Abstract
Epidemiological studies suggest that coffee consumption reduces the risk of cancers, including colon cancer, but the molecular mechanisms and target(s) underlying the chemopreventive effects of coffee and its active ingredient(s) remain unknown. Based on serving size or daily units, coffee contains larger amounts of phenolic phytochemicals than tea or red wine. Coffee or chlorogenic acid inhibited CT-26 colon cancer cell-induced lung metastasis by blocking phosphorylation of ERKs. Coffee or caffeic acid (CaA) strongly suppressed mitogen-activated MEK1 and TOPK activities and bound directly to either MEK1 or TOPK in an ATP-noncompetitive manner. Coffee or CaA, but not caffeine, inhibited ERKs phosphorylation, AP-1 and NF-κB transactivation and subsequently inhibited TPA-, EGF- and H-Ras-induced neoplastic transformation of JB6 P+ cells. Coffee consumption was also associated with a significant attenuation of ERKs phosphorylation in colon cancer patients. These results suggest that coffee and CaA target MEK1 and TOPK to suppress colon cancer metastasis and neoplastic cell transformation.
Introduction
B-Raf mutations occur frequently in colon polyps and are an early initiating event in colon cancer and melanoma (1). The mitogen-activated protein kinase/extracellular signal-regulated protein kinase kinase (MEK) proteins are downstream targets of Raf. MEK1 and MEK2 have unique characteristics among the components of the mitogen-activated protein kinase pathways. Constitutive activation of MEK1 results in transformation and colon cancer (2), whereas a small molecule inhibitor of MEKs suppresses transformation and tumor growth in cell culture and mouse models (3). Lymphokine-activated killer T-cell-originated protein kinase (TOPK) is a serine/threonine kinase expressed at high levels in leukemias, lymphomas, myelomas and colorectal cancers (CRCs) (4–6). TOPK is an upstream activator of extracellular signal-regulated kinase (ERKs) and TOPK kinase activity appears to be involved in TOPK’s oncogenic function in colon cancer (7).
Coffee is among the most widely consumed beverages in the world. More than 50% of Americans drink coffee daily. Epidemiological studies suggest that coffee consumption might lower the risk of type 2 diabetes (8), Parkinson’s disease (9) and liver disease (10). Recent meta-analyses demonstrate inverse associations between coffee intake and colon, liver, breast and endometrial cancer risks (11–14). In a prospective population-based cohort study, consumption of at least one cup of coffee per day, compared with no coffee, was associated with a 49% lower risk for upper gastrointestinal cancers (15).
Coffee is a rich source of dietary phenolic phytochemicals (supplementary Figure 1A is available at Carcinogenesis Online), including caffeic acid (CaA; 3,4-dihydroxycinnamic acid) and chlorogenic acid (ChA; 5-O-caffeoylquinic acid). The ChA content of a 200 ml (7-oz) cup of coffee ranges from 70 to 350 mg, which would provide about 35–175 mg of CaA. Previous studies suggested that ∼33% of ingested ChA and 95% of CaA are absorbed intestinally (16). Thus, about two-thirds of ingested ChA reaches the colon where it is probably metabolized to CaA (17). Bioavailability data suggest that the biological effects of ChA would become apparent after its metabolism to CaA, and hence studying the effects of CaA is necessary.
ChA was reported to reduce chemical carcinogenesis in animals (18,19). It was shown to inhibit lipid peroxidation-induced DNA adduct formation (19) and to suppress reactive oxygen species-mediated nuclear factor (NF)-κB, activator protein-1 (AP-1) and mitogen-activated protein kinase activation by upregulating antioxidant enzymes (20). CaA protected male NMRI mice against 7,12-dimethyl-benz[a)anthracene-induced skin tumors (21). These studies suggested that coffee phenolic phytochemicals are potent chemopreventive agents, but the underlying mechanisms and molecular target(s) remain unknown. Here, we report that coffee and CaA inhibit colon cancer metastasis in mice and neoplastic cell transformation by suppressing ERKs phosphorylation. These effects are associated with direct inhibition of MEK1 or TOPK activity, suggesting that these are potential molecular targets of coffee and CaA. Furthermore, we found that coffee consumption was associated with attenuated phosphorylation of ERKs in the human colon.
Materials and methods
Sample preparation
For this study, commercial instant coffee powder was obtained from Dongsuh Food, Incheon, South Korea. The coffee (2 g) was extracted with 180 ml of boiling water under reflux for 30 min. After the extraction, the solution was filtered to collect the extract and this process was repeated twice.
Cell culture
JB6 P+ mouse epidermal (JB6 P+) and H-Ras-transformed JB6 P+ (H-Ras JB6 P+) cell lines were cultured at 37°C in a 5% CO2 incubator in modified Eagle's medium (MEM) containing 5% fetal bovine serum (FBS), 2 mM L-glutamine and 25 μg/ml gentamicin. JB6 cells were stably transfected with an AP-1 or NF-κB luciferase reporter plasmid and maintained in MEM supplemented with 5% FBS containing 200 μg/ml G418. CT-26 cells from the Korea Cell Line Bank were cultured in monolayers at 37°C/5% CO2 in 10% FBS/MEM and penicillin/streptomycin.
Western blotting
Cells (1.5 × 106) were cultured 48 h and starved in serum-free medium for 24 h to eliminate FBS activation of kinases. Cells were treated with coffee (0–40 μg/ml), CaA (0–80 μM), ChA (0–80 μM) or caffeine (0–80 μM) for 1 h before exposure to 20 ng/ml 12-O-tetradecanoylphorbol 13-acetate (TPA). Harvested cells were disrupted and protein concentrations were determined using a dye-binding protein assay kit (Bio-Rad Laboratories, Hercules, CA). Lysate protein (20 μg) was subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Amersham Pharmacia Biotech). Membranes were incubated with a specific primary antibody at 4°C overnight. Proteins were visualized using a chemiluminescence detection kit (Amersham Pharmacia Biotech, Piscataway, NJ) after hybridization with the horseradish peroxidase-conjugated secondary antibody.
Luciferase assay
AP-1 or NF-κB luciferase reporter JB6 P+ cells (8 × 103/ml) suspended in 100 μl of 5% FBS/MEM were added to each well of a 96-well plate and incubated at 37°C/5% CO2. At 80–90% confluence, cells were cultured in 0.1% FBS–MEM for 24 h. Cells were treated for 1 h with coffee (0–40 μg/ml), CaA (0–80 μM), ChA (0–80 μM) or caffeine (0–80 μM) and then exposed to 20 ng/ml TPA for 24 h. After treatment, cells were disrupted with 100 μl of lysis buffer [0.1 M potassium phosphate buffer (pH 7.8), 1% Triton X-100, 1 mM dithiothreitol (DTT) and 2 mM ethylenediaminetetraacetic acid], and luciferase activity was measured using a luminometer (Luminoskan Ascent; Thermo Electron, Helsinki, Finland).
Anchorage-independent transformation assay
JB6 P+ cells (8 × 103/ml) were exposed to TPA or epidermal growth factor (EGF) with or without coffee phytochemicals in 1 ml of 0.33% Basal Media Eagle (BME) agar containing 10% FBS or in 3.5 ml of 0.5% BME agar containing 10% FBS. H-Ras-transformed JB6 cells (8 × 103/ml) were incubated with or without each compound in 1 ml of 0.33% BME agar containing 10% FBS or in 3.5 ml of 0.5% BME agar containing 10% FBS. Cultures were maintained at 37°C/5% CO2 for 14 days and colonies were counted under a microscope with the aid of the Image-Pro Plus v.4 (Media Cybernetics, Silver Spring, MD) (22).
Adenosine triphosphate and CaA competition assay
Briefly, 0.2 μg of active MEK1 or TOPK were incubated with 100 μl of CaA-Sepharose 4B or 100 μl of Sepharose 4B in reaction buffer (see pull-down assay) for 12 h at 4°C and adenosine triphosphate (ATP) was added (0, 10 or 100 μM) to a final volume of 500 μl for 30 min. Samples were washed and proteins detected by western blotting.
Immunohistochemistry
For phosphorylated ERKs immunostaining, antigen retrieval was performed by microwave after deparaffinization and rehydration of tissue sections (6 μm) for 10 min in sodium citrate buffer. Sections were cooled to room temperature, treated with 3% H2O2 in methanol 10 min and blocked with 6% horse serum 40 min at room temperature. Sections were incubated at 4°C overnight with an antibody against phospho-ERK (diluted 1:150; Cell Signaling Technology, Danvers, NA). Sections were washed in phosphate-buffered saline and incubated with the secondary antibody (biotinylated goat anti-rabbit, 1:150; Vector Laboratories, Burlingame, CA) for 30 min. After washing, color was developed by the indirect avidin/biotin-enhanced horseradish peroxidase method with the DAKO Cytomation ABC complex/horseradish peroxidase kit and with 3,3′-diaminobenzidine tetrahydrochloride as substrate. For evaluation, 10 representative ×200 power photomicrographs were taken with a digital camera, avoiding gross necrotic areas. The positively stained cells within each photomicrograph were counted.
Molecular modeling
Insight II (Accelrys, San Diego, CA) was used for the docking study and structure analysis with the crystal coordinates of MEK1 (accession code 1S9J), which are available in the Protein Data Bank (http://www.rcsb.org/pdb/).
Total phenolic phytochemical content
The total phenolic phytochemical concentration was measured using the Folin–Ciocalteu method as described (23). The total phenolic contents of five replicate samples are expressed in milligrams of gallic acid equivalents (GAEs).
In vivo metastasis assay
To determine the effect of CaA on metastasis, a pulmonary colonization assay was performed as described by Fidler (24). CT-26 colon cancer cells (1.5 × 105 CT-26 cells/ml) were washed and resuspended in Hank's balanced salt solution. Aliquots (200 μl) were injected into the tail vein of 6-week-old male Balb/c mice divided into several groups (n = 10 each) as follows: (i) an untreated uninjected group; (ii) a group injected with CT-26 cells and no further treatment; (iii) three groups injected with CT-26 cells and coffee treated (0.5, 1.0 and 2.0 g/kg body wt); (iv) three groups injected with CT-26 cells and ChA treated (0.1, 0.5 and 1.0 g/kg body wt) and (v) two groups injected with CT-26 cells and CaA treated (0.1 and 0.5 g/kg body wt). One hour before intravenous injection of the CT-26 cells, the mice were treated orally with 0.5% carboxymethyl cellulose solution as a control and coffee or ChA diluted with 0.5% carboxymethyl cellulose solution, followed by daily doses. Two weeks after tumor inoculation, mice were killed and lungs were removed, weighed, fixed in Bouin's solution or kept in phosphate-buffered saline. Tumors on the five lobes of the lungs were counted and tumor volume was measured with a caliper and expressed as average tumor volume (cubic millimeter). Tumor volume was calculated using the following formula: tumor volume (mm3) = largest diameter × shortest diameter × depth × (π/6). The lung tissues with tumor nodules were homogenized in liquid nitrogen and stored at −70°C.
Gelatin zymography for matrix metalloproteinases
CT-26 cells (5 × 105) were plated on six-well dishes and grown to 90% confluence. Cells were maintained in serum-free media for 24 h with various coffee compounds. Conditioned medium was collected and concentrated at 10 000g for 30 min in a SpeedVac (Savant/E-C Instruments, Niantic, CT). Protein concentration was measured using the bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL). Equal amounts of conditioned media were mixed with non-reducing sample buffer, incubated for 15 min at room temperature and then subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis in gels containing 1 mg/ml gelatin. Gels were washed twice with 2.5% Triton X-100 for 30 min each time, rinsed three times for 30 min with 50 mM Tris–HCl buffer (pH 7.6) containing 5 mM CaCl2, 0.02% Brij-35 and 0.2% sodium azide and incubated overnight at 37°C. The gels were stained 30 min with 0.5% Coomassie brilliant blue R-250 solution containing 10% acetic acid and 20% methanol and then destained with 7.5% acetic acid solution containing 10% methanol. Areas of gelatinase activity were detected as clear bands against the blue-stained gelatin background.
Kinase assays
The MEK1 kinase assay reaction contained 20 μl of dilution buffer [20 mM 3-(N-morpholino) propanesulfonic acid (pH 7.2), 25 mM β-glycerol phosphate, 5 mM ethylene glycol-bis (2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid , 1 mM sodium orthovanadate (Na3VO4) and 1 mM DTT] and a magnesium-ATP cocktail buffer. For MEK1, 1 μg of the inactive ERK2 substrate peptide was also included. The TOPK kinase reactions contained 5 μl of 2× kinase buffer [25 mM Tris–HCl (pH 7.5), 5 mM β-glycerophosphate, 2 mM DTT, 0.1 mM Na3VO4 and 10 mM MgCl2] and 1 μl of ATP (10 mM). For TOPK, 1 μg of the inactive ERK2 substrate peptide was also included. Then, 25 μl aliquots were transferred onto p81 filter paper and washed 3× with 0.75% phosphoric acid for 5 min/wash, followed by washing once with acetone 2 min. The incorporation of radioactivity was determined by scintillation counter. The effects of coffee phytochemicals were evaluated by separately incubating each compound with the reaction mixtures at 30°C for 15 or 30 min. Each experiment was performed three times.
Pull-down assays
Recombinant MEK1 (1 μg) or TOPK (1 μg) was incubated with the coffee-, CaA- or ChA-Sepharose 4B (or Sepharose 4B as control) beads (100 μl, 50% slurry) in reaction buffer [50 mM Tris–HCl, (pH 7.5), 5 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40, 2 μg/ml bovine serum albumin, 0.02 mM phenylmethanesulphonylfluoride and 1× protease inhibitor mixture]. After incubation with gentle rocking overnight at 4°C, the beads were washed 5× with buffer (50 mM Tris–HCl pH 7.5, 5 mM ethylenediaminetetraacetic acid, 150 mM NaCl, 1 mM DTT, 0.01% Nonidet P-40 and 0.02 mM phenylmethanesulphonylfluoride) and proteins bound to the beads were analyzed by immunoblotting.
Study subject identification
CRC patients were included from the Biobank for Gastrointestinal Health Research, a prospective repository composed of annotated biospecimens from individuals with CRC evaluated at Mayo Clinic (Rochester, MN) from April 2000 until August 2008 and who consented to be in the repository. Immunohistochemical staining for hMLH1, hMSH2 and hMSH6 and microsatellite instability (MSI) testing were performed on tumors from all the CRC patients as described previously (25). Microsatellite markers BAT26, D17S250, D5S346, ACTC, BAT40, BAT25, BAT34C4, D10S197, MYCL and D18S55 were utilized to assess MSI status, and a tumor was microsatellite stable if none of the markers showed MSI and all immunostains showed intact expression of mismatch repair proteins. No MSI (low) or MSI (high) tumors were included in this study. Persons with chronic ulcerative colitis, classical familial adenomatous polyposis or mutation-confirmed attenuated familial adenomatous polyposis were also excluded from the study. The study was approved by Mayo Clinic's research Institutional Review Board.
Tumor processing
Tissue was macrodissected from snap frozen CRC specimens harvested at the time of surgical resection for CRC. For CRC tumors, areas with >70% tumor cells were selected for macrodissection. The cryostat stage was decontaminated with ethanol and the microtome blade changed between sectioning of each sample. For each specimen, six unstained 6 μ thick sections were placed on glass slides and one 50 μ thick section was placed in an additive-free cryotube. CRC tissues were divided into two groups based on coffee consumption (10, no coffee; 10, 1+ cup of coffee/day). Of the 10 patients that consumed coffee, 4 drank decaffeinated coffee (DC) and 6 drank regular coffee.
Statistical analysis
The Student's t-test was used for single statistical comparisons. A probability value of P < 0.05 indicated statistical significance.
Results
Coffee contains higher levels of phenolic phytochemicals than tea or red wine
Tea was reported to be the best source of dietary antioxidant phenolics, followed by chocolate, apple, onion and red wine, which have been investigated as potential chemopreventive agents (26,27). Here, we found that a cup of DC (1186 ± 42.1 mg GAEs) contains higher levels of total phenolic phytochemicals than a cup of tea (128 ± 3.6 mg GAEs) or a glass of red wine (327 ± 4.1 mg GAEs) (supplementary Figure 1B, left, is available at Carcinogenesis Online). Moreover, the amount of total phenolic phytochemicals is also higher in coffee than tea or red wine on a per-daily basis (supplementary Figure 1B, right, is available at Carcinogenesis Online). Therefore, coffee appears to be an important source of phenolic phytochemicals.
DC, ChA or CaA suppresses CT-26 cell-induced mouse lung metastasis
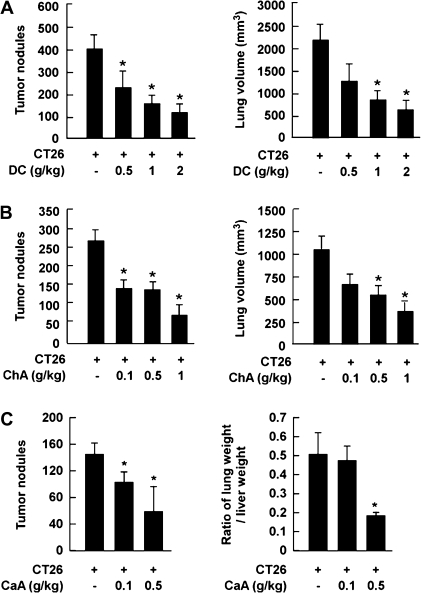
CT-26 colon cancer cells were injected into the tail vein of Balb/c mice. The animals were given various oral doses of DC, ChA or CaA daily for 2 weeks. Mice injected with CT-26 cells exhibited marked increases in tumor nodules in the lung, but DC significantly attenuated tumor nodule formation (Figure 1A, left) and suppressed CT-26 cell-induced lung volume dose dependently (Figure 1A, right). ChA had similar effects (Figure 1B). CaA reduced not only tumor nodule number (Figure 1C, left) but also reduced CT-26 cell-injected lung weight by 65% compared with a group injected with CT-26 cells only (Figure 1C, right).
Fig. 1.
Effects of DC, ChA and CaA on CT-26 cell-induced lung metastasis. (A) DC, (B) ChA and (C) CaA inhibited CT-26 cell-induced tumor nodule formation (left) and CT-26 cell-induced lung volume or weight (right) in Balb/c mice. For A, B and C, mice were treated orally daily for 2 weeks with DC, ChA or CaA after injection of CT-26 cells (1 × 105 cells/200 μl). Results are shown as means ± SEMs (n = 7). The asterisk (*) indicates a significant difference between the group injected with CT-26 cells alone and treated groups (P < 0.05). The tumor volume (cubic millimeter) was calculated as tumor volume = largest diameter × shortest diameter × height × (π/6).
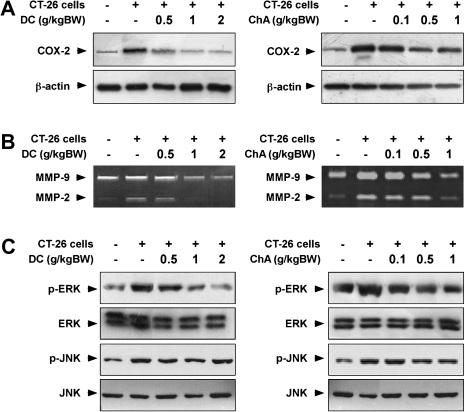
DC or ChA inhibits CT-26 cell-induced COX-2 expression, matrix metalloproteinase-2 and -9 activity and ERKs phosphorylation
The abundance of COX-2 in the lung was increased in CT-26 cell-injected mice and consumption of DC or ChA repressed this expression (Figure 2A). Further, consumption of DC or ChA attenuated matrix metalloproteinase-2 or -9 expression in lungs of CT-26 cell-injected mice (Figure 2B). Activation of the ERKs signaling pathway is involved in the development of colon cancer (2) and results confirmed that DC or ChA inhibited phosphorylation of ERKs, but not c-jun N-terminal kinases, in CT-26 cell-induced mouse lung metastasis (Figure 2C).
Fig. 2.
Effects of DC or ChA on CT-26 cell-induced COX-2 and matrix metalloproteinase (MMP) abundance and ERKs and c-jun N-terminal kinases (JNKs) phosphorylation. (A) DC or ChA inhibited CT-26 cell-induced COX-2 expression in Balb/cmice as determined by western blotting. (B) DC or ChA suppressed CT-26 cell-induced MMP-2 and -9 abundance in Balb/c mice as determined by gelatin zymography. (C) DC or ChA inhibited CT-26 cell-induced ERKs and JNKs phosphorylation in Balb/c mouse lung tissue homogenates. Data are representative of three independent experiments.
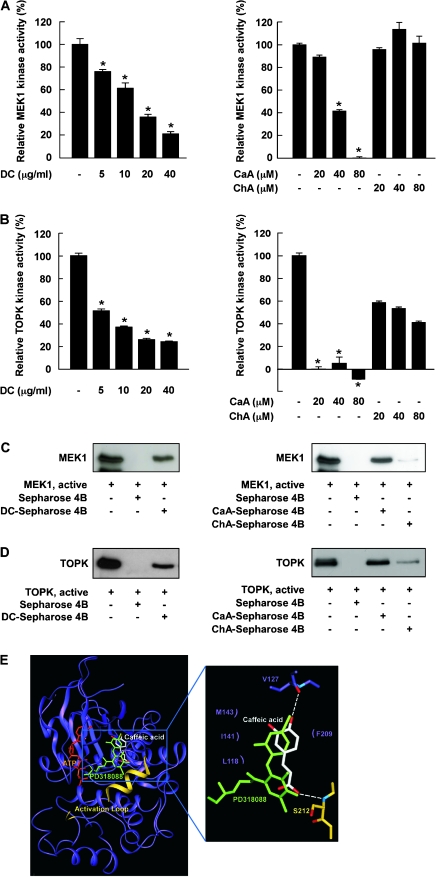
DC or CaA suppresses MEK1 or TOPK kinase activity
MEK1 and TOPK are upstream activators of ERKs and results showed that DC or CaA suppressed MEK1 activity, whereas ChA had no effect (Figure 3A). All three compounds inhibited TOPK activity (Figure 3B), and the effect of CaA was greater than ChA. These results indicated that MEK1 and TOPK are molecular targets of DC and CaA in the inhibition of lung cancer metastasis.
Fig. 3.
Effects of DC, CaA or ChA on MEK1 or TOPK kinase activity and direct binding with MEK1 or TOPK. (A, left) DC and (right) CaA, but not ChA, inhibit MEK1 activity. (B, left) DC and (right) CaA and ChA inhibit TOPK activity. Kinase activities are expressed as percent inhibition relative to respective untreated control. Data are presented as means ± SDs from three independent experiments. Asterisks (*) indicate a significant decrease in kinase activity in groups treated with active MEK1 or TOPK and DC, CaA or ChA and the group treated with active MEK1 or TOPK alone (P < 0.05). (C, left) DC or (right) CaA, but not ChA, binds specifically with MEK1. (D, left) DC or (right) CaA, but not ChA, binds with TOPK. Binding was confirmed by immunoblotting: lane 1 (input control), MEK1 or TOPK protein standard; lane 2 (negative control); lane 3, MEK1 or TOPK pulled down using DC-Sepharose 4B (left) or CaA-Sepharose 4B (right) affinity beads and lane 4, MEK1 or TOPK pulled down using ChA-Sepharose 4B (right) affinity beads. (E) Modeling study of the binding of CaA to MEK1.
DC or CaA binds specifically with MEK1 and TOPK noncompetitively with ATP
MEK1 (Figure 3C) and TOPK (Figure 3D) bind to DC- or CaA-Sepharose 4B beads but not ChA-Sepharose 4B beads. Moreover, ATP did not compete with CaA for binding with MEK1 or TOPK (supplementary Figure 2A is available at Carcinogenesis Online) indicating that CaA suppresses MEK1 or TOPK activity through direct binding in an ATP-noncompetitive manner. In this mode of inhibition, the inhibitor reversibly binds to an allosteric site, which is a different site than the active site and prevents the substrate from binding to the active site. Thus, no competition occurs between the inhibitor and the substrate. A modeling study (Figure 3E) suggested that CaA bound to a pocket of MEK or TOPK that is separate from the ATP-binding site similar to that observed for PD318088 binding in the crystal structure of the MEK1–PD318088 complex (28).
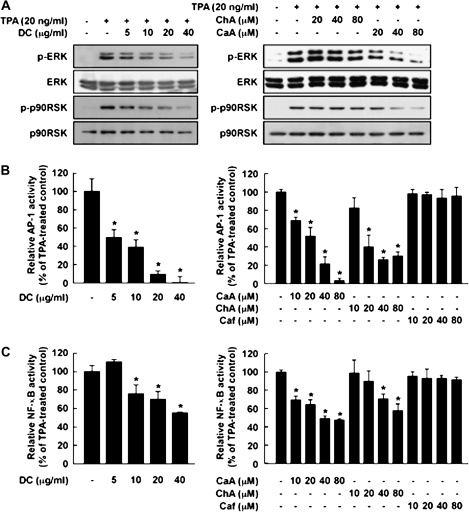
DC or CaA suppresses TPA-induced ERK/p90RSK phosphorylation and AP-1 and NF-κB transactivation
ERKs comprise one of the most important pathways in TPA-induced JB6 P+ cell transformation (29). DC (Figure 4A, left) or CaA (Figure 4A, right) inhibited TPA-induced phosphorylation of ERKs and p90RSK. AP-1 and NF-κB play major roles in tumor promoter-induced neoplastic transformation of JB6 P+ cells (22,30). In JB6 cell lines stably transfected with an AP-1 or NF-κB luciferase plasmid, DC (Figure 4B and C, left) or CaA (Figure 4B and C, right), but not caffeine, suppressed TPA-induced transactivation of AP-1 or NF-κB. These results suggested that inhibition of ERK/p90RSK signaling by DC or CaA leads to the suppression of AP-1 or NF-κB transactivation.
Fig. 4.
Effects of DC, CaA, ChA or Caf on TPA-induced phosphorylation of ERKs or p90RSK or activation of AP-1 and NF-κB in JB6 P+ cells. (A, left) DC or (right) CaA, but not ChA, inhibits TPA-induced phosphorylation of ERKs and p90RSK. Data are representative of three independent experiments. (B, left) DC or (right) CaA or ChA, but not Caf, inhibits TPA-induced AP-1 transactivation. (C, left) DC or (right) CaA or ChA, but not Caf, suppresses TPA-induced NF-κB transactivation. AP-1 and NF-κB activities are expressed as percent inhibition relative to cells treated with TPA alone. Data are presented as means ± SDs of AP-1 or NF-κB luciferase activity calculated from three independent experiments. Asterisks (*) indicate a significant difference between groups treated with TPA and each compound and the group treated with TPA alone (P < 0.05).
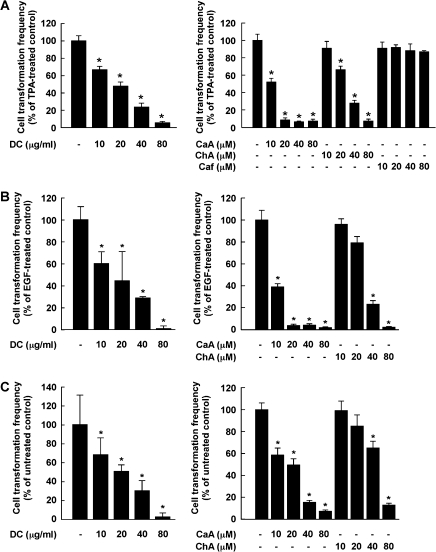
Coffee phenolic phytochemicals, but not caffeine, suppress neoplastic transformation
Treatment with DC markedly inhibits TPA-promoted neoplastic transformation of JB6 P+ cells (Figure 5A, left). CaA attenutated TPA-induced transformation of JB6 P+ cells more strongly than ChA, whereas caffeine had no effect (Figure 5A, right). Similar results were also found for EGF- or H-Ras-induced transformation of JB6 P+ cells (Figure 5B and C, left). The inhibitory effects of CaA on EGF- or H-Ras-induced cell transformation were more potent than ChA at the same concentration (Figure 5B and C, right). The inhibitory effects of the coffee phytochemicals were not caused by cytotoxicity because the concentration ranges for effective inhibition of cell transformation did not affect the viability of JB6 P+ cells (data not shown). These findings indicated that DC or CaA suppresses cell transformation mainly by targeting ERKs signaling. Overall, CaA is more potent than ChA in suppressing TPA-induced neoplastic transformation of JB6 P+ cells and might contribute to the antitumor-promoting activity of coffee.
Fig. 5.
Effects of DC, CaA, ChA or Caf on TPA-, EGF- or H-Ras-induced neoplastic transformation of JB6 P+ cells. (A, left) DC or (right) CaA or ChA, but not Caf, inhibited TPA-induced JB6P+ cell transformation. (B, left) DC or (right) CaA or ChA suppressed EGF-induced transformation of JB6 P+ cells. (C, left) DC or (right) CaA or ChA inhibited H-Ras-induced transformation of JB6 P+ cells. Colonies were counted under a microscope after 14 days. The effects of compounds on transformation are presented as percent inhibition of cells treated only with TPA-, EGF- or H-Ras. Data are shown as means ± SDs of the percent inhibition from three independent experiments. Asterisks (*) indicate a significant difference between groups treated with tumor promoters and compounds and the group treated with only TPA, EGF or H-Ras alone (P < 0.05).
Coffee phenolic phytochemicals inhibit ERKs phosphorylation in humans
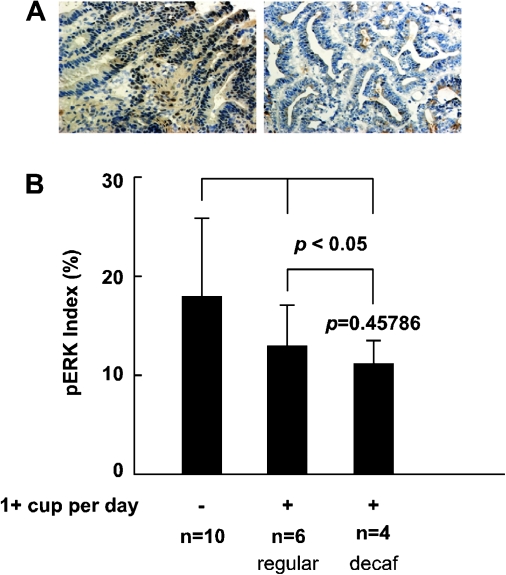
Immunohistochemical results of the staining of excised human tumor sections showed nuclear localization of phosphorylated ERKs (Figure 6A). Tumor tissues from colon cancer patients who often drink over one cup of coffee daily showed a trend of decreased abundance of phosphorylated ERKs compared with the tissues of patients who did not drink coffee (Figure 6B). This is consistent with the previous result showing that DC or ChA blocks ERKs signaling ex vivo.
Fig. 6.
Effects of coffee consumption on ERKs phosphorylation in CRC tissues. (A) Photographic data (×200) of immunohistochemistry staining of ERKs in tumor tissues from representative colon cancer patients who reported drinking more than one cup of coffee daily (right) and no coffee consumption (left). (B) Level of ERKs phosphorylation. The asterisk (*) indicates a significant decrease (P < 0.005) in ERKs phosphorylation in patients who consumed either decaffeinated or regular coffee compared with the ‘no coffee consumption’ group. No significant difference was observed in ERKs phosphorylation in patients who consumed regular or DC.
Discussion
Epidemiological studies suggest that consumption of coffee decreases the risk of certain cancers (10,12) and that caffeine has substantial health-promoting effects. A serving of coffee contains 60–115 mg of caffeine (31,32) and the plasma concentration of caffeine physiologically obtainable is approximately 10–30 μM. However, caffeine concentrations up to 500 μM might be required to suppress EGF- or TPA-induced malignant cell transformation (33), which is not feasible. We showed that coffee contains higher levels of phenolic phytochemicals than tea or red wine and treatment with DC or ChA attenuated tumor nodule formation and tumor volume dose dependently.
COX-2 is overexpressed in many cancers and matrix metalloproteinases play a pivotal role in cancer cell invasion. Oral administration of DC or ChA inhibited COX-2 expression and matrix metalloproteinase-2 or -9 activation. Ras-activating mutations occur in most colorectal carcinomas, and Ras induces proliferation by activating ERKs in colon cancer. We found that DC or ChA inhibited CT-26 cell-induced ERKs phosphorylation suggesting that ERKs might be associated with the effects of phenolic phytochemicals on CT-26 colon cancer cell-induced metastasis.
ERKs are abundant MEK-activated protein kinases that occupy a strategic downstream position in critical intracellular signaling cascades (34). The MEK/ERK cascade is linked to oncogenic transformation and cancer progression (35). Constitutive activation of MEK causes transformation, whereas dominant-negative MEKs reverse the process (36). ERKs are constitutively active in CRC cells, suggesting that MEK is activated in primary colorectal tumors (37) and might be useful as an anticancer target. Evidence also suggested that TOPK constitutively activates mitogen-activated protein kinase signaling leading to increased levels of phosphorylated ERKs and transformation. Knockdown of TOPK in HCT116 CRC cells reduced proliferation (38), raising the possibility that TOPK might also be a potential target. Our results demonstrated that DC or CaA inhibited MEK kinase activity. Moreover, DC, CaA or ChA suppressed TOPK activity, and CaA appeared most effective. Thus, MEK or TOPK is an important target for the antitumor-promoting activities of these compounds.
Data suggested that mechanisms of chemoprevention attributed to phenolic phytochemicals are not related to their antioxidant activities but instead to their binding of target molecules (39,40). Data indicated that DC or CaA binds directly with MEK1 or TOPK, and ChA binds with TOPK but not MEK1. However, CaA binds more strongly with TOPK than does ChA and does not compete with ATP for binding. In contrast to other protein kinase inhibitors, MEK1 inhibitors like PD098059, U0126, PD184352 and PD318088 do not compete with ATP, which accounts for their high selectivity (41). PD184352 is another MEK1-specific inhibitor that binds to a pocket that lies adjacent to the ATP-binding site (28). Compared with the homologous ATP-binding site, this binding pocket of MEK contains distinctive sequences unshared with other kinases. The binding of PD184352 with MEK1 results in a stabilized inactive conformation and deformation of the catalytic sites (28). Our modeling study (Figure 3E) suggested that CaA binds to a pocket adjacent to the ATP-binding site, similar to that observed for PD318088 in the crystal structure of the MEK1–PD318088 complex (28). The predicted binding mode of CaA is also similar to that of PD318088. The hydroxyl group at the para position of the benzene ring forms a hydrogen bond with the backbone carbonyl group of Val127 in the ATP-noncompetitive binding site. In addition, several van der Waals interactions occur with the hydrophobic surface formed by Leu118, Ile141, Phe209 and Met143. The carboxylic group of CaA forms a critical hydrogen bond with the backbone amide group of Ser212 of the activation loop of MEK1. Interaction of CaA with the activation loop would lock MEK1 into a catalytically inactive form by stabilizing the inactive conformation of the activation loop. A structural analysis of TOPK has not yet been reported. Further study will be needed to use X-ray crystallography for investigating the direct MEK or TOPK inhibitory mechanism of CaA.
Our data indicated that DC or CaA block ERKs signaling and TPA-induced phosphorylation of ERKs and p90RSK in JB6 P+ cells. AP-1 and NF-κB signaling is important in tumor promoter-induced tumorigenesis (39,42), and results indicated that coffee phytochemicals suppressed TPA-induced AP-1 or NF-κB transactivation. Additionally, results suggested that DC or CaA effectively inhibited TPA-induced transformation of JB6 P+ cells. Previous studies showed that EGF or H-Ras activated ERKs signaling leads to anchorage-independent growth of JB6 P+ cells (29,43) and either compound suppressed both EGF- and H-Ras-induced transformation. These results suggest that these compounds inhibit transformation mainly by targeting ERKs signaling.
Results also revealed that tumor tissues from colon cancer patients, who drank over one cup of coffee daily, showed decreased phosphorylated ERKs compared with patients who did not drink coffee. This is consistent with results showing that coffee phytochemicals block ERKs signaling ex vivo. Coffee is an important source of dietary phenolic phytochemicals. Results suggested that MEK1 or TOPK are targets of CaA for suppressing colon cancer metastasis and transformation. The chemopreventive effects of DC are probably attributable to CaA. These studies provide a better understanding of the mechanisms responsible for the beneficial effects of coffee phenolic phytochemicals in human health.
Supplementary data
Supplementary Figures 1–3 can be found at http://carcin.oxfordjournals.org/
Funding
The Hormel Foundation, National Institutes of Health (CA027502, CA120388, CA111536, CA077646 and CA081064); Basic Science Research Program (2008-331-F00072, 2010-0011345), WCU program (R31-2008-00-10056-0); Leap Research Program (2010-0029233); National Research Foundation of Korea; Republic of Korea.
Supplementary Material
Acknowledgments
Some of the work reported in this manuscript was performed by B.H.K who passed away unexpectedly before the work was published. We wish to express our condolences to her family and loved ones. She will be greatly missed by all who knew her.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AP-1
activator protein-1
- ATP
adenosine triphosphate
- BME
Basal Media Eagle
- CaA
caffeic acid
- ChA
chlorogenic acid
- CRC
colorectal cancer
- DC
decaffeinated coffee
- DTT
dithiothreitol
- EGF
epidermal growth factor
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- GAE
gallic acid equivalent
- MEM
modified Eagle's medium
- MSI
microsatellite instability
- NF
nuclear factor
- TPA
12-O-tetradecanoylphorbol 13-acetate
- TOPK
T-cell-originated protein kinase
References
- 1.Pratilas CA, et al. Therapeutic strategies for targeting BRAF in human cancer. Rev. Recent Clin. Trials. 2007;2:121–134. doi: 10.2174/157488707780599393. [DOI] [PubMed] [Google Scholar]
- 2.Komatsu K, et al. Oncogenic potential of MEK1 in rat intestinal epithelial cells is mediated via cyclooxygenase-2. Gastroenterology. 2005;129:577–590. doi: 10.1016/j.gastro.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Cowley S, et al. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 4.Abe Y, et al. Cloning and expression of a novel MAPKK-like protein kinase, lymphokine-activated killer T-cell-originated protein kinase, specifically expressed in the testis and activated lymphoid cells. J. Biol. Chem. 2000;275:21525–21531. doi: 10.1074/jbc.M909629199. [DOI] [PubMed] [Google Scholar]
- 5.Gaudet S, et al. Characterization of PDZ-binding kinase, a mitotic kinase. Proc. Natl Acad. Sci. USA. 2000;97:5167–5172. doi: 10.1073/pnas.090102397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simons-Evelyn M, et al. PBK/TOPK is a novel mitotic kinase which is upregulated in Burkitt's lymphoma and other highly proliferative malignant cells. Blood Cells Mol. Dis. 2001;27:825–829. doi: 10.1006/bcmd.2001.0452. [DOI] [PubMed] [Google Scholar]
- 7.Park JH, et al. PDZ-binding kinase/T-LAK cell-originated protein kinase, a putative cancer/testis antigen with an oncogenic activity in breast cancer. Cancer Res. 2006;66:9186–9195. doi: 10.1158/0008-5472.CAN-06-1601. [DOI] [PubMed] [Google Scholar]
- 8.Dam RM, et al. Coffee consumption and risk of type 2 diabetes mellitus. Lancet. 2002;360:1477–1488. doi: 10.1016/S0140-6736(02)11436-X. [DOI] [PubMed] [Google Scholar]
- 9.Ascherio A, et al. Coffee consumption, gender, and Parkinson's disease mortality in the cancer prevention study II cohort: the modifying effects of estrogen. Am. J. Epidemiol. 2004;160:977–984. doi: 10.1093/aje/kwh312. [DOI] [PubMed] [Google Scholar]
- 10.Ruhl CE, et al. Coffee and tea consumption are associated with a lower incidence of chronic liver disease in the United States. Gastroenterology. 2005;129:1928–1936. doi: 10.1053/j.gastro.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 11.Bravi F, et al. Coffee drinking and endometrial cancer risk: a metaanalysis of observational studies. Am. J. Obstet. Gynecol. 2009;200:130–135. doi: 10.1016/j.ajog.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Je Y, et al. Coffee consumption and risk of colorectal cancer: a systematic review and meta-analysis of prospective cohort studies. Int. J. Cancer. 2009;124:1662–1668. doi: 10.1002/ijc.24124. [DOI] [PubMed] [Google Scholar]
- 13.Larsson SC, et al. Coffee consumption and risk of liver cancer: a meta-analysis. Gastroenterology. 2007;132:1740–1745. doi: 10.1053/j.gastro.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 14.Tang N, et al. Coffee consumption and risk of breast cancer: a metaanalysis. Am. J. Obstet. Gynecol. 2009;200:e1–e9. doi: 10.1016/j.ajog.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Naganuma T, et al. Coffee consumption and the risk of oral, pharyngeal, and esophageal cancers in Japan: the Miyagi Cohort Study. Am. J. Epidemiol. 2008;168:1425–1432. doi: 10.1093/aje/kwn282. [DOI] [PubMed] [Google Scholar]
- 16.Olthof MR, et al. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001;131:66–71. doi: 10.1093/jn/131.1.66. [DOI] [PubMed] [Google Scholar]
- 17.Olthof MR, et al. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 2003;133:1806–1814. doi: 10.1093/jn/133.6.1806. [DOI] [PubMed] [Google Scholar]
- 18.Huang MT, et al. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid, and ferulic acid on tumor promotion in mouse skin by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1988;48:5941–5946. [PubMed] [Google Scholar]
- 19.Kasai H, et al. Action of chlorogenic acid in vegetables and fruits as an inhibitor of 8-hydroxydeoxyguanosine formation in vitro and in a rat carcinogenesis model. Food Chem. Toxicol. 2000;38:467–471. doi: 10.1016/s0278-6915(00)00014-4. [DOI] [PubMed] [Google Scholar]
- 20.Feng R, et al. Inhibition of activator protein-1, NF-kappaB, and MAPKs and induction of phase 2 detoxifying enzyme activity by chlorogenic acid. J. Biol. Chem. 2005;280:27888–27895. doi: 10.1074/jbc.M503347200. [DOI] [PubMed] [Google Scholar]
- 21.Kaul A, et al. Polyphenols inhibit promotional phase of tumorigenesis: relevance of superoxide radicals. Nutr. Cancer. 1998;32:81–85. doi: 10.1080/01635589809514723. [DOI] [PubMed] [Google Scholar]
- 22.Li JJ, et al. Inhibitors of both nuclear factor-kappaB and activator protein-1 activation block the neoplastic transformation response. Cancer Res. 1997;57:3569–3576. [PubMed] [Google Scholar]
- 23.Singleton VL, et al. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 24.Zhang RD, et al. Malignant potential of cells isolated from lymph node or brain metastases of melanoma patients and implications for prognosis. Cancer Res. 1991;51:2029–2035. [PubMed] [Google Scholar]
- 25.Cunningham JM, et al. The frequency of hereditary defective mismatch repair in a prospective series of unselected colorectal carcinomas. Am. J. Hum. Genet. 2001;69:780–790. doi: 10.1086/323658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arts IC, et al. Chocolate as a source of tea flavonoids. Lancet. 1999;354:488. doi: 10.1016/S0140-6736(99)02267-9. [DOI] [PubMed] [Google Scholar]
- 27.Bode AM, et al. Post-translational modification of p53 in tumorigenesis. Nat. Rev. Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 28.Ohren JF, et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat. Struct. Mol. Biol. 2004;11:1192–1197. doi: 10.1038/nsmb859. [DOI] [PubMed] [Google Scholar]
- 29.Huang C, et al. Shortage of mitogen-activated protein kinase is responsible for resistance to AP-1 transactivation and transformation in mouse JB6 cells. Proc. Natl Acad. Sci. USA. 1998;95:156–161. doi: 10.1073/pnas.95.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomura M, et al. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-kappaB activation by tea polyphenols, (-)-epigallocatechin gallate and theaflavins. Carcinogenesis. 2000;21:1885–1890. doi: 10.1093/carcin/21.10.1885. [DOI] [PubMed] [Google Scholar]
- 31.Barone JJ, et al. Caffeine consumption. Food Chem. Toxicol. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 32.Lelo A, et al. Assessment of caffeine exposure: caffeine content of beverages, caffeine intake, and plasma concentrations of methylxanthines. Clin. Pharmacol. Ther. 1986;39:54–59. doi: 10.1038/clpt.1986.10. [DOI] [PubMed] [Google Scholar]
- 33.Nomura M, et al. Inhibition of epidermal growth factor-induced cell transformation and Akt activation by caffeine. Mol. Carcinog. 2005;44:67–76. doi: 10.1002/mc.20120. [DOI] [PubMed] [Google Scholar]
- 34.Yoon S, et al. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 35.Shaul YD, et al. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim. Biophys. Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Wolf I, et al. The mitogen-activated protein kinase signaling cascade: from bench to bedside. Isr. Med. Assoc. J. 2002;4:641–647. [PubMed] [Google Scholar]
- 37.Hoshino R, et al. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–822. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- 38.Zhu F, et al. Bidirectional signals transduced by TOPK-ERK interaction increase tumorigenesis of HCT116 colorectal cancer cells. Gastroenterology. 2007;133:219–231. doi: 10.1053/j.gastro.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 39.Bode AM, et al. Signal transduction pathways in cancer development and as targets for cancer prevention. Prog. Nucleic Acid Res. Mol. Biol. 2005;79:237–297. doi: 10.1016/S0079-6603(04)79005-4. [DOI] [PubMed] [Google Scholar]
- 40.Watts RG, et al. Expression of dominant negative Erk2 inhibits AP-1 transactivation and neoplastic transformation. Oncogene. 1998;17:3493–3498. doi: 10.1038/sj.onc.1202259. [DOI] [PubMed] [Google Scholar]
- 41.Favata MF, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 42.Suzukawa K, et al. AP-1, NF-kappa-B, and ERK activation thresholds for promotion of neoplastic transformation in the mouse epidermal JB6 model. Environ. Health Perspect. 2002;110:865–870. doi: 10.1289/ehp.02110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung JY, et al. Mechanisms of inhibition of the Ras-MAP kinase signaling pathway in 30.7b Ras 12 cells by tea polyphenols (-)-epigallocatechin-3-gallate and theaflavin-3,3'-digallate. FASEB J. 2001;15:2022–2024. doi: 10.1096/fj.01-0031fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.