.1158/1078-0432.CCR-09-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124(6):1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Alison MR, Islam S, Wright NA. Stem cells in cancer: instigators and propagators? Journal of Cell Science. 2010;123(14):2357–2368. doi: 10.1242/jcs.054296. [DOI] [PubMed] [Google Scholar]
- 27.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Pleniceanu O, Harari-Steinberg O, Dekel B. Concise review: kidney stem/progenitor cells: differentiate, sort out, or reprogram? Stem Cells. 2010;28(9):1649–1659. doi: 10.1002/stem.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dekel B, Metsuyanim S, Schmidt-Ott KM, et al. Multiple imprinted and stemness genes provide a link between normal and tumor progenitor cells of the developing human kidney. Cancer Research. 2006;66(12):6040–6049. doi: 10.1158/0008-5472.CAN-05-4528. [DOI] [PubMed] [Google Scholar]
- 30.Bussolati B, Bruno S, Grange C, et al. Isolation of renal progenitor cells from adult human kidney. American Journal of Pathology. 2005;166(2):545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sagrinati C, Netti GS, Mazzinghi B, et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. Journal of the American Society of Nephrology. 2006;17(9):2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 32.Sallustio F, De Benedictis L, Castellano G, et al. TLR2 plays a role in the activation of human resident renal stem/progenitor cells. FASEB Journal. 2010;24(2):514–525. doi: 10.1096/fj.09-136481. [DOI] [PubMed] [Google Scholar]
- 33.Bruno S, Bussolati B, Grange C, et al. CD133+ renal progenitor cells contribute to tumor angiogenesis. American Journal of Pathology. 2006;169(6):2223–2235. doi: 10.2353/ajpath.2006.060498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemmati HD, Nakano I, Lazareff JA, et al. Cancerous stem cells can arise from pediatric brain tumors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Alterio C, Cindolo L, Portella L, et al. Differential role of CD133 and CXCR4 in renal cell carcinoma. Cell Cycle. 2010;9(22):4492–4500. doi: 10.4161/cc.9.22.13680. [DOI] [PubMed] [Google Scholar]
- 36.Bussolati B, Bruno S, Grange C, Ferrando U, Camussi G. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB Journal. 2008;22(10):3696–3705. doi: 10.1096/fj.08-102590. [DOI] [PubMed] [Google Scholar]
- 37.Addla SK, Brown MD, Hart CA, Ramani VAC, Clarke NW. Characterization of the Hoechst 33342 side population from normal and malignant human renal epithelial cells. American Journal of Physiology. 2008;295(3):F680–F687. doi: 10.1152/ajprenal.90286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes C, Liew M, Sachdeva A, et al. SR-FTIR spectroscopy of renal epithelial carcinoma side population cells displaying stem cell-like characteristics. Analyst. 2010;135(12):3133–3141. doi: 10.1039/c0an00574f. [DOI] [PubMed] [Google Scholar]
- 39.Zhong Y, Guan K, Guo S, et al. Spheres derived from the human SK-RC-42 renal cell carcinoma cell line are enriched in cancer stem cells. Cancer Letters. 2010;299(2):150–160. doi: 10.1016/j.canlet.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Gupta S, Verfaillie C, Chmielewski D, et al. Isolation and characterization of kidney-derived stem cells. Journal of the American Society of Nephrology. 2006;17(11):3028–3040. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 41.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of Cell Science. 2006;119(11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 42.Plotkin MD, Goligorsky MS. Mesenchymal cells from adult kidney support angiogenesis and differentiate into multiple interstitial cell types including erythropoietin-producing fibroblasts. American Journal of Physiology. 2006;291(4):F902–F912. doi: 10.1152/ajprenal.00396.2005. [DOI] [PubMed] [Google Scholar]
- 43.Lazzeri E, Crescioli C, Ronconi E, et al. Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. Journal of the American Society of Nephrology. 2007;18(12):3128–3138. doi: 10.1681/ASN.2007020210. [DOI] [PubMed] [Google Scholar]
- 44.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. Journal of Experimental Medicine. 1996;183(4):1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong J, Zhao Y, Huang Q, et al. Glioma stem/progenitor cells contribute to neovascularization via transdifferentiation. Stem Cell Reviews and Reports. 2011;7(1):141–152. doi: 10.1007/s12015-010-9169-7. [DOI] [PubMed] [Google Scholar]
- 46.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468(7325):824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 47.Wang R, Chadalavada K, Wilshire J, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468(7325):829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 48.Zhao Y, Dong J, Huang Q, Lou M, Wang A, Lan Q. Endothelial cell transdifferentiation of human glioma stem progenitor cells in vitro. Brain Research Bulletin. 2010;82(5-6):308–312. doi: 10.1016/j.brainresbull.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Xiong YQ, Sun HC, Zhang W, et al. Human hepatocellular carcinoma tumor-derived endothelial cells manifest increased angiogenesis capability and drug resistance compared with normal endothelial cells. Clinical Cancer Research. 2009;15(15):4838–4846. doi: 10.1158/1078-0432.CCR-08-2780. [DOI] [PubMed] [Google Scholar]
- 50.Alvero AB, Fu HH, Holmberg J, et al. Stem-like ovarian cancer cells can serve as tumor vascular progenitors. Stem Cells. 2009;27(10):2405–2413. doi: 10.1002/stem.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bussolati B, Grange C, Sapino A, Camussi G. Endothelial cell differentiation of human breast tumour stem/progenitor cells. Journal of Cellular and Molecular Medicine. 2009;13(2):309–319. doi: 10.1111/j.1582-4934.2008.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heldwein FL, Escudier B, Smyth G, Souto CAV, Vallancien G. Metastatic renal cell carcinoma management. International Brazilian Journal of Urology. 2009;35(3):256–270. doi: 10.1590/s1677-55382009000300002. [DOI] [PubMed] [Google Scholar]
- 53.Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M. Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. American Journal of Physiology. 2008;294(1):C22–C28. doi: 10.1152/ajpcell.00227.2007. [DOI] [PubMed] [Google Scholar]