Abstract
Salt-inducible kinase 1 (SIK1) in epithelial cells mediates the increases in active sodium transport (Na+,K+-ATPase-mediated) in response to elevations in the intracellular concentration of sodium. In lung alveolar epithelial cells increases in active sodium transport in response to β-adrenergic stimulation increases pulmonary edema clearance. Therefore, we sought to determine whether SIK1 is present in lung epithelial cells and to examine whether isoproterenol-dependent stimulation of Na+,K+-ATPase is mediated via SIK1 activity. All three SIK isoforms were present in airway epithelial cells, and in alveolar epithelial cells type 1 and type 2 from rat and mouse lungs, as well as from human and mouse cell lines representative of lung alveolar epithelium. In mouse lung epithelial cells, SIK1 associated with the Na+,K+-ATPase α-subunit, and isoproterenol increased SIK1 activity. Isoproterenol increased Na+,K+-ATPase activity and the incorporation of Na+,K+-ATPase molecules at the plasma membrane. Furthermore, those effects were abolished in cells depleted of SIK1 using shRNA, or in cells overexpressing a SIK1 kinase-deficient mutant. These results provide evidence that SIK1 is present in lung epithelial cells and that its function is relevant for the action of isoproterenol during regulation of active sodium transport. As such, SIK1 may constitute an important target for drug discovery aimed at improving the clearance of pulmonary edema.
Keywords: sodium transport, Na+, K+-ATPase activity, lung edema clearance
1. Introduction
Keeping the alveolar space free of fluid is essential for adequate lung function, e.g. maintaining the surface tension and proper gas exchange. Excess fluid in the lung alveoli is reabsorbed across the alveolar epithelial cells type 1 (AEC1) and type 2 (AEC2), via active sodium transport across the alveolo-capillary barrier. Sodium is transported from the alveolar lumen through amiloride sensitive- and insensitive epithelial sodium channels (ENaC) and cGMP gated cation channels located on the apical domain of the alveolar cells [1] and [2], whereas the main driving force for sodium transport over the lung-capillary barrier is the Na+,K+-ATPase localized in the basolateral domain of the membrane [3]. The Na+,K+-ATPase actively pump sodium from the interior of the epithelial cell into the interstitium and/or lung capillaries [4]. The generated sodium gradient drives the transport of water molecules through aquaporins that are localized in the apical domain of the cells, or via paracellular absorption [5].
The regulation of the Na+,K+-ATPase activity is tightly controlled in response to circulating hormones either by modulating its catalytic activity, or by regulating the abundance of active molecules at the plasma membrane [6]. The long-term regulation is achieved by affecting gene transcription and/or protein degradation [6] and [7]. Also, short-term regulation of the catalytic activity (housekeeping function) could be accomplished primarily via antagonist binding which results in inhibition of the activity (e.g. ouabain) or via changes in substrate abundance (e.g. Na+, ATP, cAMP) [6]. Different circulating hormones (e.g. dopamine [8] and [9], aldosterone [10], angiotensin II [11], insulin [12] and [13], isoproterenol [14]) regulate the abundance of active Na+,K+-ATPase molecules at the plasma membrane, in a tissue specific manner, via endocytosis or by recruitment of active Na+,K+-ATPase molecules from intracellular compartments. Similarly, exposing alveolar epithelial cells to hypoxia or hypercapnia leads to modulation of Na+,K+-ATPase activity by regulating the number of active molecules present in the plasma membrane [15] and [16].
Activation of β-adrenergic receptors accelerate lung edema clearance [17], [18] and [19], and this effect is associated with increases in Na+,K+-ATPase activity and active sodium transport. The proposed cellular mechanisms involved incorporation of Na+,K+-ATPase molecules at the plasma membrane of alveolar epithelial cells [14] and changes in the state of phosphorylation of the Na+,K+-ATPase α-subunit [20] and [21].
We recently described in renal epithelial cells the presence of a sodium-sensing network that is responsible for the regulation of Na+,K+-ATPase activity [22]. At the core of this network is the salt-inducible kinase 1 (SIK1) that is activated in a calcium-dependent manner leading to increases in Na+,K+-ATPase activity in response to elevated intracellular sodium [22]. SIK1 is also responsible for the increases in Na+,K+-ATPase activity in renal tubules from Milan hypertensive rats [23]. SIK1 is a member of the AMPK family of kinases [24] and expressed in various transporting epithelia [25] and [26]. Various functions have been ascribed to SIK1, such as a regulator of gene expression [27], cardiac development [28], and steroidogenesis [29].
Within this background, we sought to determine whether SIK1 is present in lung epithelial cells and whether it mediates the stimulatory effect of isoproterenol an alveolar cell Na+,K+-ATPase activity.
2. Materials and Methods
2.1. Reagents
SIK1, SIK2 and SIK3 antibodies and SIK1 pT182 antibody were described elsewhere [30], β-Tubulin (T4026) antibody was from Sigma-Aldrich, E-cadherin from Santa Cruz Biotechnology, CA, USA, Hemagglutinin (HA) antibody was from Covance (Denver, Pennsylvania, USA. 5α monoclonal antibody directed to the NK α-subunit and T1α (Developmental Studies Hybridoma Bank, University of Iowa). FITC-conjugated goat anti rabbit IgG from Invitrogen (Carlsbad, CA, USA), LB180 was provided by Dr. Mary Williams and Dr. Antoinette Wetterwald, and Texas Red-labeled goat anti-mouse IgG from Invitrogen (Carlsbad, CA, USA). EZ-link NHS-SS-biotin and streptavidin-Sepharose beads were from Pierce Chemical Co. (Rockford, Il, USA). Isoproterenol and ouabain were from Sigma, and [86Rb+] RbCl from Perkin Elmer (Norwalk, Connecticut, USA). All other reagents were of highest grade available.
2.2. Cell lines
A model of pulmonary epithelial cells from mouse, MLE-12 cells (ATCC) were grown in DMEM/F12 (Gibco/Invitrogen) supplemented with 10 % FBS and 1 % Penicillin/streptomycin. A549 cells, a human lung carcinoma cell line, and Opossum Kidney (OK) cells from the proximal tubules, were grown in DMEM supplemented with 10 % FBS and 1 % Penicillin/streptomycin. NCI-H441 cells, a human lung adenocarcinoma epithelial cell line, exhibiting a number of alveolar epithelial cells type II characteristics, were cultured in RPMI 1640 medium, supplemented with 10% FBS and 1% Penicillin/streptomycin.
2.3. Plasmids and transfection
Cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to manufacturer's instructions, with expression times between 24 and 48h. Stable clones of shRNA constructs were selected using Hygromycin B (Gibco/Invitrogen). Transient transfection performed with SIK1 wild type (WT) or SIK1 mutant (K56M), both bearing a hemagglutinin tag (HA) [22], and SIK1-GST [30]. SIK1 was also silenced using shRNA. pSilencer 3.1-H1-hygro containing shRNA for SIK1 (Forward 5'→3'GATCCGGGAGTACGAGGGTCCCCAGTTCAAGAGACTGGGGACCCTCGTACTCCTTTTTTGG AAA; Reverse 5'→3'AGCTTTTCCAAAAAAGGAGTACGAGGGTCCCCAGTCTCTTGAACTGGGGACCCTCGT ACTCCCG) or a negative control containing a scramble sequence (Forward 5'→3'GATCCGTTACACTTTTTTGGAAA; Reverse 5'→3' CTAGGCAATGTGAAAAAACCTTT). All constructs were verified by DNA sequence analysis and the expression validated by western blot.
2.4. Isolation of rat AEC1 and AEC2 cells
AEC1 and AEC2 were isolated from male Sprague-Dawley rats as described [31]. The Northwestern University Animal Use and Care Committee approved all animal procedures performed in this study. Rats were anaesthetized with ketamine (40 mg/kg/BW) and xylazine (8 mg/kg/BW). A tracheotomy was performed and rats were ventilated with a rodent ventilator. The heart was then transected and a catheter placed in the pulmonary artery. After perfusion, the lungs were removed for the isolation of AEC1 and AEC2. The purities of the final AEC1 and AEC2 preparations were >90% and >98% (assessed by immunocytochemistry using anti-T1α antibodies anti-LB180 antibodies, respectively). Cross-contaminations of AEC1 and AEC2 were less than 0.5%. The viabilities of both cell preparations were >95%.
2.5. Reverse transcription-PCR
Total RNAs were isolated using RNeasy Mini kit (Qiagen, Valencia, CA) and incubated with DNase I enzyme (Ambion, Austin, TX). First-strand cDNA synthesis was performed using Superscript™ first-strand synthesis system (Invitrogen, Carlsbad, CA). Primers for SIK1, SIK2, SIK3 and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were listed in Table 1. Conditions for PCR amplification were: 10 mM of Tris-HCl (pH 9.0 at 25 °C), 50 mM of KCl, 0.1% Triton X-100, 1.8 mM of MgCl2, 0.16 mM of dNTP mix, 1.6 μM of each primer and 1 unit of DNA Taq polymerase, with 20 ng of cDNA in a final reaction volume of 25μl. Agarose-gel (1.5%) electrophoresis and ethidium bromide staining were used to visualize PCR bands.
Table 1.
primers list
| Primer sequence (5' - 3') | Size | GenBank ID | |
|---|---|---|---|
| Mouse sik1f | CTTCGACATGCAAGGCTACA | 262 | NM_010831 |
| Mouse sik1r | AGGAACCTCCAGACTGCTGA | ||
| Mouse sik2f | TCCAAGACCTTTCGAGCAGT | 255 | AB067780 |
| Mouse sik2r | GGAAGAGTCGCTTCTGTTGG | ||
| Mouse sik3f | GGCTTCAGCAACCTCTTCAC | 323 | NM_27498 |
| Mouse sik3r | TTGTGCCTGCAGATCTGTTC | ||
| Rat sik1f | GCTTTTACGACGTGGAACG | 677 | AC096909 |
| Rat sik1r | GTCTTGAGACATGAAGAAGG | ||
| Rat sik2f | TCTCCATGAGTGATAGCCCC | 428 | DQ188032 |
| Rat sik2r | ACTGCTCGAAAGGTCTTGGA | ||
| Rat sik3f | GAACAGTGCAGCTCCTTTCC | 684 | XM_001068984 |
| Rat sik3r | ACCAGTTGGTGACTTGGAGG | ||
| Human sik1f | GAGTCACCAAAACGCAGGTT | 456 | NM_173354 |
| human sik1r | CCTTCCCCTCAAAGACTTCC | ||
| human sik2f | TCCCAGTGGAGCAGAGACTT | 431 | NM_015191 |
| human sik2r | TTCTGACAGAGTGTGCCGTC | ||
| human sik3f | GGCTACTACGAGATCGACCG | 622 | NM_025164 |
| human sik3r | GATTCTGCAGTGTGCTTCCA | ||
| GAPDHf | ACCACAGTCCATGCCATCAC | 452 | BC064681 |
| GAPDHr | TCCACCACCCTGTTGCTGTA |
2.6. Real-time PCR
Sub-confluent cells were harvested and lysed and RNA extraction was performed using Omega Biotech E.Z.N.A.™ Total RNA purification kit according to manufacturer's instructions. Genomic DNA was digested using Omega Biotech E.Z.N.A. RNase free DNase Kit 1. Total RNA (500 ng), was reversely transcribed into cDNA using RevertAID™ H Minus M-MuLV Reverse Transcriptase, Random Hexamer primer and RiboLock RNase Inhibitor from Fermentas Life Science (Vilnius, Lithuania). SIK1 gene expression levels were analyzed using Mm00440317_m1 Gene Expression Assay and Master mix from Applied Biosystems (Foster City, CA, USA) and normalized for the expression of RPLP0 (ribosomal protein, large, P0) by Mm00725448_s1, using ABI PRISM 7000 Sequence Detection System using the 7000 System SDS Software Version 1.2.3 (Applied Biosystems). Samples were assayed in duplicates using the standard curve method.
2.7. Immunohistochemistry
Paraffin-embedded lung tissue sections (4 μm thick) were devoid of paraffin with xylene and rehydrated. Antigen retrieval was used before blocking in PBS with 10% normal goat serum, 0.1%BSA, 0.3% TX-100. The antigen retrieval solution is 0.5M Tris-HCl with 5% Urea, pH 9.5. The dilutions for rabbit against SIK1, SIK2, SIK3, mouse against LB180 and mouse against rat T1α antibodies are 1:100, 1:100, 1:100, 1:50 and 1:200, respectively. Secondary antibody for SIK1, SIK2, and SIK3 was FITC-conjugated goat anti rabbit IgG (1:1000) and for T1α or LB180, Texas Red-labeled goat anti-mouse IgG (1:1000). An anti-fade mounting media (Innovex Biosciences) was used to fix the coverslip to a slide. The slides were examined using a Nikon Eclipse E800 fluorescence microscope, and the images were processed by MetaMorph software (Molecular Devices, Inc.).
2.8. Determination of SIK1 activity
MLE-12 cells were transformed with SIK1-GST. After 24h expression time, the cells were incubated with or w/o agonist. Thereafter, cells were then placed on ice, rinsed with PBS, and lysed. The lysates were centrifuged and the supernatant added to MicroSpin GST Purification Module (GE Healthcare), eluted in 10 mM glutathione and then analysed by SDS-PAGE and Western blot. Protein concentration determined according to Bradford [32] using a commercial reagent (Bio-Rad). Proteins were separated using the Laemmli buffer system [33]. The SIK1 activity was assessed by measuring the levels of SIK1 phosphorylated at Threonine 182 and compared with the total amount of SIK1. ImageJ software (http://rsb.info.nih.gov/ij/) was used for quantification.
2.9. Immunoprecipitation
Cells were lysed in immunoprecipitation (IP) buffer: 50 mM TRIS, 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 50 mM NaF, 1% Triton X-100, and protease inhibitors (1 mM PMSF, 5 μg/ml leupeptin, 5 μg/ml antipain, 5 μg/ml pepstatin A and 10 μg/ml aprotinin). Post-nuclear supernatants (PNS) were pre-cleared with protein A/G (Santa Cruz Biotechnology). The PNS were incubated in IP with NK 5α antibody for 16 h with end-over-end rotation at 4°C. Protein A/G was added, and after additional 2h at end-over-end rotation at 4°C, the complexes were spun down and washed with IP buffer. Samples were warmed at 54°C for 20 minutes in Laemmli loading buffer [33], and the proteins were analysed by SDS-PAGE and Western blot.
2.10. Determination of Na+,K+-ATPase activity
Na+,K+-ATPase activity was assayed using 86Rb+ transport as described previously [34]. Briefly, MLE-12 cells transiently transfected with SIK1 WT or K56M mutant were seeded in 24-well plates to a density of 100 000 cells/ well. Media was exchanged to serum free DMEM with 50 mM HEPES (pH 7,4) EGTA (2 mM) was added for 20 minutes incubation at 37°C. Trace amounts of [86RB+] RbCl was added and cells were incubated 5 minutes at room temperature before Isoproterenol was added. The reaction was terminated by washing with ice-cold NaCl and lysing of the cells in 3% SDS. Radioactivity was quantified by using a scintillation counter and corrected for protein concentration. Na+,K+-ATPase mediated transport was calculated by subtracting the activity in samples treated with ouabain from the total activity.
2.11. Plasma membrane biotinylation
Cells were labeled for one hour using 0.5 mg/ml EZ-link NHS-SS-biotin. Thereafter, the cells were rinsed with PBS containing 50 mM glycine to quench unreacted biotin and then lysed in modified RIPA buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 1% NP-40, and 1% sodium deoxycholate, 1 μg/ml leupeptin, 100 μg/ml TPCK, and 1 mM PMSF). Aliquots (150 μg of total protein) were incubated overnight at 4°C with end-over-end shaking in the presence of streptavidin beads. The beads were thoroughly washed and then re-suspended in 30 μl of Laemmli sample buffer solution [33]. SDS-PAGE and Western blotting were used to determine the plasma membrane abundance of the α-subunit.
2.13. Statistical analysis
To estimate the significance of the differences between the samples, the unpaired Student's t-test was used. P-values ≤ 0.05 were considered statistically significant.
3. Results and Discussion
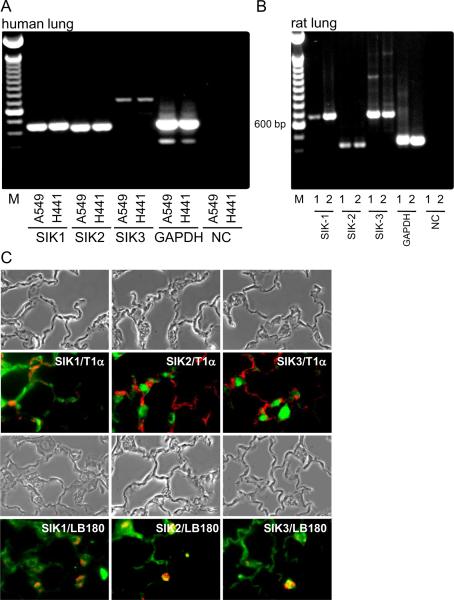
We examined whether all three SIK isoforms were present in airways/alveolar epithelial cells from several species. Using reverse transcription PCR, we identified the presence of mRNA for all three isoforms in A549 and H441 cells, both cell lines derived from human lungs and that retain certain characteristics of alveolar epithelial cells (Fig. 1A). Similarly, all three SIK isoforms were present in AEC1 and AEC2 from rat lungs (Fig. 1B). At the protein levels, using immunofluorescence, all three isoforms were present in AEC1 and AEC2 from rat lung sections (Fig. 1C).
Fig. 1.
Presence and localization of SIK isoforms in rat alveolar epithelium and in cell lines of human origin. (A) SIK mRNA detection by reverse transcription-PCR in human cell lines, A549 and H441 cells. GAPDH used as positive control and samples processed without reverse transcriptase as negative control. (B) SIK mRNA detection by reverse transcription-PCR in isolated AEC1 and AEC2 from rats. DNA ladder (1kb) used in (A) and (B). (C) SIK isoforms detected by immunohistochemistry in sections derived from rat lungs. AEC1 and AEC2 stained with T1 and LB180, respectively. Magnification: 400×.
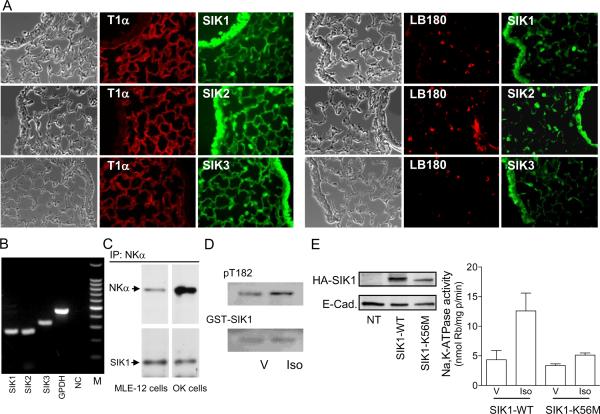
SIK isoforms were also present in lung epithelial cells and in small airways in mice lung sections. Immunohistochemistry revealed the presence of all three isoforms in AEC1 and AEC2 (Fig. 2A). The presence and localization of all three SIK isoforms is of relevance because of the wide range of cellular functions in which these isoforms are reported to be involved. The fact that lung sections revealed the presence of the SIK1 isoform in both, AEC1 and AEC2 suggest that its role during regulation of Na+,K+-ATPase activity and active sodium transport could take place theoretically in both cell types. Most functional studies aimed at understanding the role and regulation of Na+,K+-ATPase activity during lung edema clearance has been performed in isolated AEC2 or cell line models of AEC2. In a cell line (MLE-12) representative of a mouse model of AEC2 cells mRNA was detected for all three SIK isoforms (Fig. 2B). Because the SIK1 isoform associates with the Na+,K+-ATPase and regulates the activity in response to increases in sodium permeability [22], we further examined whether SIK1 also associates with the Na+,K+-ATPase in MLE-12 cells. Similar to OK cells, the SIK1 protein coimmunoprecipitated with the NK α-subunit (Fig. 2C), and may therefore participate in its regulation. Thus, we examined whether isoproterenol, a known activator of Na+,K+-ATPase activity, regulates SIK1 activity in MLE-12 cells. SIK1 activity could be determined in intact cells by measuring the state of phosphorylation of its Thr-182 residue. Isoproterenol increased SIK1 activity (% increase: 27 ± 3, n=4, P = 0.013) (Fig. 2D). Isoproterenol increases both SIK1- and Na+,K+-ATPase activity. To examine whether SIK1 plays a role during stimulation of Na+,K+-ATPase, MLE-12 cells were transiently transfected with SIK1 wild type or a kinase deficient mutant (K56M). Isoproterenol increased Na+,K+-ATPase activity in cells transiently overexpressing the SIK1 wild type (similar to what has been previously reported for non-transfected cells [14]), but this effect was absent in cells overexpressing an inactive SIK1 (Fig. 2E). These results suggest that SIK1 is part of the cell-signaling network utilized by isoproterenol during stimulation of Na+,K+-ATPase activity.
Fig. 2.
Localization of SIK isoforms in sections of mouse lungs and the role of SIK1 during regulation of Na+,K+-ATPase activity. (A) SIK isoforms in sections derived from mouse lungs. AEC1 and AEC2 stained with T1 and LB180, respectively. Magnification: 400×. (B) SIK mRNA detection by reverse transcription-PCR in MLE-12 cells. GAPDH was used to verify quantity of cDNA and reaction without enzyme was used as the negative control. DNA ladder (100bp) was used. (C) Na+,K+-ATPase α-subunit was co-immunoprecipitated with SIK1 from MLE-12 and OK cells. Representative Western blot. (D) SIK1 activity was measured in MLE-12 cells transformed with GST-SIK1 and pretreated with isoproterenol (Iso) 1 μM for 15 min at room temperature, or vehicle (V). Samples were analyzed by SDS-PAGE and Western blot with a SIK1 or pT182 antibody. Representative experiment of three performed independently. (E) Left panel: representative Western blot of lysates obtained from MLE-12 cells transfected with the cDNA for SIK1 wild type (SIK-WT), for kinase-deficient mutant (SIKK56M), or non-transfected (NT) cells. Right panel: determination of Na+,K+-ATPase activity in MLE-12 cells overexpressing the SIK-WT or SIK1-K56M that have been incubated with (Iso) or w/o (V) isoproterenol as indicated in (D). Each bar is the mean + SEM of three experiments performed independently and in triplicate determinations.
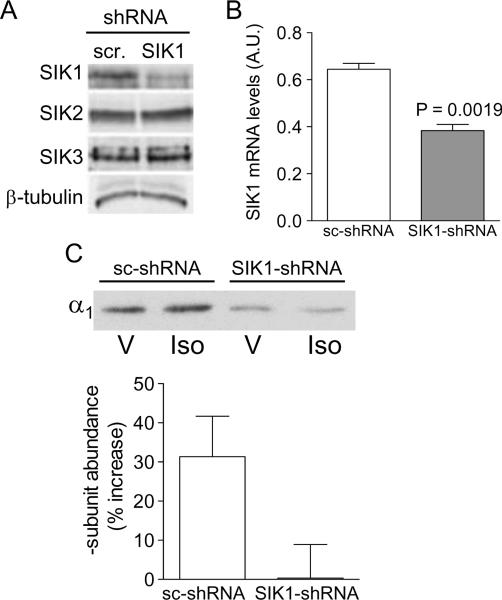
Isoproterenol increased Na+,K+-ATPase activity by incorporation of new molecules at the plasma membrane [14]. To further examine whether SIK is involved in such process, we utilized MLE-12 cells in which the SIK1 protein expression has been stably suppressed. Expression of SIK1-shRNA promoted a reduction in SIK1 protein expression only (Fig. 3A) as well as in the SIK1 mRNA level (Fig. 3B) when compared to cells expressing a scrambled sc-shRNA. Plasma membrane biotinylation was performed to assess the incorporation of Na+,K+-ATPase molecules at the plasma membrane. Whereas isoproterenol increased the α-subunit abundance at the plasma membrane in cells expressing sc-shRNA, this effect was absent in cells expressing SIK1-shRNA (lacking SIK1) (Fig. 3C). In order to influence the intracellular cellular trafficking of Na+,K+-ATPase-containing vesicles, hormones regulate the activity of molecular motors that ultimately propel these vesicles to the plasma membrane. Because such motors (i.e.: kinesin) contain SIK-consensus phosphorylation sites it is plausible that SIK1 might be part of a distinct signaling network specifically regulating Na+,K+-ATPase trafficking to the plasma membrane in response to isoproterenol. We have previously reported that SIK1 controls the Na+,K+-ATPase activity of molecules that are present at the plasma membrane via activation of phosphatases and de-phosphorylation of the catalytic subunit [22]. Thus, the present results may also suggest that SIK1 could mediated the trafficking of active molecules to the plasma membrane, and this event alone or in combination with increases in the catalytic activity of Na+,K+-ATPase molecules that are already the plasma membrane account for the increases in active sodium transport and alveolar fluid clearance.
Fig. 3.
Role of SIK1 during regulation of Na+,K+-ATPase trafficking by isoproterenol in MLE-12 lacking SIK1 isoform. (A) Western blot analysis of MLE-12 cells lysates lacking (SIK1-shRNA)- vs cells expressing SIK1 (sc-shRNA) using a SIK1, SIK2, SIK3 and a b-tubulin antibody for loading control. A representative blot is shown. (B) Expression of SIK1 (mRNA levels) in cells lacking (SIK1-shRNA)- vs cells expressing SIK1 (sc-shRNA). Each bar represents the mean + SEM value of three experiments. (C) Upper panel: Abundance of Na+,K+-ATPase α-subunit at the plasma membrane of MLE-12 cells lacking (SIK1-shRNA)- vs cells expressing SIK1 (sc-shRNA) and treated w (Iso) or w/o (V) isoproterenol 1 μM for 15 min at room temperature. A representative blot is shown. Lower panel: Quantitative analysis is expressed as % change. Each bar represents the mean + SEM of six experiments performed independently.
In conclusion, these data indicate that SIK1 is present in lung epithelial cells as well as in small airways epithelia, and that its function is relevant for the action of isoproterenol during regulation of active sodium transport. Therefore, SIK1 may constitute an important target for drug discovery aimed at improving the clearance of pulmonary edema.
Acknowledgements
We thank Dr Laura Brion for constructive advice. The study was supported in part by grants from the Swedish Research Council (10860), Swedish Heart and Lung Foundation, and the National Institutes of Health (HL48129).
Abbreviations
- AEC1
alveolar epithelial cells type 1
- AEC2
alveolar epithelial cells type 2
- SIK1
salt-inducible kinase 1
- siRNA
small inhibitory ribonucleic acid
- shRNA
short hairpin ribonucleic acid
- Iso
isoproterenol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Yue G, Russell WJ, Benos DJ, Jackson RM, Olman MA, Matalon S. Increased expression and activity of sodium channels in alveolar type II cells of hyperoxic rats. Proc Natl Acad Sci U S A. 1995;92:8418–22. doi: 10.1073/pnas.92.18.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jain L, Chen XJ, Malik B, Al-Khalili O, Eaton DC. Antisense oligonucleotides against the alpha-subunit of ENaC decrease lung epithelial cation-channel activity. Am J Physiol. 1999;276:L1046–51. doi: 10.1152/ajplung.1999.276.6.L1046. [DOI] [PubMed] [Google Scholar]
- [3].Sznajder JI, Factor P, Ingbar DH. Invited review: lung edema clearance: role of Na(+)-K(+)-ATPase. J Appl Physiol. 2002;93:1860–6. doi: 10.1152/japplphysiol.00022.2002. [DOI] [PubMed] [Google Scholar]
- [4].Factor P, Senne C, Dumasius V, Ridge K, Jaffe HA, Uhal B, Gao Z, Sznajder JI. Overexpression of the Na+,K+-ATPase alpha1 subunit increases Na+,K+-ATPase function in A549 cells. Am J Respir Cell Mol Biol. 1998;18:741–9. doi: 10.1165/ajrcmb.18.6.2918. [DOI] [PubMed] [Google Scholar]
- [5].Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- [6].Therien AG, Blostein R. Mechanisms of sodium pump regulation. Am J Physiol Cell Physiol. 2000;279:C541–66. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- [7].Lecuona E, Sun H, Vohwinkel C, Ciechanover A, Sznajder JI. Ubiquitination participates in the lysosomal degradation of Na,K-ATPase in steady-state conditions. Am J Respir Cell Mol Biol. 2009;41:671–9. doi: 10.1165/rcmb.2008-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aperia A, Bertorello A, Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol. 1987;252:F39–45. doi: 10.1152/ajprenal.1987.252.1.F39. [DOI] [PubMed] [Google Scholar]
- [9].Bertorello AM, Komarova Y, Smith K, Leibiger IB, Efendiev R, Pedemonte CH, Borisy G, Sznajder JI. Analysis of Na+,K+-ATPase motion and incorporation into the plasma membrane in response to G protein-coupled receptor signals in living cells. Mol Biol Cell. 2003;14:1149–57. doi: 10.1091/mbc.E02-06-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].O'Neil RG. Aldosterone regulation of sodium and potassium transport in the cortical collecting duct. Semin Nephrol. 1990;10:365–74. [PubMed] [Google Scholar]
- [11].Bharatula M, Hussain T, Lokhandwala MF. Angiotensin II AT1 receptor/signaling mechanisms in the biphasic effect of the peptide on proximal tubular Na+,K+-ATPase. Clin Exp Hypertens. 1998;20:465–80. doi: 10.3109/10641969809053225. [DOI] [PubMed] [Google Scholar]
- [12].Sweeney G, Klip A. Regulation of the Na+/K+-ATPase by insulin: why and how? Mol Cell Biochem. 1998;182:121–33. [PubMed] [Google Scholar]
- [13].Comellas AP, Kelly AM, Trejo HE, Briva A, Lee J, Sznajder JI, Dada LA. Insulin regulates alveolar epithelial function by inducing Na+/K+-ATPase translocation to the plasma membrane in a process mediated by the action of Akt. J Cell Sci. 2010;123:1343–51. doi: 10.1242/jcs.066464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bertorello AM, Ridge KM, Chibalin AV, Katz AI, Sznajder JI. Isoproterenol increases Na+-K+-ATPase activity by membrane insertion of alpha-subunits in lung alveolar cells. Am J Physiol. 1999;276:L20–7. doi: 10.1152/ajplung.1999.276.1.L20. [DOI] [PubMed] [Google Scholar]
- [15].Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest. 2003;111:1057–64. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Vadasz I, Dada LA, Briva A, Trejo HE, Welch LC, Chen J, Toth PT, Lecuona E, Witters LA, Schumacker PT, Chandel NS, Seeger W, Sznajder JI. AMP-activated protein kinase regulates CO2-induced alveolar epithelial dysfunction in rats and human cells by promoting Na,K-ATPase endocytosis. J Clin Invest. 2008;118:752–62. doi: 10.1172/JCI29723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Atabai K, Ware LB, Snider ME, Koch P, Daniel B, Nuckton TJ, Matthay MA. Aerosolized beta(2)-adrenergic agonists achieve therapeutic levels in the pulmonary edema fluid of ventilated patients with acute respiratory failure. Intensive Care Med. 2002;28:705–11. doi: 10.1007/s00134-002-1282-x. [DOI] [PubMed] [Google Scholar]
- [18].Litvan J, Briva A, Wilson MS, Budinger GR, Sznajder JI, Ridge KM. Beta-adrenergic receptor stimulation and adenoviral overexpression of superoxide dismutase prevent the hypoxia-mediated decrease in Na,K-ATPase and alveolar fluid reabsorption. J Biol Chem. 2006;281:19892–8. doi: 10.1074/jbc.M602064200. [DOI] [PubMed] [Google Scholar]
- [19].Folkesson HG, Matthay MA. Alveolar epithelial ion and fluid transport: recent progress. Am J Respir Cell Mol Biol. 2006;35:10–9. doi: 10.1165/rcmb.2006-0080SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Blanco G, Sanchez G, Mercer RW. Differential regulation of Na,K-ATPase isozymes by protein kinases and arachidonic acid. Arch Biochem Biophys. 1998;359:139–50. doi: 10.1006/abbi.1998.0904. [DOI] [PubMed] [Google Scholar]
- [21].Lecuona E, Ridge K, Pesce L, Batlle D, Sznajder JI. The GTP-binding protein RhoA mediates Na,K-ATPase exocytosis in alveolar epithelial cells. Mol Biol Cell. 2003;14:3888–97. doi: 10.1091/mbc.E02-12-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sjostrom M, Stenstrom K, Eneling K, Zwiller J, Katz AI, Takemori H, Bertorello AM. SIK1 is part of a cell sodium-sensing network that regulates active sodium transport through a calcium-dependent process. Proc Natl Acad Sci U S A. 2007;104:16922–7. doi: 10.1073/pnas.0706838104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Stenstrom K, Takemori H, Bianchi G, Katz AI, Bertorello AM. Blocking the salt-inducible kinase 1 network prevents the increases in cell sodium transport caused by a hypertension-linked mutation in human alpha-adducin. J Hypertens. 2009;27:2452–7. doi: 10.1097/HJH.0b013e328330cf15. [DOI] [PubMed] [Google Scholar]
- [24].Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J. 2004;23:833–43. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Feldman JD, Vician L, Crispino M, Hoe W, Baudry M, Herschman HR. The salt-inducible kinase, SIK, is induced by depolarization in brain. J Neurochem. 2000;74:2227–38. doi: 10.1046/j.1471-4159.2000.0742227.x. [DOI] [PubMed] [Google Scholar]
- [26].Wang Z, Takemori H, Halder SK, Nonaka Y, Okamoto M. Cloning of a novel kinase (SIK) of the SNF1/AMPK family from high salt diet-treated rat adrenal. FEBS Lett. 1999;453:135–9. doi: 10.1016/s0014-5793(99)00708-5. [DOI] [PubMed] [Google Scholar]
- [27].Li S, Zhang C, Takemori H, Zhou Y, Xiong ZQ. TORC1 regulates activity-dependent CREB-target gene transcription and dendritic growth of developing cortical neurons. J Neurosci. 2009;29:2334–43. doi: 10.1523/JNEUROSCI.2296-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Romito A, Lonardo E, Roma G, Minchiotti G, Ballabio A, Cobellis G. Lack of sik1 in mouse embryonic stem cells impairs cardiomyogenesis by down-regulating the cyclin-dependent kinase inhibitor p57kip2. PLoS One. 5:e9029. doi: 10.1371/journal.pone.0009029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Okamoto M, Takemori H, Katoh Y. Salt-inducible kinase in steroidogenesis and adipogenesis. Trends Endocrinol Metab. 2004;15:21–6. doi: 10.1016/j.tem.2003.11.002. [DOI] [PubMed] [Google Scholar]
- [30].Lin X, Takemori H, Katoh Y, Doi J, Horike N, Makino A, Nonaka Y, Okamoto M. Salt-inducible kinase is involved in the ACTH/cAMP-dependent protein kinase signaling in Y1 mouse adrenocortical tumor cells. Mol Endocrinol. 2001;15:1264–76. doi: 10.1210/mend.15.8.0675. [DOI] [PubMed] [Google Scholar]
- [31].Chen J, Chen Z, Narasaraju T, Jin N, Liu L. Isolation of highly pure alveolar epithelial type I and type II cells from rat lungs. Lab Invest. 2004;84:727–35. doi: 10.1038/labinvest.3700095. [DOI] [PubMed] [Google Scholar]
- [32].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- [33].Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- [34].Efendiev R, Bertorello AM, Zandomeni R, Cinelli AR, Pedemonte CH. Agonist-dependent regulation of renal Na+,K+-ATPase activity is modulated by intracellular sodium concentration. J Biol Chem. 2002;277:11489–96. doi: 10.1074/jbc.M108182200. [DOI] [PubMed] [Google Scholar]