Abstract
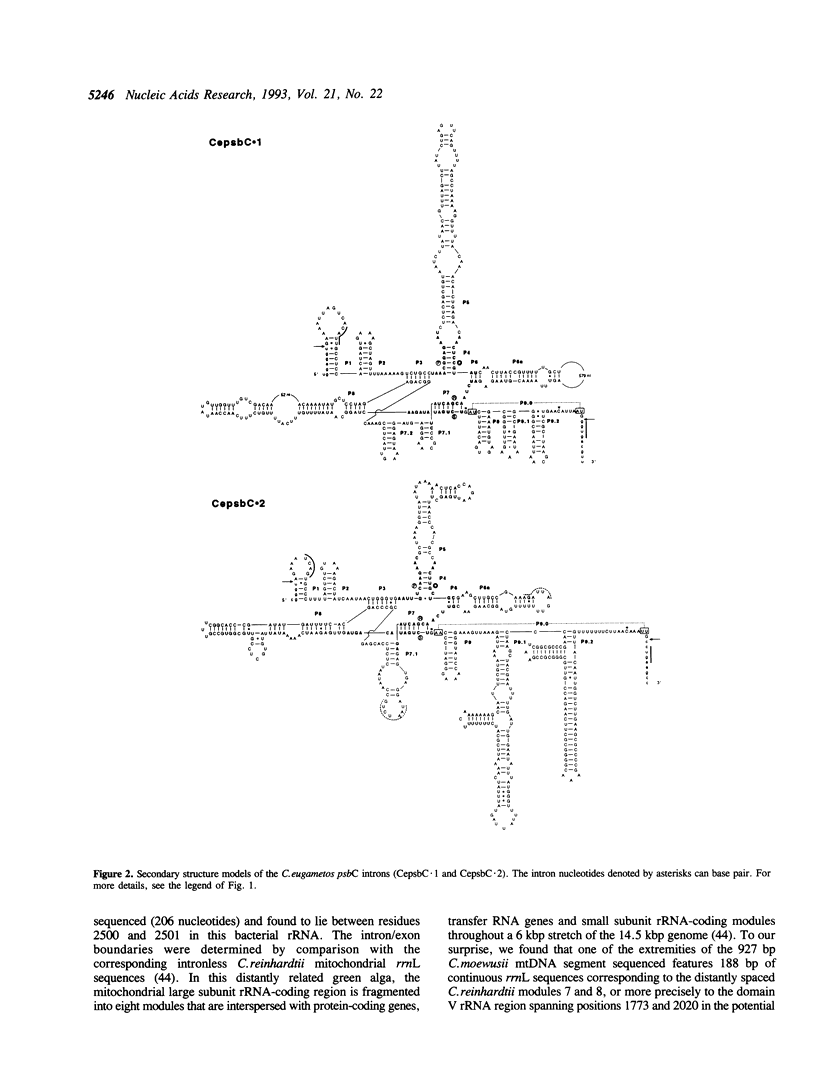
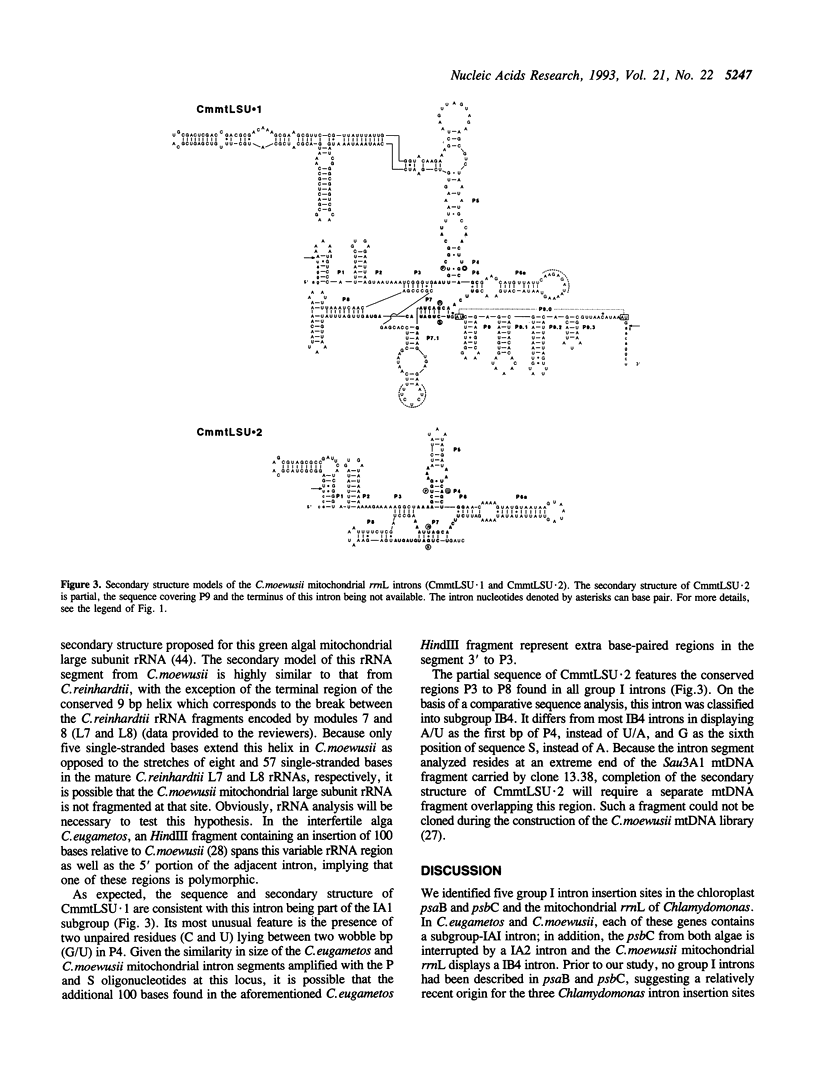
The polymerase chain reaction was used to identify novel IAI subgroup introns in cpDNA-enriched preparations from the interfertile green algae Chlamydomonas eugametos and Chlamydomonas moewusii. These experiments along with sequence analysis disclosed the presence, in both green algae, of a single IA1 intron in the psaB gene and of two group I introns (IA2 and IA1) in the psbC gene. In addition, two group I introns (IA1 and IB4) were found in the peptidyltransferase region of the mitochondrial large subunit rRNA gene at the same positions as previously reported Chlamydomonas chloroplast introns. The 188 bp segment preceding the first mitochondrial intron revealed extensive sequence similarity to the distantly spaced rRNA-coding modules L7 and L8 in the Chlamydomonas reinhardtii mitochondrial DNA, indicating that these two modules have undergone rearrangements in Chlamydomonas. The IA1 introns in psaB and psbC were found to be related in sequence to the first intron in the C. moewusii chloroplast psbA gene. The similarity between the former introns extends to the immediate 5' flanking exon sequence, suggesting that group I intron transposition occurred from one of the two genes to the other through reverse splicing.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boer P. H., Gray M. W. Scrambled ribosomal RNA gene pieces in Chlamydomonas reinhardtii mitochondrial DNA. Cell. 1988 Nov 4;55(3):399–411. doi: 10.1016/0092-8674(88)90026-8. [DOI] [PubMed] [Google Scholar]
- Bowman C. M., Barker R. F., Dyer T. A. In wheat ctDNA, segments of ribosomal protein genes are dispersed repeats, probably conserved by nonreciprocal recombination. Curr Genet. 1988 Aug;14(2):127–136. doi: 10.1007/BF00569336. [DOI] [PubMed] [Google Scholar]
- Burke J. M., Esherick J. S., Burfeind W. R., King J. L. A 3' splice site-binding sequence in the catalytic core of a group I intron. Nature. 1990 Mar 1;344(6261):80–82. doi: 10.1038/344080a0. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Intron phylogeny: a new hypothesis. Trends Genet. 1991 May;7(5):145–148. [PubMed] [Google Scholar]
- Cech T. R. Conserved sequences and structures of group I introns: building an active site for RNA catalysis--a review. Gene. 1988 Dec 20;73(2):259–271. doi: 10.1016/0378-1119(88)90492-1. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Domenico J. M., Nelson J. DNA sequence and secondary structures of the large subunit rRNA coding regions and its two class I introns of mitochondrial DNA from Podospora anserina. J Mol Evol. 1989 Mar;28(3):242–255. doi: 10.1007/BF02102482. [DOI] [PubMed] [Google Scholar]
- Cushman J. C., Hallick R. B., Price C. A. The two genes for the P700 chlorophyll a apoproteins on the Euglena gracilis chloroplast genome contain multiple introns. Curr Genet. 1988 Feb;13(2):159–171. doi: 10.1007/BF00365651. [DOI] [PubMed] [Google Scholar]
- Côté V., Mercier J. P., Lemieux C., Turmel M. The single group-I intron in the chloroplast rrnL gene of Chlamydomonas humicola encodes a site-specific DNA endonuclease (I-ChuI). Gene. 1993 Jul 15;129(1):69–76. doi: 10.1016/0378-1119(93)90697-2. [DOI] [PubMed] [Google Scholar]
- Davies R. W., Waring R. B., Ray J. A., Brown T. A., Scazzocchio C. Making ends meet: a model for RNA splicing in fungal mitochondria. Nature. 1982 Dec 23;300(5894):719–724. doi: 10.1038/300719a0. [DOI] [PubMed] [Google Scholar]
- De Wachter R., Neefs J. M., Goris A., Van de Peer Y. The gene coding for small ribosomal subunit RNA in the basidiomycete Ustilago maydis contains a group I intron. Nucleic Acids Res. 1992 Mar 25;20(6):1251–1257. doi: 10.1093/nar/20.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denovan-Wright E. M., Lee R. W. Comparative analysis of the mitochondrial genomes of Chlamydomonas eugametos and Chlamydomonas moewusii. Curr Genet. 1992 Mar;21(3):197–202. doi: 10.1007/BF00336841. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dron M., Rahire M., Rochaix J. D. Sequence of the chloroplast 16S rRNA gene and its surrounding regions of Chlamydomonas reinhardii. Nucleic Acids Res. 1982 Dec 11;10(23):7609–7620. doi: 10.1093/nar/10.23.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B. Group I introns as mobile genetic elements: facts and mechanistic speculations--a review. Gene. 1989 Oct 15;82(1):91–114. doi: 10.1016/0378-1119(89)90034-6. [DOI] [PubMed] [Google Scholar]
- Durocher V., Gauthier A., Bellemare G., Lemieux C. An optional group I intron between the chloroplast small subunit rRNA genes of Chlamydomonas moewusii and C. eugametos. Curr Genet. 1989 Apr;15(4):277–282. doi: 10.1007/BF00447043. [DOI] [PubMed] [Google Scholar]
- Dávila-Aponte J. A., Huss V. A., Sogin M. L., Cech T. R. A self-splicing group I intron in the nuclear pre-rRNA of the green alga, Ankistrodesmus stipitatus. Nucleic Acids Res. 1991 Aug 25;19(16):4429–4436. doi: 10.1093/nar/19.16.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürrenberger F., Rochaix J. D. Chloroplast ribosomal intron of Chlamydomonas reinhardtii: in vitro self-splicing, DNA endonuclease activity and in vivo mobility. EMBO J. 1991 Nov;10(11):3495–3501. doi: 10.1002/j.1460-2075.1991.tb04913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. M., Rahire M., Rochaix J. D. Chlamydomonas reinhardii gene for the 32 000 mol. wt. protein of photosystem II contains four large introns and is located entirely within the chloroplast inverted repeat. EMBO J. 1984 Dec 1;3(12):2753–2762. doi: 10.1002/j.1460-2075.1984.tb02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier A., Turmel M., Lemieux C. A group I intron in the chloroplast large subunit rRNA gene of Chlamydomonas eugametos encodes a double-strand endonuclease that cleaves the homing site of this intron. Curr Genet. 1991 Jan;19(1):43–47. doi: 10.1007/BF00362086. [DOI] [PubMed] [Google Scholar]
- Jaeger L., Westhof E., Michel F. Function of P11, a tertiary base pairing in self-splicing introns of subgroup IA. J Mol Biol. 1991 Oct 20;221(4):1153–1164. doi: 10.1016/0022-2836(91)90925-v. [DOI] [PubMed] [Google Scholar]
- Joyce P. B., Gray M. W. Chloroplast-like transfer RNA genes expressed in wheat mitochondria. Nucleic Acids Res. 1989 Jul 25;17(14):5461–5476. doi: 10.1093/nar/17.14.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhsel M. G., Strickland R., Palmer J. D. An ancient group I intron shared by eubacteria and chloroplasts. Science. 1990 Dec 14;250(4987):1570–1573. doi: 10.1126/science.2125748. [DOI] [PubMed] [Google Scholar]
- Kück U., Choquet Y., Schneider M., Dron M., Bennoun P. Structural and transcription analysis of two homologous genes for the P700 chlorophyll a-apoproteins in Chlamydomonas reinhardii: evidence for in vivo trans-splicing. EMBO J. 1987 Aug;6(8):2185–2195. doi: 10.1002/j.1460-2075.1987.tb02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambowitz A. M., Belfort M. Introns as mobile genetic elements. Annu Rev Biochem. 1993;62:587–622. doi: 10.1146/annurev.bi.62.070193.003103. [DOI] [PubMed] [Google Scholar]
- Lee R. W., Dumas C., Lemieux C., Turmel M. Cloning and characterization of the Chlamydomonas moewusii mitochondrial genome. Mol Gen Genet. 1991 Dec;231(1):53–58. doi: 10.1007/BF00293821. [DOI] [PubMed] [Google Scholar]
- Michel F., Netter P., Xu M. Q., Shub D. A. Mechanism of 3' splice site selection by the catalytic core of the sunY intron of bacteriophage T4: the role of a novel base-pairing interaction in group I introns. Genes Dev. 1990 May;4(5):777–788. doi: 10.1101/gad.4.5.777. [DOI] [PubMed] [Google Scholar]
- Michel F., Westhof E. Modelling of the three-dimensional architecture of group I catalytic introns based on comparative sequence analysis. J Mol Biol. 1990 Dec 5;216(3):585–610. doi: 10.1016/0022-2836(90)90386-Z. [DOI] [PubMed] [Google Scholar]
- Michel H., Hunt D. F., Shabanowitz J., Bennett J. Tandem mass spectrometry reveals that three photosystem II proteins of spinach chloroplasts contain N-acetyl-O-phosphothreonine at their NH2 termini. J Biol Chem. 1988 Jan 25;263(3):1123–1130. [PubMed] [Google Scholar]
- Montandon P. E., Vasserot A., Stutz E. Euglena gracilis chloroplast DNA: analysis of a 1.6 kb intron of the psb C gene containing an open reading frame of 458 codons. Curr Genet. 1986;11(1):35–39. doi: 10.1007/BF00389423. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Logsdon J. M., Jr The recent origins of introns. Curr Opin Genet Dev. 1991 Dec;1(4):470–477. doi: 10.1016/s0959-437x(05)80194-7. [DOI] [PubMed] [Google Scholar]
- Reinhold-Hurek B., Shub D. A. Self-splicing introns in tRNA genes of widely divergent bacteria. Nature. 1992 May 14;357(6374):173–176. doi: 10.1038/357173a0. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., Kuchka M., Mayfield S., Schirmer-Rahire M., Girard-Bascou J., Bennoun P. Nuclear and chloroplast mutations affect the synthesis or stability of the chloroplast psbC gene product in Chlamydomonas reinhardtii. EMBO J. 1989 Apr;8(4):1013–1021. doi: 10.1002/j.1460-2075.1989.tb03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochaix J. D., Rahire M., Michel F. The chloroplast ribosomal intron of Chlamydomonas reinhardii codes for a polypeptide related to mitochondrial maturases. Nucleic Acids Res. 1985 Feb 11;13(3):975–984. doi: 10.1093/nar/13.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin M. L., Ingold A., Karlok M., Nielsen H., Engberg J. Phylogenetic evidence for the acquisition of ribosomal RNA introns subsequent to the divergence of some of the major Tetrahymena groups. EMBO J. 1986 Dec 20;5(13):3625–3630. doi: 10.1002/j.1460-2075.1986.tb04691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmel M., Boulanger J., Lemieux C. Two group I introns with long internal open reading frames in the chloroplast psbA gene of Chlamydomonas moewusii. Nucleic Acids Res. 1989 May 25;17(10):3875–3887. doi: 10.1093/nar/17.10.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmel M., Boulanger J., Schnare M. N., Gray M. W., Lemieux C. Six group I introns and three internal transcribed spacers in the chloroplast large subunit ribosomal RNA gene of the green alga Chlamydomonas eugametos. J Mol Biol. 1991 Mar 20;218(2):293–311. doi: 10.1016/0022-2836(91)90713-g. [DOI] [PubMed] [Google Scholar]
- Turmel M., Gutell R. R., Mercier J. P., Otis C., Lemieux C. Analysis of the chloroplast large subunit ribosomal RNA gene from 17 Chlamydomonas taxa. Three internal transcribed spacers and 12 group I intron insertion sites. J Mol Biol. 1993 Jul 20;232(2):446–467. doi: 10.1006/jmbi.1993.1402. [DOI] [PubMed] [Google Scholar]
- Turmel M., Lemieux B., Lemieux C. The chloroplast genome of the green alga Chlamydomonas moewusii: localization of protein-coding genes and transcriptionally active regions. Mol Gen Genet. 1988 Nov;214(3):412–419. doi: 10.1007/BF00330474. [DOI] [PubMed] [Google Scholar]
- Wilcox L. W., Lewis L. A., Fuerst P. A., Floyd G. L. Group I introns within the nuclear-encoded small-subunit rRNA gene of three green algae. Mol Biol Evol. 1992 Nov;9(6):1103–1118. doi: 10.1093/oxfordjournals.molbev.a040781. [DOI] [PubMed] [Google Scholar]
- Woodson S. A., Cech T. R. Reverse self-splicing of the tetrahymena group I intron: implication for the directionality of splicing and for intron transposition. Cell. 1989 Apr 21;57(2):335–345. doi: 10.1016/0092-8674(89)90971-9. [DOI] [PubMed] [Google Scholar]
- Xu M. Q., Kathe S. D., Goodrich-Blair H., Nierzwicki-Bauer S. A., Shub D. A. Bacterial origin of a chloroplast intron: conserved self-splicing group I introns in cyanobacteria. Science. 1990 Dec 14;250(4987):1566–1570. doi: 10.1126/science.2125747. [DOI] [PubMed] [Google Scholar]



