Abstract
Artemisia annua L. produces the sesquiterpene lactone, artemisinin, a potent antimalarial drug that is also effective in treating other parasitic diseases, some viral infections and various neoplasms. Artemisinin is also an allelopathic herbicide that can inhibit the growth of other plants. Unfortunately, the compound is in short supply and thus, studies on its production in the plant are of interest as are low cost methods for drug delivery. Here we review our recent studies on artemisinin production in A. annua during development of the plant as it moves from the vegetative to reproductive stage (flower budding and full flower formation), in response to sugars, and in concert with the production of the ROS, hydrogen peroxide. We also provide new data from animal experiments that measured the potential of using the dried plant directly as a therapeutic. Together these results provide a synopsis of a more global view of regulation of artemisinin biosynthesis in A. annua than previously available. We further suggest an alternative low cost method of drug delivery to treat malaria and other neglected tropical diseases.
Keywords: Artemisinin pharmacokinetics, ROS, DMSO, Artemisia annua development, Trichomes
Introduction
In low income and developing nations, malaria is the fifth most prevalent infectious disease and the tenth overall cause of death, and is projected to remain at that level until at least 2030 (Mathers et al. 2006). The World Health Organization (WHO 2005) estimates that more than 380 million cases of malaria occur each year and account for more than 1 million deaths especially in developing countries (Rathore et al. 2005). Artemisinin and its derivatives also have been shown to be effective against a number of viruses, Pnuemocystis carinii, Toxoplasma gondii, a number of human cancer cell lines (Efferth 2007), and a variety of other parasitic tropical diseases including schistosomiasis (Utzinger et al. 2001), leishmaniasis (Sen et al. 2007), Chagas disease, and African sleeping sickness (Mishina et al. 2007). All of these diseases probably can be successfully treated with artemisinin, if enough of the drug is made available and, for developing countries, also at a cost that is affordable.
Although total chemical synthesis of artemisinin has been achieved, it is not cost effective (Haynes 2006). Current technology for artemisinin production is based on cultivated A. annua with best cultivars giving yields of artemisinin of ca. 1.5% of dry plant material and 70 kg/ha (Kumar et al. 2004). Artemisinin is solvent-extracted from plant material, crystallized, and typically used for semi-synthesis of artemisinin derivatives (Haynes 2006). While A. annua is relatively easy to grow in temperate climates, low yields of artemisinin result in relatively high costs for isolation and purification of the useful chemical. The relatively long agricultural timeframe also results in wide swings in supply and price as demand changes. Although scientists at University of York, UK and elsewhere are breeding cultivars of A. annua for higher trichome densities and, thus, artemisinin production (Grove et al. 2007), and transgenic production schemes are in progress (Arsenault et al. 2008), there is still a worldwide shortage of the drug just for treating malaria let alone any other diseases against which artemisinin holds such promise (De Ridder et al. 2008). Clearly more low cost production and delivery of artemisinin as WHO recommended Artemisinin Combination Therapy (ACT) are needed.
Considering that this drug must be produced cheaply in much greater quantities than currently available we summarize here our recent work to better explain artemisinin production in planta. We also provide preliminary data from feeding studies with mice that suggest a new approach for drug delivery could be implemented using encapsulated dried leaves of the plant and an ACT counterpart to minimize the emergence of resistance. This same drug delivery approach, without the ACT, could also be used to treat other neglected tropical diseases that are apparently susceptible to artemisinin such as schistosomiasis, Chagas disease, and African sleeping sickness.
Artemisinin biosynthesis: current understanding
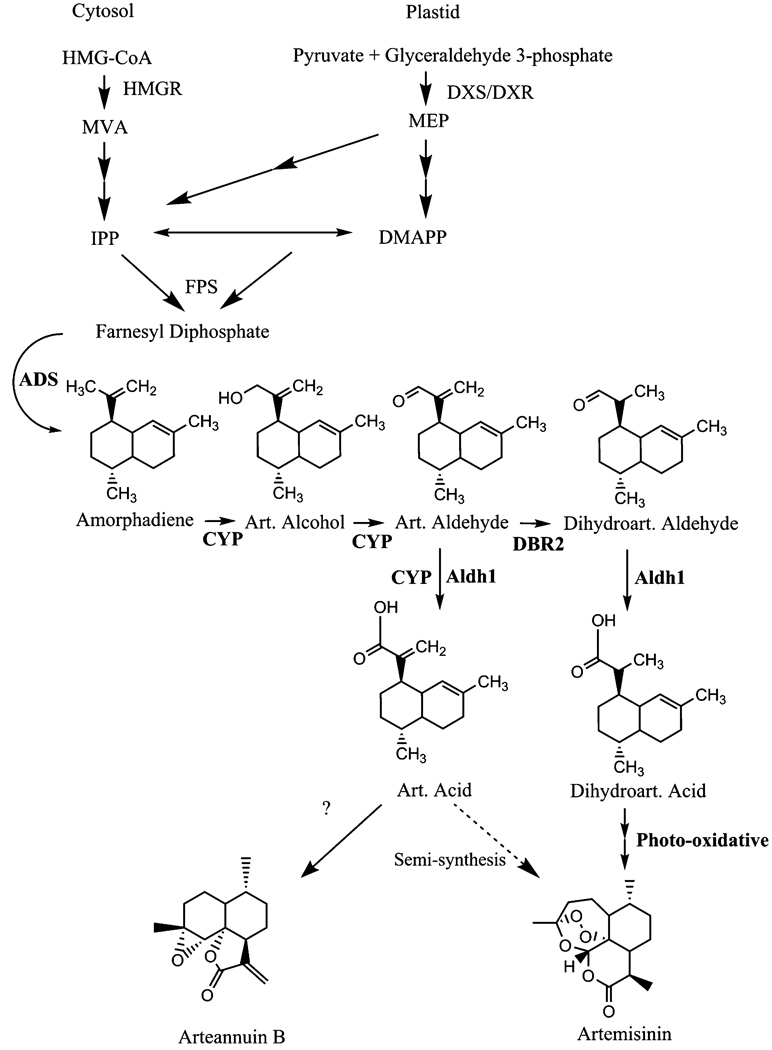
Through recent work from several groups, the biosynthesis of the sesquiterpene, artemisinin, is almost completely resolved (Fig. 1). Artemisinin derives from the condensation of three 5-carbon isoprenoid molecules that originate from both the plastid and cytosol (Towler and Weathers 2007; Schramek et al. 2010). These two arms of the terpenoid pathway up to farnesyl diphosphate are regulated in large part by 1-deoxyxylulose 5-phosphate synthase (DXS), and 1-deoxyxylulouse 5-phosphate reductoisomerase (DXR) or 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), respectively, finally leading to the production of farnesyl diphosphate via farnesyl diphosphate synthase (FPS). Farnesyl diphosphate is then converted to amorpha-4, 11-diene via amorphadiene synthase (ADS; Bouwmeester et al. 1999; Picaud et al. 2005). The majority of data support the role of dihydroartemisinic acid (DHAA) as a late intermediate in artemisinin biosynthesis (Fig. 1; Sy and Brown 2002; Zhang et al. 2008). DHAA is formed from amorpha-4, 11-diene via artemisinic aldehyde by the action of the cytochrome P450, CYP71AV1 (Teoh et al. 2006; Ro et al. 2006), DBR2 (Zhang et al. 2008) and probably ALDH1 (Teoh et al. 2009). DHAA is believed to be converted to artemisinin (AN) nonenzymatically (Sy and Brown 2002; Covello 2008). The pathway also branches at artemisinic aldehyde to give artemisinic acid (AA) by the action of CYP71AV1 and/orALDH1, and arteannuin B (AB), possibly nonenzymatically (Brown and Sy 2007). The genes encoding ADS, CYP71AV1, DBR2 and ALDH1 are all preferentially expressed in glandular trichomes (Covello et al. 2007; Olsson et al. 2009).
Fig. 1.
Artemisinin structure and simplified biosynthetic pathway. ADS amorphadiene synthase, Aldh1 aldehyde dehydrogenase 1, CYP CYP71AV1, DBR2 double bond reductase 2, DMAPP dimethylallyl diphosphate, DXS 1-deoxyxylulose 5-phosphate synthase, DXR 1-deoxyxylulouse 5-phosphate reductoisomerase, HMGR 3-hydroxy- 3-methylglutaryl-CoA reductase, IPP isopentenyl diphosphate, MEP methyl erythritol phosphate, MVA mevalonic acid
Artemisinin production and trichomes are intimately related
Artemisinin is produced in glandular trichomes that are found on leaves, floral buds, and flowers (Ferreira and Janick 1995; Tellez et al. 1999). During vegetative growth of A. annua plants, trichome numbers increase on the leaf surface, but when leaf expansion halts, the numbers begin to decline, possibly a result of their collapse (Lommen et al. 2006). AN increased with trichome numbers, but in some cases AN levels continue to rise even after trichome populations begin collapsing; this was attributed to maturation effects within the trichome (Lommen et al. 2006).
AN content can vary widely among different cultivars or ecotypes of A. annua (Wallaart et al. 2000), and to the time of harvest, light intensity, and developmental stage (Ferreira and Janick 1995). AN levels reach their peak either just before or at anthesis (Acton and Klayman 1985; Woerdenbag et al. 1993), yet transgenic plants with the flower promoting factor 1 (Fpf1) flowered earlier, but did not produce more AN (Wang et al. 2004). Thus, other factors linked to flowering are likely more involved in AN increases.
Little is known about how artemisinin and its metabolites are affected throughout plant development and in relation to trichomes. Artemisinin transcripts, metabolite levels (AA, AB, AN, and DHAA), and trichomes populations were therefore analyzed in three types of leaves, in floral buds and flowers, and in three developmental stages: vegetative, floral budding and full flower. Although the maximum production of AN occurs when flowers are fully emerged, expression levels in the leaves of early pathway genes, HMGR, PFS, DXS, and DXR did not show close correlation with either AN or its precursors. However, later pathway genes, ADS and CYP, did correlate well with AN’s immediate precursor, DHAA, in all leaf tissues tested. A close correlation between AN levels and leaf trichome populations (as trichomes mm−2) was also observed (Arsenault et al. 2010a, manuscript submitted).
DMSO helps elucidate a possible ROS role of DHAA in AN biosynthesis
Prior work showed that dimethyl sulfoxide (DMSO) increased artemisinin in A. annua seedling shoots (Towler and Weathers 2007), but the mechanism of this serendipitous response was not understood. Interestingly it was the roots that were key to this DMSO response; artemisinin levels were not increased when only shoots of either rooted or unrooted shoots were treated with DMSO. This is not surprising, however, because the roots of A. annua are reported to play an important, but not as yet understood role in the production of artemisinin in the shoots (Ferreira and Janick 1996). Indeed rooted shoots of A. annua produce about 8 times the artemisinin of unrooted shoots, and in rooted shoots DMSO doubles that amount (Mannan et al. 2009). In contrast, unrooted shoots are not responsive to DMSO. To determine if there is an optimum DMSO response, both the concentration of DMSO and duration of exposure were examined. At concentrations of DMSO between 0 and 2%, rooted shoots exhibited biphasic artemisinin production compared to the untreated controls with 2 peaks at 0.25 and 2% DMSO, both at about 2.26 times that of the control. At 0.5% DMSO, however, artemisinin production significantly decreased relative to the production at the peaks. Using the 0.25% DMSO concentration peak to determine the kinetics of the effect, we determined that the production of AN along with its precursor, DHAA, persisted for 7 days (Mannan et al. 2009).
To investigate this DSMO response further, real time PCR was used to measure the transcriptional response of the artemisinic pathway genes, ADS and CYP, in both the shoot and root tissues of A. annua rooted shoot cultures after incubation in DMSO. The first gene in the artemisinin biosynthetic pathway, ADS, showed no significant increase in transcript level in response to DMSO compared to controls. On the other hand, the second gene in the pathway, CYP, did respond to DMSO but at a level of transcripts inverse to the amount of artemisinin (Mannan et al. 2009). These results suggested that DMSO may be altering artemisinin production in some other way.
DMSO can act as both a reducing and an oxidizing agent, and can also associate with unshared pairs of electrons in the oxygen of alcohols, and may even act as a “radical trap” whereby as an intermediate in radical transfer, it may promote peroxidation (Kharasch and Thyagarajan 1983). Weathers et al. (1999) had previously shown that in a highly oxygenated environment more artemisinin is produced than in a hypoxic one. Wallaart et al. (1999, 2001) had suggested earlier that DHAA may be acting as a reactive oxygen species (ROS) scavenger and indeed the DMSO data are consistent with the hypothesis that the DMSO-induced ROS were possibly causing the increase in production of DHAA and, thus, providing the extra oxygens needed for the final biosynthetic step leading to AN.
To explore this further, rooted shoots were incubated in increasing DMSO concentrations and then stained with 3, 3′-diaminobenzidine-HCl (DAB), which is specific for the ROS, H2O2. Although the increasing DMSO concentrations did not affect growth, the level of DAB staining in the leaves of rooted plantlets showed an increased in situ production of H2O2 in the foliage. In contrast, unrooted shoots showed no ROS formation in the presence of DMSO; roots were required for the ROS response in the shoots (Mannan et al. 2009).
If DMSO was indeed increasing ROS production in the leaves of A. annua plantlets, then a natural ROS scavenger like ascorbic acid should inhibit both ROS and AN production. DMSO-induced hydrogen peroxide levels and artemisinin levels were both inhibited by addition of ascorbate. Together these data show that at least in response to DMSO, artemisinin production and hydrogen peroxide increase, and that when in situ hydrogen peroxide is reduced, so also is artemisinin suggesting that the ROS stimulated hydrogen peroxide may play a role in artemisinin production in A. annua.
Sugar metabolism may also play a role in regulating artemisinin biosynthesis
In A. annua seedlings, glucose in particular was shown to stimulate artemisinin production (Wang and Weathers 2007). Indeed it is the ratio of glucose to fructose that is important in regulating AN production. When seedlings were grown in sucrose-free medium, increasing artemisinin levels were directly proportional to increasing glucose as the ratio of glucose to fructose was increased from 0 to 100%. In comparison to sucrose or glucose, fructose inhibits the production of artemisinin. Other primary and secondary metabolites have been shown to be sugar responsive including products of the glyoxylate cycle (Graham et al. 1994) and anthocyanins (Vitrac et al. 2000). Although in both Vitis and Arabidopsis, a number of anthocyanin genes have been shown to be upregulated in response to sucrose (Gollop et al. 2001, 2002; Solfanelli et al. 2006), the mechanism of action is not entirely known.
Using Artemisia annua seedlings, artemisinic metabolites and gene transcript responses were measured (Arsenault et al. 2010b, manuscript submitted) after growth for 0–14 days on sucrose, glucose, or fructose. The six genes measured by real time RT-PCR were: HMGR, FPS, DXS, DXR, ADS, and CYP. Compared to seedlings grown in sucrose, HMGR, FPS, DXS, DXR, ADS and CYP transcript levels increased in varied amounts and with varied kinetics after growth in glucose, but not in fructose. The kinetics of these transcripts over 14 days, however, was very different both in timing and intensity of response (Arsenault et al. 2010b, manuscript submitted).
Using LC/MS intracellular concentrations of AN, DHAA, AA, and AB were also measured in response to the three sugars. Compared to sucrose-fed seedlings, AN levels were significantly increased in seedlings fed glucose, but inhibited in fructose-fed seedlings. In contrast, AB levels doubled in seedlings grown in fructose compared to those grown in glucose. The level of mRNA transcripts of many of the genes analyzed was often negatively correlated with the observed metabolite concentrations.
AN is a known phytotoxin, even against A. annua (Duke et al. 1987), suggesting that it may also inhibit its own synthesis in planta. When seedlings were grown in increasing levels of AN, root elongation was inhibited and, interestingly, levels of AA fell to below detectable limits (Arsenault et al. 2010b, manuscript submitted). Together these results show there is a complex interplay between exogenous sugars and early developmental cues in the biosynthesis of artemisinin and its precursor metabolites. The results also suggest that the dynamics of shifting sugar concentrations in the plant play a role in the in situ control of artemisinin metabolism.
Artemisia annua as a delivery system for artemisinin: mouse studies
Artemisia annua has a rich ethnopharmacological history in the Chinese Materia Medica as a therapeutic tea (Willcox et al. 2007), and the plant, although not highly palatable, also has been used as a condiment by various Asian cultures (http://pfaf.org/database/plants.php?Artemisia+annua). While use of the tea is no longer recommended due to emergence of resistance, to our knowledge there has been no investigation of the use of A. annua plant material to treat patients. Considering that some plant secondary metabolites appear to have a more synergistic effect when provided in planta than in a purified form (Gilbert and Alves 2003), eating A. annua via a compacted capsule in combination with an ACT partner, may offer an alternative, safe, inexpensive mode of drug delivery. Towards that goal it was necessary to also show that artemisinin could actually move from ingested plant material in the gut into the bloodstream.
Artemisia annua L. seeds from a Chinese strain (PEG01; a gift to PJW from CZ Liu (Chinese Academy of Sciences) were germinated in soil and then transplanted to small (3 inches × 3 inches × 2.5 inches deep) pots and grown in a growth chamber at 25°C under full spectrum fluorescent lights at ~90 µmol m−2 s−1 with a 16 h photoperiod to inhibit flowering. Plant material was harvested, dried and leaves stripped from stems.
To determine the bioavailability of artemisinin in mice from oral ingestion of A. annua plant material A. annua leaves were dried at room temperature and then pulverized into a homogenous mixture that was aliquoted both for assay to determine the level of artemisinin and to use as feed. Ground leaf samples were suspended in water, pelletized, and then fed once via orogastric gavage to anesthetized BL6xICR mice to insure quantitative ingestion of the plant material. Prior to feeding, mice were fasted for 24 h with water given ad libitum and prior to gastric intubation. Mice were fed one of the following at a volume ≤0.4 ml per mouse: pelletized A. annua plant material containing 30.7 µg AN in toto, or pure artemisinin mixed into pelleted feed at either 30.7 or 1,400 µg per mouse. Animals were then anesthetized and exsanguinated in groups of three at 30, and 60 min post feeding. At the end of the study, gross pathological examination of animals’ digestive system was performed to ensure that animals suffered no internal damage.
Artemisinin and related metabolic constituents were extracted from plant material and from mouse blood using toluene and petroleum ether, respectively. Samples from each were subsequently dried and resuspended in ethyl acetate before injection onto a GC–MS. GC separation was achieved using a DB-5MS column (30m × 25 mm × 0.25 um) and a temperature gradient programmed at 2°C/min from 120 to 160°C and held at 160°C for 10 min and then heated to 300°C at 10°/min. All heated zones (injector and detector) were maintained at 200°C. MS scans from 50–400 m/z and EI with 70 eV. Artemisinin was detected and quantified via total ion count and retention time based on a genuine external standard and corrected via an internal standard.
Artemisinin from ingested Artemisia annua leaves passes readily into the bloodstream of mice
To our knowledge, there has been no bioavailability study of artemisinin from oral ingestion of A. annua leaves. One of the key concerns is the relative bioavailability of artemisinin to a patient from a drug that is administered in planta. Mice were used in this study to determine how much artemisinin, if any, would move from the plant material in the gut into the bloodstream.
The pharmacokinetics of AN administered to mice as either dried A. annua leaves or pure compound mixed with mouse chow were compared (Fig. 2). In this preliminary study, measurements were only taken up to 60 min after feeding. However, some general conclusions can be drawn. When ~31 µg pure AN was fed, no AN was detectable in blood up to 60 min. Upon feeding 1,400 µg AN, the levels in the blood rose to 0.074 mg l−1 after 60 min. On the other hand, feeding A. annua leaves equivalent to 31 µg AN led to a Cmax of 0.087 mg l−1 at a tmax of 30 min. These results are similar to those of Rath et al. (2004) who compared the pharmacokinetics in humans of AN delivered as a tea, to pure AN. The tea showed a tmax of 30 min and the pure compound a tmax of 2.3 h, consistent with our mouse data measured up to 60 min.
Fig. 2.
Pharmacokinetics of artemisinin in mice. Artemisinin supplied via leaves appears at about the same time in the mouse bloodstream as it does from the pure drug, but may not persist as long. Bars = SD; n ≥ 3 per point
Of particular interest is the comparatively high level of transfer of artemisinin into the bloodstream from the plant material vs. the pure drug. There was 45 times more pure artemisinin fed to the mice than the amount fed via A. annua leaves, yet almost the same amount of AN appeared in the bloodstream. Furthermore, when equal amounts of pure drug and plant delivered drug (~31 µg) were fed to each mouse, the amount of artemisinin found in the blood from the plant-fed material (~87 µg l−1 blood) far surpassed the level from delivered pure drug (undetectable). Taken together these results show that compared to the pure drug, the bioavailability of AN from dried plant material is apparently greater (Table 1). These results suggest that an alternative mode of delivery of artemisinin may be possible.
Table 1.
Comparison of maximum artemisinin detected in serum or plasma from orally ingested pure artemisinin, whole plant A. annua, or prepared tea
| Drug delivery form | Dose per individual | Subject | Plasma/serum concentration Artemisinin Cmax (mg l−1) |
Reference |
|---|---|---|---|---|
| Pure artemisinin | 500 mg | Healthy human males | 0.6 | Dien et al. (1997) |
| 500 mg | 0.3 | de Vries and Dien (1996) | ||
| Tea extract | ||||
| 5 g dried leaves | 57.5 mg | Healthy human males | 0.2 | Rath et al. (2004) |
| Pure artemisinin control | 500 mg | 0.5 | ||
| Intragastric delivery in 1:9 dimethyl-acetamide-oila |
10 mg kg−1 2.320 µg mouse−1 |
Rats | 0.8 | Li et al. (1998) |
| Whole plant-dried leaves | 30.7 µg mouse−1 | Mice | 0.087 | This study |
| Pure artemisinin control | 30.7 µg mouse−1 | Not detectable | ||
| Pure artemisinin control | 1,400 µg mouse−1 | ≥0.074 |
Delivered as dihydroartemisinin
Rat body weights ranged from 210–254 g; we used 232 as an average to calculate total µg delivered to each animal
Bioavailability of artemisinin after oral intake is crucial for assessing the potential of using an edible botanical drug. Equally important are pharmacokinetic studies to insure proper formulation of the drug dose to be delivered from plants to patient. Current oral bioavailability data on artemisinin are mainly based on studies with artemisinin capsules or tea prepared from A. annua leaves. For example, Rath et al. (2004) measured artemisinin plasma concentrations in healthy male volunteers after oral ingestion of either traditionally prepared A. annua tea or in solid form. Although the intake as tea showed a faster absorption than the solid form, there was no difference in bioavailability (Table 1; Rath et al. 2004). On the other hand, bioavailability after oral intake was reported at 32% of the drug administered via an intramuscular route (Titulaer et al. 1990). Pharmacokinetic studies done with healthy male volunteers showed artemisinin has an absorption lag-time of 0.5–2 h, with peak plasma concentrations at 1–3 h post-administration and a relatively short half-life of 1–3 h (Alin et al. 1996; Ashton et al. 1998; Titulaer et al. 1990).
Possible synergistic and broader effects of an in planta delivered drug
Artemisinin may have a more synergistic effect when provided in planta than as a pure drug. Inhibition of human cytochrome P450s by herbal extracts of numerous species, including a number of traditional medicinal plants, has been extensively studied (Rodeiro et al. 2009), thereby increasing serum half-life. Indeed Liu et al. (1992) showed that although several methoxylated flavonoids, e.g. chrysosplenol-D, isolated from A. annua leaves had no direct effect on P. falciparum, when combined with pure AN, there was a significant enhancement of AN activity that could only be attributed to the presence of these compounds. A number of these constitutive flavonoids are present at all stage of A. annua’s growth (Baraldi et al. 2008), and also show some antimalarial activity, albeit at levels that are orders of magnitude less than AN (Willcox 2009). Chrysosplenetin, casticin, eupatin, and chrysosplenol-D appear to help activate AN in its interaction with hemin (Bilia et al. 2002, 2006). Thus, these in planta constituents in A. annua likely enhance the overall activity of the drug. Another possible benefit to ingestion of whole leaf material is that there may be less chance of resistance occurring because there is a combination of active agents acting in concert to attack the pathogen. Eating A. annua combined with an ACT partner, may, therefore, offer an alternative, safe, inexpensive mode of drug delivery via a compacted capsule. Indeed should future studies prove successful in patients (clinical trials are still needed), this approach may also eventually prove more useful than purified compounds for production and delivery of other drugs produced in edible plants that survive the digestive tract in planta. Likewise, use of this plant may also prove useful in treating a variety of other diseases and parasitic ailments.
Is oral delivery via A. annua plants even reasonable?
To answer this question we used data from Rath et al. (2004) where AN was administered as a tea. From 5 g DW of A. annua leaves (>1% DW AN), 57.5 mg of AN were measured and provided to humans and 240 µg l−1 appeared in the bloodstream. The minimum effective concentration of AN in the blood is ~10 µg l−1 (Alin and Bjorkman 1994). An adult human male weighing 70 kg has about 5 l of blood. The AN in a tea extract from 5 g of dried leaves containing 1% (w/w) AN, therefore, provides considerably more AN (240 µg l−1) than the minimum required in the blood suggesting that 1 g of ingested dried leaves could be more than adequate to deliver a single dose of AN to an adult patient.
As another comparison, mice have about 1.4 ml blood, while a 70 kg human male has about 5 l. Our mice were fed about 31 µg of AN and contained an average total of 0.12 µg AN in their blood, so to obtain the necessary total amount of AN in human blood, 50 µg are needed (10 µg AN ml−1 is considered therapeutic) for a single AN dose. Assuming similar uptake, a patient would have to ingest 17 mg of AN from plant leaves. Assuming also a 1% AN content, which is possible to consistently obtain from some A. annua strains (e.g. the Artemis strain has ~1.4%; Ferreira et al. 2005), 1–2 g of dried leaves would be adequate and reasonable to deliver a single dose of the drug to a 70 kg adult. For children smaller amounts would be required, which is easily accomplished using smaller capsules.
Controlling the dose of a plant-delivered drug?
To provide a controlled delivery of the drug via oral delivery of dried plant material, plants must be harvested, dried, powdered, homogenized, and pooled into large containers where they can be assayed for artemisinin content using strategies that are easy, low cost, and quantitative (Widmer et al. 2007; Koobkokkraud et al. 2007). Capsules would then be loaded with compacted leaf powder of a known dosage to which the ACT drug partner can be added. Alternatively the ACT drug partner could be administered separately. This processing strategy (Fig. 3) is inexpensive, and also reliable for preparing known doses of artemisinin as dried plant material. If this processing facility were centered within a region where local farmers are growing the plant, the entire process could be self-sustaining thereby not only strengthening local health, but also the local economy.
Fig. 3.
Schematic illustrating the concept of localized culture of A. annua for dose controlled therapeutic use and delivery as an ACT. Larger outer circle represents the medicinal crop cultivation area, while inner boxes show the required sequence of unit operations for dose controlled preparation of encapsulated A. annua leaves and ACT combination drug
Not a monotherapy
Although our data compare favorably with studies in rats and A. annua teas (Table 1), use of a tea is a monotherapy, involves no ACT, and is thus, counter-indicated by the WHO in an effort to minimize emergence of resistant strains of the pathogen. In contrast, our drug delivery plan would also incorporate an ACT drug partner as follows: plants are harvested and dried (WHO 2006); leaves are pulverized and homogenized in large vessels; samples are then taken to measure AN content to ensure preparation of adequate and controlled doses for patients; the assayed leaves are then compacted into capsules into which appropriate amounts of ACT partner drugs are added. As an example see Fig. 4. The caplet shown is about 1,300 mg so if the assayed dry leaves only contained 1% AN, about 1–2 capsules would need to be ingested per dose to treat a 70 kg human. WHO guidelines for human treatment specify additional AN doses throughout the day.
Fig. 4.
ACT delivery involving A. annua leaves. Capsule sizes are reasonable for human ingestion of A. annua leaves in combination with approved WHO combination therapy drugs
We therefore, submit that oral delivery of artemisinin via dried A. annua plant material and in conjunction with an ACT drug partner could provide an effective, low cost therapy for treating malaria in developing countries, but with the following caveats. (1) Human trials first must be conducted using healthy subjects to validate and quantitate absorption of artemisinin from A. annua leaves and to determine if there are any side effects fromingestion of the whole plant material. (2) If positive, then the ACT mixtures we have proposed would also have to be tested again on healthy subjects to determine if both artemisinin and the ACT partner enter the bloodstream. (3)After successful completion of both of these steps, tests could begin on diseased patients. Clearly for cerebral malaria or other severe cases of malaria cases where oral administration of the drug is not possible due to vomiting (De Ridder et al. 2008), other means of administering artemisinin or one of its derivatives would have to be implemented.
Conclusions
Despite the prevalence and preference of the modern medical community for single-ingredient drugs, there are examples that illustrate the often ignored benefits of using complex botanical drugs vs. pure ones (Raskin et al. 2002). With the potential for synergistic benefits, drug delivery via natural sources may be preferable to that in an isolated form (Fabricant and Farnsworth 2001; Raskin et al. 2002; Gilbert and Alves 2003). We have shown that when provided directly from plant material, high levels of artemisinin can be detected in the bloodstream of mice. We further proposed a simple method for insuring a controlled dose of artemisinin via in planta delivery that when combined with the simple methods for stimulating increases of the drug while the crop is in the field, may provide significant relief to the shortage of low cost artemisinin available for use to treat malaria and other neglected diseases in developing countries.
Acknowledgments
Thanks to Professor Carole Cramer, Arkansas Bioscience Institute, for suggesting the mouse feeding experiments. Approval for animal studies was obtained from the Institutional Animal Care and Use Committee (IACUC) of Arkansas State University. Support was provided in part by NIH 2R15GM069562-02 and the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000.
Contributor Information
Pamela J. Weathers, Email: weathers@wpi.edu, Department of Biology/Biotechnology, Worcester Polytechnic Institute, 100 Institute Rd, Worcester, MA 01609, USA.
Patrick R. Arsenault, Department of Biology/Biotechnology, Worcester Polytechnic Institute, 100 Institute Rd, Worcester, MA 01609, USA
Patrick S. Covello, Plant Biotechnology Institute, 110 Gymnasium Place, Saskatoon, SK S7N OW9, Canada
Anthony McMickle, Arkansas Bioscience Institute, Jonesboro, AR 72401, USA.
Keat H. Teoh, Arkansas Bioscience Institute, Jonesboro, AR 72401, USA
Darwin W. Reed, Plant Biotechnology Institute, 110 Gymnasium Place, Saskatoon, SK S7N OW9, Canada
References
- Acton N, Klayman DL. Artemisitene, a new sesquiterpene lactone endoperoxide from Artemisia annua. Planta Med. 1985;51:441–442. doi: 10.1055/s-2007-969543. [DOI] [PubMed] [Google Scholar]
- Alin MH, Bjorkman A. Concentration and time dependency of artemisinin efficacy against Plasmodium falciparum in vitro. Am J Trop Med Hyg. 1994;50:771–776. doi: 10.4269/ajtmh.1994.50.771. [DOI] [PubMed] [Google Scholar]
- Alin MH, Ashton M, Kihamia CM, Mtey GJ, Bjorkman A. Multiple dose pharmacokinetics of oral artemisinin and comparison of its efficacy with that of oral artesunate in falciparum malaria patients. Trans R Soc Trop Med Hyg. 1996;90:61–65. doi: 10.1016/s0035-9203(96)90480-0. [DOI] [PubMed] [Google Scholar]
- Arsenault PR, Wobbe KK, Weathers PJ. Recent advances in artemisinin production through heterologous expression. Curr Medicin Chem. 2008;15:2886–2896. doi: 10.2174/092986708786242813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault PR, Vail D, Wobbe KK, Erickson K, Weathers PJ. Reproductive development modulates artemisinin related gene expression and metabolite levels in Artemisia annua. 2010a doi: 10.1104/pp.110.162552. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault PR, Vail D, Wobbe KK, Weathers PJ. Effect of sugars on artemisinin production in Artemisia annua L.: transcript and metabolite measurements. 2010b doi: 10.3390/molecules15042302. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton M, Hai TN, Sy ND, Huong DX, Huong NV, Nieu NT, Cong LD. Artemisinin pharmacokinetics is time-dependent during repeated oral administration in healthy male adults. Drug Metab Dispos. 1998;26:25–27. [PubMed] [Google Scholar]
- Baraldi R, Isacchi B, Predieri S, Marconi G, Vincieri FF, Bili AR. Distribution of artemisinin and bioactive flavonoids from Artemisia annua L. during plant growth. Biochem System Ecol. 2008;36:340–348. [Google Scholar]
- Bilia AR, Lazari D, Messori L, Taglioli V, Temperini C, Vincieri FF. Simple and rapid physico-chemical methods to examine action of antimalarial drugs with hemin: its application to Artemisia annua constituents. Life Sci. 2002;70:769–778. doi: 10.1016/s0024-3205(01)01447-3. [DOI] [PubMed] [Google Scholar]
- Bilia AR, de Malgalhães PM, Bergonzi MC, Vincieri FF. Simultaneous analysis of artemisinin and flavonoids of several extracts of Artemisia annua L. obtained form a commercial sample and a selected cultivar. Phytomed. 2006;13:487–493. doi: 10.1016/j.phymed.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Wallaart TE, Janssen MH, van Loo B, Jansen BJ, Posthumus MA, Schmidt CO, De Kraker JW, Konig WA, Franssen MC. Amorpha-4, 11-diene synthase catalyses the first probable step in artemisinin biosynthesis. Phytochem. 1999;52:843–854. doi: 10.1016/s0031-9422(99)00206-x. [DOI] [PubMed] [Google Scholar]
- Brown GD, Sy LK. In vivo transformations of artemisinic acid in Artemisia annua plants. Tetrahedron. 2007;63:9548–9566. [Google Scholar]
- Covello PS. Making artemisinin. Phytochemistry. 2008;69:2881–2885. doi: 10.1016/j.phytochem.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Covello PS, Teoh KH, Polichuk DR, Reed DW. Functional genomics and the biosynthesis of artemisinin. Phytochemistry. 2007;68:1864–1871. doi: 10.1016/j.phytochem.2007.02.016. [DOI] [PubMed] [Google Scholar]
- De Ridder S, van der Kooy F, Verpoorte R. Artemisia annua as a self-treatment for malaria in developing countries. J Ethnopharmacol. 2008;120:302–314. doi: 10.1016/j.jep.2008.09.017. [DOI] [PubMed] [Google Scholar]
- de Vries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52:818–836. doi: 10.2165/00003495-199652060-00004. [DOI] [PubMed] [Google Scholar]
- Dien TK, de Vries PJ, Khanh NX, Koopmans R, Binh LN, Duc DD, Kager PA, van Boxtel CJ. Effect of food intake on pharmacokinetics of oral artemisinin in healthy Vietnamese subjects. Antimicrob Agents Chemother. 1997;41:1069–1072. doi: 10.1128/aac.41.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke SO, Vaughn KC, Croom EM, ElSohly HN. Artemisinin, a constituent of annual wormwood (Artemisia annua) is a selective phytotoxin. Weed Sci. 1987;35:499–505. [Google Scholar]
- Efferth T. William Schwabe Award 2006: antiplasmodial and antitumor activity of artemisinin–from bench to bedside. Planta Med. 2007;73:299–309. doi: 10.1055/s-2007-967138. [DOI] [PubMed] [Google Scholar]
- Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspectives. 2001;109:69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JFS, Janick J. Floral morphology of Artemisia annua with special reference to trichomes. Int J Plant Sci. 1995;156:807–815. [Google Scholar]
- Ferreira JFS, Janick J. Roots as an enhancing factor for the production of artemisinin in shoot cultures of Artemisia annua. Plant Cell Tiss Organ Cult. 1996;44:211–217. [Google Scholar]
- Ferreira JFS, Laughlin JC, Delabays N, de Magalhães PM. Cultivation and genetics of Artemisia annua L. for increased production of the antimalarial artemisinin. Plant Genet Resour. 2005;3:206–229. [Google Scholar]
- Gilbert B, Alves LF. Synergy in plant medicines. Curr Medicin Chem. 2003;10:13–20. doi: 10.2174/0929867033368583. [DOI] [PubMed] [Google Scholar]
- Gollop R, Farhi S, Peri A. Regulation of the leucoanthocyanidin dioxygenase gene expression in Vitis vinifera. Plant Sci. 2001;161:579–588. [Google Scholar]
- Gollop R, Even S, Colova-Solova V, Peri A. Expression of the grape dihydroflavanol reductase gene and analysis of its promoter region. J Exp Bot. 2002;53:1397–1409. [PubMed] [Google Scholar]
- Graham IA, Denby KJ, Leaver CJ. Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell. 1994;6:761–772. doi: 10.1105/tpc.6.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove N, Bowles D, Craft JC, Brewer T. Innovative new approaches to reduce economic access barriers to artemisinin-based combination therapy. Eur J Trop Med Internatl Health. 2007;12 Supplement 1:68. [Google Scholar]
- Haynes RK. From artemisinin to new artemisinin antimalarials: biosynthesis, extraction, old and new derivatives, stereochemistry and medicinal chemistry requirements. Curr Top Med Chem. 2006;6:509–537. doi: 10.2174/156802606776743129. [DOI] [PubMed] [Google Scholar]
- Kharasch N, Thyagarajan BS. Structural basis for biological activities of dimethyl sulfoxide. Annal NY Acad Sci. 1983;411:391–402. doi: 10.1111/j.1749-6632.1983.tb47334.x. [DOI] [PubMed] [Google Scholar]
- Koobkokkraud T, Chochai A, Kerdmanee C, De-eknamkul W. TLC-densitometric analysis of artemisinin for the rapid screening of high-producing plantlets of Artemisia annua L. Phtochem Anal. 2007;18:229–234. doi: 10.1002/pca.976. [DOI] [PubMed] [Google Scholar]
- Kumar S, Gupta SK, Singh P, Bajpai MM, Gupta D, Singh D, Gupta AK, Ram G, Shasany AK, Sharma S. High yields of artemisinin by multi-harvest of Artemisia annua crops. Indust Crops Products. 2004;19:77–90. [Google Scholar]
- Li Q-G, Peggins JO, Fleckenstein LL, Masonic K, Heiffer MH, Brewer TG. The pharmacokinetics and bioavailability of dihydroartemisinin, arteether, artemether, artesunic acid and artelinic acid in rats. J Pharm Pharmacol. 1998;50:173–182. doi: 10.1111/j.2042-7158.1998.tb06173.x. [DOI] [PubMed] [Google Scholar]
- Liu KC-SC, Yang S-L, Roberts MF, Elford BC, Phillipson JD. Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Rep. 1992;11:637–640. doi: 10.1007/BF00236389. [DOI] [PubMed] [Google Scholar]
- Lommen WJ, Shenk E, Bouwmeester HJ, Verstappen FW. Trichome dynamics and artemisinin accumulation during development and senescence of Artemisia annua leaves. Planta Med. 2006;72:336–345. doi: 10.1055/s-2005-916202. [DOI] [PubMed] [Google Scholar]
- Mannan A, Liu C, Arsenault P, Towler M, Vail D, Lorence A, Weathers P. DMSO stimulates production of artemisinin suggesting that the sesquiterpene may also function as a ROS sink in Artemisia annua. Plant Cell Rep. 2009 Accepted for publication. [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality, burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina Y, Krishna S, Haynes RK, Meade JC. Artemisinins inhibit Trypanosome cruzi and Trypanosoma crucei rhodesiense in vitro growth. Antimicrob Agents Chemother. 2007;51:1852–1854. doi: 10.1128/AAC.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson ME, Olofsson LM, Lindahl A-L, Lundgren A, Brodelius M, Brodelius PE. Localization of enzymes of artemisinin biosynthesis to the apical cells of glandular trichomes of Artemisia annua L. Phytochem. 2009;70:1123–1128. doi: 10.1016/j.phytochem.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Picaud S, Olofsson L, Brodelius M, Brodelius PE. Expression, purification, and characterization of recombinant amorpha-4, 11-diene synthase from Artemisia annua L. Arch Biochem Biophys. 2005;436:215–226. doi: 10.1016/j.abb.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C, Yakoby N, O’Neal JM, Cornwell T, Pastor I, Fridlender B. Plants and human health in the twenty-first century. Trends Biotechnol. 2002;20:522–531. doi: 10.1016/s0167-7799(02)02080-2. [DOI] [PubMed] [Google Scholar]
- Rath K, Taxis K, Walz G, Gleiter CH, Li SM, Heide L. Pharmacokinetic study of artemisinin after oral intake of a traditional preparation of Artemisia annua L. (Annual Wormwood) Am J Trop Med Hyg. 2004;70:128–132. [PubMed] [Google Scholar]
- Rathore D, McCutchan TF, Sullivan M, Kumar S. Antimalarial drugs: current status and new developments. Expert Opin Investig Drugs. 2005;14:871–883. doi: 10.1517/13543784.14.7.871. [DOI] [PubMed] [Google Scholar]
- Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MC, Withers ST, Shiba Y, Sarpong R, Keasling JD. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006;440:940–943. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- Rodeiro I, Donato MT, Jimenez N, Garrido G, Molina-Torres J, Menendez R, Castell JV, Gomez-Lechon MJ. Inhibition of human P450 enzymes by natural extracts used in traditional medicine. Phytother Res. 2009;23:279–282. doi: 10.1002/ptr.2613. [DOI] [PubMed] [Google Scholar]
- Schramek N, Wang H, Romisch-Margl W, Keil B, Radykewicz T, Winzenhorlein B, Beerhues L, Bacher A, Rohdich F, Gershenzon J, Liu B, Eisenreich W. Artemisinin biosynthesis in growing plants of Artemisia annua. A 13CO2 study. Phytochem. 2010;71:179–187. doi: 10.1016/j.phytochem.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Sen R, Bandyopadhyay S, Dutta A, Mandal G, Ganguly S, Saha P, Chatterjee M. Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania donovani promastigotes. J Med Microbiol. 2007;56:1213–1218. doi: 10.1099/jmm.0.47364-0. [DOI] [PubMed] [Google Scholar]
- Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006;140:634–646. doi: 10.1104/pp.105.072579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy L-K, Brown GD. The mechanism of the spontaneous autoxidation of dihydroartemisinic acid. Tetrahedron. 2002;58:897–908. [Google Scholar]
- Tellez MR, Canel C, Rimando AM, Duke SO. Differential accumulation of isoprenoids in glanded and gland-less Artemisia annua L. Photochem. 1999;52:1035–1040. [Google Scholar]
- Teoh KH, Polichuk DR, Reed DW, Nowak G, Covello PS. Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett. 2006;580:1411–1416. doi: 10.1016/j.febslet.2006.01.065. [DOI] [PubMed] [Google Scholar]
- Teoh KH, Polichuk DR, Reed DW, Covello PS. Molecular cloning of an aldehyde dehydrogenase implicated in artemisinin biosynthesis in Artemisia annua. Botany. 2009;87:635–642. [Google Scholar]
- Titulaer HA, Zuidema J, Kager PA, Wetsteyn JC, Lugt CB, Merkus FW. The pharmacokinetics of artemisinin after oral, intramuscular and rectal administration to volunteers. J Pharm Pharmacol. 1990;42:810–813. doi: 10.1111/j.2042-7158.1990.tb07030.x. [DOI] [PubMed] [Google Scholar]
- Towler MJ, Weathers PJ. Evidence of artemisinin production from IPP stemming from both the mevalonate and the nonmevalonate pathways. Plant Cell Rep. 2007;26:2129–2136. doi: 10.1007/s00299-007-0420-x. [DOI] [PubMed] [Google Scholar]
- Utzinger J, Xiao S, Keiser J, Chen M, Zheng J, Tanner M. Current progress in the development and use of artemether for chemoprophylaxis of major human schistosome parasites. Curr Medicin Chem. 2001;8:1841–1860. doi: 10.2174/0929867013371581. [DOI] [PubMed] [Google Scholar]
- Vitrac X, Larronde F, Krisa S, Decendit A, Deffieux G, Merillon JM. Sugar sensing and Ca2+calmodulin requirement in Vitis vinifera cells producing anthocyanins. Phytochem. 2000;53:659–665. doi: 10.1016/s0031-9422(99)00620-2. [DOI] [PubMed] [Google Scholar]
- Wallaart TE, Van Uden W, Lubberink HGM, Woerdenbag HJ, Pras N, Quax WJ. Isolation and identification of dihydroartemisinic acid from Artemisia annua and its possible role in the biosynthesis of artemisinin. J Nat Prod. 1999;62:430–433. doi: 10.1021/np980370p. [DOI] [PubMed] [Google Scholar]
- Wallaart TE, Pras N, Beekman AC, Quax WJ. Seasonal variation of artemisinin and its biosynthetic precursors in plants of Artemisia annua of different geographical origin: proof for the existence of chemotypes. Planta Med. 2000;66:57–62. doi: 10.1055/s-2000-11115. [DOI] [PubMed] [Google Scholar]
- Wallaart TE, Bouwmeester HJ, Hille J, Popping L, Maijers NC. Amorpha-4, 11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta. 2001;212:460–465. doi: 10.1007/s004250000428. [DOI] [PubMed] [Google Scholar]
- Wang Y, Weathers PJ. Sugars proportionately affect artemisinin production. Plant Cell Rep. 2007;26:1073–1081. doi: 10.1007/s00299-006-0295-2. [DOI] [PubMed] [Google Scholar]
- Wang H, Ge L, Ye HC, Chong K, Liu BY, Li GF. Studies of the effects of fpf1 gene on Artemisia annua flowering time and on the linkage between flowering and artemisinin biosynthesis. Planta Med. 2004;70:347–352. doi: 10.1055/s-2004-818947. [DOI] [PubMed] [Google Scholar]
- Weathers PJ, Wyslouzil BE, Wobbe KK, Kim YJ, Yigit E. The biological response of hairy roots to O2 levels in bioreactors. In Vitro Cell Dev Biol Plant. 1999;35:286–289. [Google Scholar]
- WHO. Meeting on the production of artemisinin and artemisinin-based combination therapies. Arusha: United Republic of Tanzania; 2005 June 6–7;
- WHO. 2006 http://www.who.int/medicines/publications/traditional/ArtemisiaMonograph.pdf.
- Widmer V, Handloser D, Reich E. Quantitative HPTLC analysis of artemisinin in dried Artemisia annua L.: a practical approach. J Liq Chromatog Relat Technol. 2007;30:2209–2219. [Google Scholar]
- Willcox M. Artemisia species: from traditional medicines to modern antimalarials–and back again. J Alternat Complement Med. 2009;15:101–109. doi: 10.1089/acm.2008.0327. [DOI] [PubMed] [Google Scholar]
- Willcox M, Falquet J, Ferreira JFS, Gilbert B, Hsu E, Melillo de Magalhães P, Plaizier-Vercammen J, Sharma VP, Wright CW. Artemisia annua as a herbal tea for malaria. Afr J Trad Complement Alternat Med. 2007;4:121–123. [PMC free article] [PubMed] [Google Scholar]
- Woerdenbag HJ, Luers J, van Uden W, Pras N, Malingre T, Alfermann A. Production of the new antimalarial drug artemisinin in shoot cultures of Artemisia annua L. Plant Cell Tiss Organ Cult. 1993;32:247–257. [Google Scholar]
- Zhang Y, Teoh KH, Reed DW, Maes L, Goossens A, Olson DJH, Ross ARS, Covello PS. The molecular cloning of artemisinic aldehyde Δ11(13) reductase and its role in glandular trichome-dependent biosynthesis of artemisinin in Artemisia annua. J Biol Chem. 2008;283:21501–21508. doi: 10.1074/jbc.M803090200. [DOI] [PubMed] [Google Scholar]