Abstract
This study aimed at evaluating glycemia and lipid profile of offspring from diabetic Wistar rats treated with Mentha piperita (peppermint) juice. Male offspring from nondiabetic dams (control group: 10 animals treated with water and 10 treated with peppermint juice) and from dams with streptozotocin-induced severe diabetes (diabetic group: 10 animals treated with water and 10 treated with peppermint juice) were used. They were treated during 30 days, and, after the treatment period, levels of glycemia, triglycerides, total cholesterol, and fractions were analyzed in the adult phase. The offspring from diabetic dams treated with peppermint showed significantly reduced levels of glucose, cholesterol, LDL-c, and triglycerides and significant increase in HDL-c levels. The use of the M. piperita juice has potential as culturally appropriate strategy to aid in the prevention of DM, dyslipidemia, and its complications.
1. Introduction
Diabetes mellitus (DM) is considered to be a syndrome associated with disorders in the metabolism of carbohydrates, lipids, and proteins caused by the absolute or relative lack of insulin [1], and it affects approximately 3 to 10% of the women in the gestational phase [2]. In pregnancy, DM can cause serious problems to mothers and their offspring, contributing with approximately 10% of fetal malformations and approximately 40% of neonatal death [2–4]. The lack of serious maternal glycemic control changes fetal metabolism, which leads to higher risk for fetuses to show metabolic alterations, both in the gestational period and throughout their lives [5, 6]. The alterations resulting from maternal diabetes are related to hyperglycemia and fetal hyperinsulinemia, which affect lipid and protein synthesis [7, 8]. Additionally, maternal hyperglycemia stimulates fetal growth (macrosomia) due to the greater availability of glucose in the blood flow and by the regulation of growth factors [5, 8]. High weight at birth is related to the risk for developing insulin resistance, obesity, and DM2 in the future [9].
Studies show that obesity and DM2 are more frequent in children and adolescents whose mothers were diabetic or had gestational DM. Maternal metabolic disorders affect the growth and the metabolism of descendants, leading to diabetogenesis in different phases of the offspring life, including their pregnancies. Hence, the next generation will also be affected, and the same consequences will influence other generations, thus showing the diabetogenic tendency [10–12].
The problems resulting from DM and its complications bring high costs to the Brazilian Health Care System. The search for medical alternatives to reduce costs can be highly valuable for those living below the poverty line. An alternative can be the use of medicinal plants, which have been increasingly used in the treatment of DM and other metabolic syndrome risk factors [13–15].
Mentha piperita L (family Labiatae; genus Mentha) is one of the ten most widely used plants in Brazil and is commonly used in the treatment of loss of appetite, common cold, bronchitis, fever, nausea, vomiting [16], spasmodic responses [17], and antimicrobial and antioxidant activities [18, 19]. It is also used for culinary purposes. There is evidence that it has a positive effect on glycemia of the laboratory animal [20]. However, there are no reports in the literature concerning its effects on the offspring of diabetic animals treated with the respective plant. Hence, this study aimed at evaluating glycemia and lipid profile of offspring of diabetic Wistar rats treated with Mentha piperita juice.
2. Methods
2.1. Parental Generation
The Wistar rats used were kept in the vivarium of UNIMEP (Lins Campus) under controlled conditions (12/12-hour light/dark cycle and ambient temperature of 22 ± 2°C, relative humidity of 60 ± 5%, and water and chow ad libitum). These animals were treated according to the “Guide to the care and use of experimental animals,” which delineates the principles by the Canadian Council for the care to laboratory animals. The study was initiated after its approval by the Ethics Committee under registration number 2500000764/2007-47.
2.2. Diabetogenesis Period: Diabetes Induction
Nondiabetic rats (n = 20) weighing approximately 250 g underwent a seven-day adaptation period in the room where the experiment was conducted. Thereafter, randomly selected rats received an intravenous administration (caudal vein) of 40 mg of streptozotocin (STZ)/Kg (STZ, Sigma Chemical Company, St. Louis, Mo, USA) diluted in citrate buffer (0.1 M; pH 4.5). Nondiabetic animals (n = 20) received only the vehicle (citrate buffer) in a volume that was equivalent to that of the diabetogenic drug [17, 18]. As an inclusion criterion, rats were considered to be diabetic (n = 20) when showing glycemia above 200 mg/dL and nondiabetic (control, n = 20) when showing glycemia below 120 mg/dL.
2.3. Mating Period
After diabetes confirmation and separation of the two experimental groups, the mating phase was performed at the end of the afternoon. The rats from each group were grouped, four by four, with a normoglycemic male rat. In the next morning, the males were removed, and vaginal smears were then collected. In case spermatozoa were found in the vaginal smears, that was considered to be day 0 of pregnancy [19].
2.4. Pregnancy Period
In the mornings of days 0, 7, 14, and 21 of pregnancy, a blood drop was collected from the caudal vein for glycemia measurement by using glucometer One Touch Ultra, Johnson and Johnson. Approximately on the day 21 of pregnancy, after birth of offspring, the dams remained in individual cages with their offspring until weaning (21 days).
2.5. Offspring: Weaning and Treatment
After weaning, the offspring (60 animals) were kept in collective cages until they reached adulthood. The adult animals, weighing approximately 250 g, were divided into 4 experimental groups (n = 15 animals/group): G1: offspring from control dams treated with vehicle (water), G2: offspring from control dams treated with Mentha piperita juice, G3: offspring from diabetic dams treated with vehicle, and G4: offspring from diabetic dams treated with Mentha piperita juice.
The animals in groups G2 and G4 received Mentha piperita juice at a dose of 0.29 g/kg once a day (at early morning) for 30 consecutive days. The dose administered to the animals was based on 100 g/L, which corresponds to the daily intake of 200 mL of juice by an adult man weighing 70.0 kg (such intake was based on population consultation).
2.6. Mentha piperita Juice Preparation
Mentha piperita leaves were washed, weighed (100 g/L), and triturated with water in a blender for 7 minutes. The juice was filtered and frozen in an amber flask. Each flask was thawed daily at ambient temperature two hours prior to administration.
2.7. Blood Collection and Biochemical Profile Determination
After 30 days of treatment, the animals were anesthetized with sodium pentobarbital (150 mg/kg) and killed. Subsequently, blood samples were collected in order to determine the biochemical profile (total cholesterol, HDL-c, LDL-c, triglycerides, and glucose). Tests were performed according to the methodology proposed by commercial kits: LABTEST (Lagoa Santa, Belo Horizonte, MG) for glycemia, total cholesterol and HDL-c, and triglycerides and WIENER LAB (São Paulo, SP) for LDL-c. The results were interpreted according to criteria established by the American Diabetes Association [1].
2.8. Statistical Analysis
Data analysis was performed by using Student's t test, and the level of significance adopted was P value <.05%.
3. Results
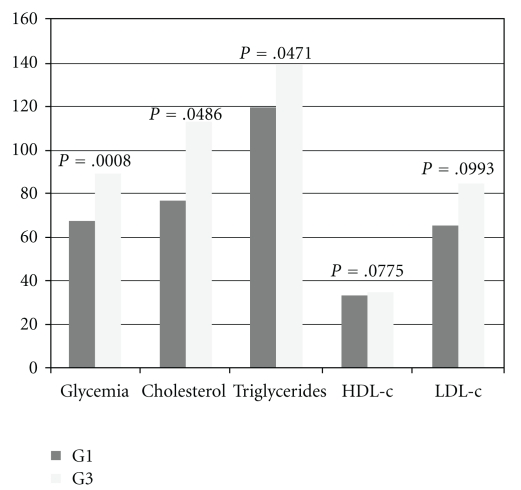
Figure 1 shows the comparison between the control groups comprising the offspring from diabetic dams and control dams. It was observed that the offspring from group G3 showed significantly higher glycemia, cholesterol, and triglycerides levels when compared to those from group G1.
Figure 1.
Representation of biochemical profile of G1 and G3.
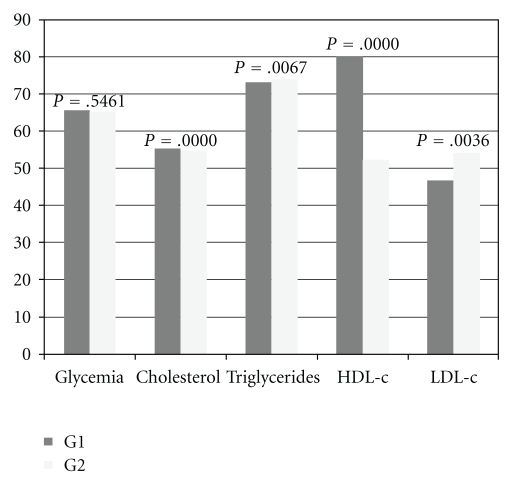
Figure 2 shows the biochemical profile results for the offspring from control dams (G1) and from nondiabetic dams treated with M. piperita (G2). No significant differences were observed for glycemia between these two groups, but there were a significant reduction in plasma levels of cholesterol, triglycerides, and LDL-c and significant increased HDL-c values in the offspring from group G2 in relation to those from group G1.
Figure 2.
Representation of biochemical profile of G1 and G2.
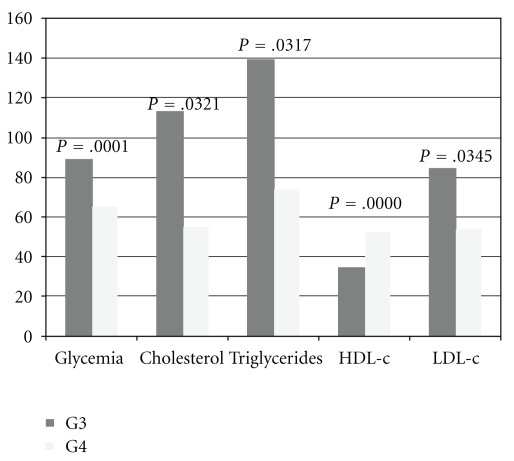
Figure 3 shows the biochemical profile results for the offspring from diabetic dams treated with vehicle (G3) and from diabetic dams treated with M. piperita (G4). The glycemia and the levels of cholesterol, LDL-c, and triglycerides were significantly reduced and the levels of HDL-c were significantly increased in group G4 as compared to group G3.
Figure 3.
Representation of biochemical profile of G3 and G4.
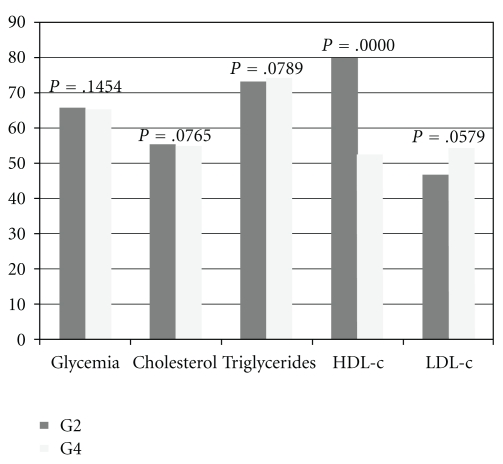
Figure 4 shows the biochemical profile results for male offspring from nondiabetic dams treated with M. piperita juice (G2) and from diabetic dams treated with M. piperita juice (G4). There were no significant differences in the glycemia, cholesterol, LDL-c, and triglycerides between the offspring from G4 and G2 groups. It was observed that offspring from G4 dams showed increased HDL-c levels in relation to those from G2.
Figure 4.
Representation of biochemical profile of G2 and G4.
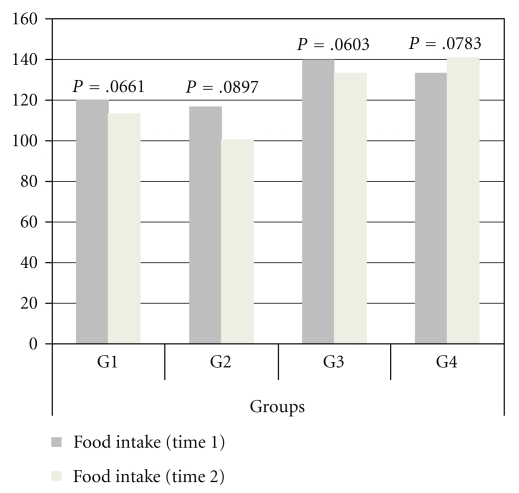
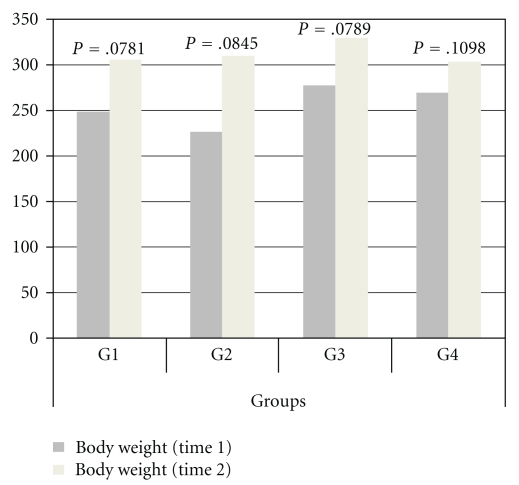
No differences were found in food intake and body weight among the groups at the beginning and end of the treatment (Figures 5 and 6).
Figure 5.
Representation of food intake of G1, G2, G3, and G4.
Figure 6.
Representation of body weight of G1, G2, G3 and G4.
4. Discussion
There are a large number of medicinal plants that are popularly used for diabetes mellitus (DM) and hypercholesterolemia treatment [13, 21–24]. Several plants used by the population have been scientifically verified [21, 25–27]. Mentha piperita (peppermint) is one of the plants most frequently used by the Brazilian population for therapeutic purposes. Its medicinal use includes anti-inflammatory, antispasmodic, and analgesic activities and the treatment of respiratory and gastrointestinal problems, as well as its antioxidant and antiperoxidant effects [28].
In this study, it was observed that the offspring from diabetic dams showed high levels of blood glucose and lipids. However, significant reductions were also found for the glycemia of offspring from diabetic mothers treated with peppermint juice, thus corroborating the findings by Narendhirakannan et al. [20], who evaluated the effect of Mentha piperita on diabetic rats. These results show that the use of peppermint juice can be beneficial in the therapy and/or prevention of DM and its complications in the offspring from dams with diabetes. Other studies in the literature show that the use of plants or antioxidant substances can help avoid damages associated with diabetes [13–15, 29–36].
The importance of peppermint can also be extrapolated to the offspring of nondiabetic dams (normoglycemic) as they also benefitted from using the plant. Peppermint juice also positively influences the concentration of plasma lipids in the studied animals, both in the offspring from nondiabetic and in those from diabetic dams. Barbalho et al. [37] observed benefits in glucose and lipid profile after using peppermint juice in normoglycemic Wistar rats. Plants contain a number of biologically active compounds able to modulate lipid and glucose metabolism, of which flavonoids and other antioxidant compounds play a central role for improving the lipid and hyperglycemic profile [38–41]. Visavadiya and Narasimhacharya [35] found hypolipidemic and antioxidant effects in asparagus root. Lee et al. [42] investigated Wasabia japonica in vitro and in vivo and observed significant antioxidant activities and anti-hypercholesterolemic effects. Kumar et al. [36] found similar effects after using Anthocephalus indicus root.
The species from the Mentha genus have substances that may be related to such effects. M. arvensis contains menthol, murol, eugenol, thymol, and hydrocarbonates. Menthol and other volatile compounds can be found in the leaves of M. piperita [28], and such compounds can be responsible, at least partly, for antioxidant and antiperoxidant effects as observed by Veerapur et al. after using Ficus racemosa Stem Bark Extract [43] and Adesegun et al. after using Phaulopsis fascisepala [44]. Samarth and Samarth (2009) showed that M. piperita leaf extract possesses high amount of phenolic content, flavonoids content, and flavonols. They also observed that this plant has radioprotective effects possibly because of the amount of phenolic compounds, flavonoids, and flavonols due to their antioxidant and radical scavenging activity [45].
As medicines used to regulate glycemia and dyslipidemia are costly, the use of peppermint juice may be an alternative low-cost strategy to treat noncommunicable diseases associated with the insulin dysfunction. It can also be used to prevent the complications of gestational DM, thus preventing fetal hyperglycemia and hyperinsulinemia, metabolic abnormalities, and the metabolic syndrome in the offspring from mothers with DM. As peppermint also shows antioxidant and antiperoxidant effects, it also can prevent oxidative damages [21, 46–49].
Species of Mentha are aromatic plants traditionally used as medicinal remedies and culinary herbs but this study suggests that the use of the M. piperita juice has potential as a culturally appropriate strategy to aid in the prevention of DM, dyslipidemia, and its complications.
Despite the promising results concerning the use of peppermint, it is fundamentally important to perform further studies in order to evaluate its effects on human beings and the ideal doses to be used.
Conflict of Interests
No competing financial interests exist.
References
- 1.American Diabetes Association (ADA) 2010, http://care.diabetesjournals.org/content/33/Supplement_1/S3.full.pdf+html.
- 2.Arteaga J, Luna L, Mutchinick OM. Diabets, pregnancy and birth defectsDiabetes, embarazo y defectos al nacimiento. Revista de Investigacion Clinica. 2008;60(2):107–114. [PubMed] [Google Scholar]
- 3.Rudge MVC, Calderon IDMP. The obstetrician ethical responsibility in the diagnosis and treatment of gestational diabetes mellitus (GDM)A responsabilidade do obstetra sobre o diagnóstico e o tratamento do diabete melito gestacional. Revista Brasileira de Ginecologia e Obstetricia. 2006;28(10):571–574. [Google Scholar]
- 4.Heiskanen N, Saarelainen H, Kärkkäinen H, et al. Gestational diabetic patients with adequate management have normal cardiovascular autonomic regulation during the third trimester of pregnancy and 3 months after delivery. Journal of Diabetes and Its Complications. 2010;24(4):234–241. doi: 10.1016/j.jdiacomp.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Nomura RMY, Costa VN, Sakamoto K, Maguanha CA, Miyadahira S, Zugaib M. Computerized cardiotocography in pregnancies complicated by pregestational diabetes mellitus: heart rate patterns in large for gestational age fetuses. Revista Brasileira de Ginecologia e Obstetrícia. 2005;27(12):712–718. [Google Scholar]
- 6.Tannus LRM, Oliveira DS, Matheus ASDM, Cunha EF, Gomes MB. Early infancy onset of type 1A Diabetes mellitus in dizygotic twins: association with genetic and environmental factors. Arquivos Brasileiros de Endocrinologia e Metabologia. 2007;51(1):142–145. doi: 10.1590/s0004-27302007000100023. [DOI] [PubMed] [Google Scholar]
- 7.Padilha PDC, Saunders C, Machado RCM, et al. Association between pre-gestational nutritional status and prediction of the risk of adverse pregnancy outcome. Revista Brasileira de Ginecologia e Obstetricia. 2007;29(10):511–518. [Google Scholar]
- 8.Maayan-Metzger A, Lubin D, Kuint J. Hypoglycemia rates in the first days of life among term infants born to diabetic mothers. Neonatology. 2009;96(2):80–85. doi: 10.1159/000203337. [DOI] [PubMed] [Google Scholar]
- 9.Hummel S, Pflüger M, Kreichauf S, Hummel M, Ziegler AG. Predictors of overweight during childhood in offspring of parents with type 1 diabetes. Diabetes Care. 2009;32(5):921–925. doi: 10.2337/dc08-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aerts L, Van Assche FA. Animal evidence for the transgenerational development of diabetes mellitus. International Journal of Biochemistry and Cell Biology. 2006;38(5-6):894–903. doi: 10.1016/j.biocel.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Buzinaro EF, Berchieri CB, Haddad ALM, Padovani CR, De Paula Pimenta W. Overweight in adolescent offspring of women with hyperglycemia during pregnancy. Arquivos Brasileiros de Endocrinologia e Metabologia. 2008;52(1):85–92. doi: 10.1590/s0004-27302008000100012. [DOI] [PubMed] [Google Scholar]
- 12.Simeoni U, Barker DJ. Offspring of diabetic pregnancy: long-term outcomes. Seminars in Fetal and Neonatal Medicine. 2009;14(2):119–124. doi: 10.1016/j.siny.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Volpato GT, Vieira FL, Damasceno DC, Câmara FLA, Di Stasi LC, Lemonica IP. Effects of leaves of Polymnia sonchifolia (yacon) aqueous extract in diabetic ratsEfeito do extrato aquoso de folhas de Polymnia sonchifolia (yacon) em ratas diabéticas. Revista Brasileira de Plantas Medicinais. 2007;9(2):88–93. [Google Scholar]
- 14.Gonçalves B, Falco V, Moutinho-Pereira J, Bacelar E, Peixoto F, Correia C. Effects of elevated CO2 on grapevine (Vitis vinifera L.): volatile composition, phenolic content, and in vitro antioxidant activity of red wine. Journal of Agricultural and Food Chemistry. 2009;57(1):265–273. doi: 10.1021/jf8020199. [DOI] [PubMed] [Google Scholar]
- 15.Okabe T, Toda T, Inafuku M, Wada K, Iwasaki H, Oku H. Antiatherosclerotic function of Kokuto, Okinawan noncentrifugal cane sugar. Journal of Agricultural and Food Chemistry. 2009;57(1):69–75. doi: 10.1021/jf802796m. [DOI] [PubMed] [Google Scholar]
- 16.Akdogan M, Kilinç I, Oncu M, Karaoz E, Delibas N. Investigation of biochemical and histopathological effects of Mentha piperita L. and Mentha spicata L. on kidney tissue in rats. Human and Experimental Toxicology. 2003;22(4):213–219. doi: 10.1191/0960327103ht332oa. [DOI] [PubMed] [Google Scholar]
- 17.Lu M, Battinelli L, Daniele C, Melchioni C, Salvatore G, Mazzanti G. Muscle relaxing activity of Hyssopus officinalis essential oil on isolated intestinal preparations. Planta Medica. 2002;68(3):213–216. doi: 10.1055/s-2002-23139. [DOI] [PubMed] [Google Scholar]
- 18.Romero-Jimenez M, Campos-Sanchez J, Analla M, Munoz-Serrano A, Alonso-Moraga A. Genotoxicity and anti-genotoxicity of some traditional medicinal herbs. Mutation Research. 2005;585(1-2):147–155. doi: 10.1016/j.mrgentox.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Mimica-Dukic N, Bozin B, Sokovic M, Mihajlovic B, Matavulj M. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Medica. 2003;69(5):413–419. doi: 10.1055/s-2003-39704. [DOI] [PubMed] [Google Scholar]
- 20.Narendhirakannan RT, Subramanian S, Kandaswamy M. Biochemical evaluation of antidiabetogenic properties of some commonly used Indian plants on streptozotocin-induced diabetes in experimental rats. Clinical and Experimental Pharmacology and Physiology. 2006;33(12):1150–1157. doi: 10.1111/j.1440-1681.2006.04507.x. [DOI] [PubMed] [Google Scholar]
- 21.Sethi J, Yadav M, Dahiya K, Sood S, Singh V, Bhattacharya SB. Antioxidant effect of Triticum aestivium (wheat grass) in high-fat diet-induced oxidative stress in rabbits. Methods and Findings in Experimental and Clinical Pharmacology. 2010;32(4):233–235. doi: 10.1358/mf.2010.32.4.1423889. [DOI] [PubMed] [Google Scholar]
- 22.Said O, Fulder S, Khalil K, Azaizeh H, Kassis E, Saad B. Maintaining a physiological blood glucose level with ’glucolevel’, a combination of four anti-diabetes plants used in the traditional Arab herbal medicine. Evidence-Based Complementary and Alternative Medicine. 2008;5(4):421–428. doi: 10.1093/ecam/nem047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo JZ, Luo L. Ginseng on hyperglycemia: effects and mechanisms. Evidence-Based Complementary and Alternative Medicine. 2009;6(4):423–427. doi: 10.1093/ecam/nem178. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Vosough-Ghanbari S, Rahimi R, Kharabaf S, et al. Effects of Satureja khuzestanica on serum glucose, lipids and markers of oxidative stress in patients with Type 2 diabetes mellitus: a double-blind randomized controlled trial. Evidence-Based Complementary and Alternative Medicine. 2010;7(4):465–470. doi: 10.1093/ecam/nen018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calderon IMP, Rudge MVC, Brasil MAM. Diabetes and experimental pregnancy in rats: I. Diabetes induction, pregnancy obtention and evolution. Acta Cirurgica Brasileira. 2002;7:9–14. [Google Scholar]
- 26.Cetinkalp S, Delen Y, Karadeniz M, Yüce G, Yilmaz C. The effect of 1α,25(OH)2D3 vitamin over oxidative stress and biochemical parameters in rats where Type 1 diabetes is formed by streptozotocin. Journal of Diabetes and Its Complications. 2009;23(6):401–408. doi: 10.1016/j.jdiacomp.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Damasceno DC, Kempinas WG, Volpato GT, Consoni M, Rudge MVC, Paumgartten FJR. Anomalias Congênitas: Estudos Experimentais. 1st edition. Belo Horizonte, Brasil: Coopmed; 2008. [Google Scholar]
- 28.Samarth RM. Protection against radiation induced hematopoietic damage in bone marrow of Swiss albino mice by Mentha piperita (Linn) Journal of Radiation Research. 2007;48(6):523–528. doi: 10.1269/jrr.07052. [DOI] [PubMed] [Google Scholar]
- 29.Fidan AF, Dündar Y. The effects of Yucca schidigera and Quillaja saponaria on DNA damage, protein oxidation, lipid peroxidation, and some biochemical parameters in streptozotocin-induced diabetic rats. Journal of Diabetes and Its Complications. 2008;22(5):348–356. doi: 10.1016/j.jdiacomp.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Helmstädter A, Schuster N. Vaccinium myrtillus as an antidiabetic medicinal plant—research through the ages. Pharmazie. 2010;65(5):315–321. [PubMed] [Google Scholar]
- 31.Kiss ACI, Takaku M, Damasceno DC, et al. Effect of Allium sativum L. (garlic) aqueous extract on biochemical parameters of the Streptozotocin-induced diabetic rats. Revista Brasileira de Plantas Medicinais. 2006;8(3):24–30. [Google Scholar]
- 32.Hürdağ C, Uyaner I, Gürel E, Utkusavas A, Atukeren P, Demirci C. The effect of α-lipoic acid on NOS dispersion in the lung of streptozotocin-induced diabetic rats. Journal of Diabetes and Its Complications. 2008;22(1):56–61. doi: 10.1016/j.jdiacomp.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Haidara MA, Mikhailidis DP, Rateb MA, et al. Evaluation of the effect of oxidative stress and vitamin E supplementation on renal function in rats with streptozotocin-induced Type 1 diabetes. Journal of Diabetes and Its Complications. 2009;23(2):130–136. doi: 10.1016/j.jdiacomp.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Shirpoor A, Salami S, Khadem-Ansari MH, Ilkhanizadeh B, Pakdel FG, Khademvatani K. Cardioprotective effect of vitamin E: rescues of diabetes-induced cardiac malfunction, oxidative stress, and apoptosis in rat. Journal of Diabetes and Its Complications. 2009;23(5):310–316. doi: 10.1016/j.jdiacomp.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Visavadiya NP, Narasimhacharya AVRL. Asparagus root regulates cholesterol metabolism and improves antioxidant status in hypercholesteremic rats. Evidence-Based Complementary and Alternative Medicine. 2009;6(2):219–226. doi: 10.1093/ecam/nem091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar V, Mahdi F, Chander R, et al. Hypolipidemic and antioxidant activity of Anthocephalus indicus (Kadam) root extract. Indian Journal of Biochemistry and Biophysics. 2010;47(2):104–109. [PubMed] [Google Scholar]
- 37.Barbalho SM, Spada APM, de Oliveira EP, et al. Mentha piperita effects on wistar rats plasma lipids. Brazilian Archives of Biology and Technology. 2009;52(5):1137–1143. [Google Scholar]
- 38.Bera TK, De D, Chatterjee K, Ali KM, Ghosh D. Effect of Diashis, a polyherbal formulation, in streptozotocin-induced diabetic male albino rats. International Journal of Ayurveda Research. 2010;1(1):18–24. doi: 10.4103/0974-7788.59939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaimal S, Sujatha KS, George S. Hypolipidaemic and antioxidant effects of fruits of Musa AAA (chenkadali) in alloxan induced diabetic rats. Indian Journal of Experimental Biology. 2010;48(2):165–173. [PubMed] [Google Scholar]
- 40.Choudhury RP, Kumar A, Garg AN. Analysis of Indian mint (Mentha spicata) for essential, trace and toxic elements and its antioxidant behaviour. Journal of Pharmaceutical and Biomedical Analysis. 2006;41(3):825–832. doi: 10.1016/j.jpba.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 41.Leifert WR, Abeywardena MY. Cardioprotective actions of grape polyphenols. Nutrition Research. 2008;28(11):729–737. doi: 10.1016/j.nutres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Lee YS, Yang JH, Bae MJ, et al. Anti-oxidant and anti-hypercholesterolemic activities of Wasabia japonica . Evidence-Based Complementary and Alternative Medicine. 2010;7(4):459–464. doi: 10.1093/ecam/nen038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Veerapur VP, Prabhakar KR, Parihar VK, et al. Ficus racemosa stem bark extract: a potent antioxidant and a probable natural radioprotector. Evidence-Based Complementary and Alternative Medicine. 2009;6(3):317–324. doi: 10.1093/ecam/nem119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adesegun SA, Fajana A, Orabueze CI, Coker HAB. Evaluation of antioxidant properties of Phaulopsis fascisepala C.B.Cl. (Acanthaceae) Evidence-Based Complementary and Alternative Medicine. 2009;6(2):227–231. doi: 10.1093/ecam/nem098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samarth RM, Samarth M. Protection against radiation-induced testicular damage in Swiss Albino Mice by Mentha piperita (Linn.) Basic and Clinical Pharmacology and Toxicology. 2009;104(4):329–334. doi: 10.1111/j.1742-7843.2009.00384.x. [DOI] [PubMed] [Google Scholar]
- 46.Edris AE, Girgis BS, Fadel HHM. Recovery of volatile aroma components from aqueous waste streams using an activated carbon column. Food Chemistry. 2003;82(2):195–202. [Google Scholar]
- 47.Schmidt E, Bail S, Buchbauer G, et al. Chemical composition, olfactory evaluation and antioxidant effects of essential oil from Mentha piperita . Natural Product Communications. 2009;4(8):1107–1112. [PubMed] [Google Scholar]
- 48.Dorman HJD, Koşar M, Başer KHC, Hiltunen R. Phenolic profile and antioxidant evaluation of Mentha piperita L. (peppermint) extracts. Natural Product Communications. 2009;4(4):535–542. [PubMed] [Google Scholar]
- 49.López V, Martín S, Gómez-Serranillos MP, Carretero ME, Jäger AK, Calvo MI. Neuroprotective and neurochemical properties of mint extracts. Phytotherapy Research. 2010;24(6):869–874. doi: 10.1002/ptr.3037. [DOI] [PubMed] [Google Scholar]