Abstract
NR4A1 (Nur77, TR3) is overexpressed in pancreatic tumors and activation of TR3 by 1,1-bis(3′-indolyl)-1-(p-methoxyphenyl)methane (DIM-C-pPhOCH3) inhibits cell and tumor growth and induces apoptosis. Microarray analysis demonstrates that in L3.6pL pancreatic cancer cells DIM-C-pPhOCH3 induces genes associated with metabolism, homeostasis, signal transduction, transcription, stress, transport, immune responses, growth inhibition and apoptosis. Among the most highly induced growth inhibitory and proapoptotic genes including activating transcription factor 3 (ATF3), p21, cystathionase, dual specificity phosphatase 1 and growth differentiation factor 15, RNA interference studies demonstrated that induction of all but the later gene by DIM-C-pPhOCH3 were TR3-dependent. We also observed that DIM-C-pPhOCH3 induced Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and induction of TRAIL was ATF3 dependent. Results of this and previous studies demonstrate that TR3 is unique among nuclear receptors since nuclear TR3 is activated or deactivated by diindolylmethane derivatives to induce different apoptotic and growth inhibitory pathways that inhibit pancreatic cancer cell and tumor growth.
Introduction
Pancreatic ductal adenocarcinoma is a major cause of cancer-related deaths in developed countries, and it is estimated that in 2010, >43 140 new cases will be diagnosed in the USA (1). Pancreatic ductal adenocarcinoma is a highly aggressive disease that invariably evades early diagnosis (2–5) and the mean survival time for patients with metastatic disease is only 3–6 months. Several factors are associated with increased risk for pancreatic cancer and these include chronic pancreatitis, prior gastric surgery, smoking, diabetes, exposure to certain classes of organic solvents and radiation (2,3). Heritable germ line mutations in several genes are also associated with increased risks for pancreatic cancer and these include Peutz–Jeghers, hereditary pancreatitis, familial atypical multiple melanoma, familial breast cancer 2 and hereditary non-polyposis colorectal cancer syndromes (4–7). In addition to heritable mutations, several acquired gene mutations have been identified in sporadic pancreatic tumors and these include the K-ras oncogene, the cyclin-dependent kinase inhibitor p16, the tumor suppressor gene p53 and SMAD4.
5-Fluorouracil and more recently gemcitabine have been extensively used for treatment of advanced pancreatic cancer (8,9), and several other drugs are being investigated and these include ‘antimetabolites’, taxanes, topoisomerase I inhibitors and various combinations of these drugs as well as other novel mechanism-based agents (8,9). Diindolylmethane is a metabolite of indole-3-carbinol, one of the cancer chemopreventive agents in cruciferous vegetables (10), and diindolylmethane inhibits pancreatic cancer growth through activation of multiple pathways (11,12). Studies in this laboratory have synthesized a series of 1,1-bis(3′-indolyl)-1-(p-substituted phenyl)methane (C-DIM) analogs and the addition of the bulky substituted phenyl group gives compounds that bind or activate multiple nuclear receptors (NRs), including peroxisome proliferator-activated receptor γ and the orphan nuclear receptors NR4A1 (TR3, Nur77) and NR4A2 (Nurr1) (13–21). TR3 is overexpressed in human colon, pancreatic and bladder tumors and cancer cells (16–21), and compounds such as 1,1-bis(3′-indolyl)-1-(p-methoxyphenyl)methane (DIM-C-pPhOCH3) activate nuclear TR3 in colon and pancreatic cancer cells and tumors in athymic nude mouse xenograft experiments (16–20). Previous reports have shown that several apoptosis-inducing agents activate cell death pathways that involve nuclear export of TR3 which can form a proapoptotic TR3-bcl-2 complex (22–27) and this contrasts to the activation of nuclear TR3 by DIM-C-pPhOCH3 (16,17).
Treatment of pancreatic cancer cells with DIM-C-pPhOCH3 induces tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas ligand (FasL) and other induced proapoptotic genes identified using microarrays include activating transcription factor 3 (ATF3), p21, cystathionase (CTH), dual specificity phosphatase 1 (DUSP1) and growth differentiation factor 15/non-steroidal anti-inflammatory drug-activated gene 1 (GDF15/NAG1). RNA interference studies demonstrated that, with the exception of NAG1, induction of the remaining genes was TR3 dependent and induction of TRAIL was also ATF3 dependent. DIM-C-pPhOCH3 also inhibited pancreatic tumor growth in a xenograft (L3.6pL cells) model and induced apoptosis in these tumors, indicating that TR3 is a novel target for pancreatic cancer chemotherapy where activation of nuclear TR3 by DIM-C-pPhOCH3 and deactivation of TR3 by 1,1-bis-(3′-indolyl)-1-(p-hydroxyphenyl)methane (DIM-C-pPhOH) (21) induce different proapoptotic genes and pathways.
Materials and methods
Cell lines and cell culture
L3.6pL cell line was developed at the M. D. Anderson Cancer Center (Houston, TX) and kindly provided by Dr I.J.Fidler. Panc1, Panc28 and MiaPaCa-2 human pancreatic cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA). Panc1, Panc28 and MiaPaCa-2 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) nutrient mixture with Ham’s F-12 (DMEM/Ham’s F-12; Sigma–Aldrich, St Louis, MO) supplemented with 0.22% sodium bicarbonate, 0.011% sodium pyruvate, 10% fetal bovine serum (FBS) and 10 ml/l 100× antibiotic antimycotic solution (Sigma–Aldrich). L3.6pL cells were maintained in RPMI-1640 medium supplemented with 10% FBS and 10 ml/l 100× antibiotic antimycotic solution. Cells were maintained at 37°C in the presence of 5% CO2 and the solvent [dimethyl sulfoxide (DMSO)] used in the experiments was ≤0.1%.
Antibodies and reagents
TR3/Nur77 and Sp1 antibodies were purchased from Imgenex (San Diego, CA) and Upstate (Temecula, CA), respectively. ATF3, TRAIL and Fas-L antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The specific antibodies for cleaved poly(ADP-ribose)polymerase (PARP) (#9541), caspase 8 (#9496), caspase 3 (#9661) and caspase 7 (#9491) were purchased from Cell Signaling Technology (Beverly, MA). All the small inhibitory RNAs were prepared by Dharmacon Research (Lafayette, CO). ATF3 expression construct (pCG-ATF3) was generously provided by Dr S.J.Baek (University of Tennessee, Knoxville, TN). DIM-C-Ph and DIM-C-pPhOCH3 were >98% pure as determined by GC–MS. Both compounds are synthesized by condensation of p-methoxybenzaldehyde or benzaldehyde with indole as described (13).
Cell proliferation assay and apoptosis detection
Cells (1 × 105 per well) were plated in 12-well plates and allowed to attach for 16 h. The medium was then changed to DMEM/Ham’s F-12 medium containing 2.5% charcoal-stripped FBS and either vehicle (DMSO) or different concentrations of the compound were added. Fresh medium and compounds were added every 48 h, and cells were then trypsinized and counted after 24, 48, and 72 h using a Coulter Z1 cell counter (Beckman Coulter, Brea, CA). Apoptosis was detected using 4′,6-diamidino-2-phenylindole (2 μg/ml) staining, and fluorescent images were collected and analyzed using a Zeiss Axioplan2 fluorescence microscope (Carl Zeiss, Jena, Germany).
Western blot analysis and quantitative real-time polymerase chain reaction
Cells (2 × 105) were plated in six-well plates in DMEM/Ham’s F-12 media containing 10% charcoal-stripped FBS for 16 h and then treated with different concentrations of the compounds. Cellular lysates and their subsequent separation by electrophoresis was carried out as described previously using β-actin as a loading control (17,21) and for the high dose of DIM-C-pPhOCH3 (20 μM), combined lysates from adherent and floating cells were analyzed. Total RNA was extracted, real-time polymerase chain reaction (PCR) was carried out as described previously, and messenger RNA (mRNA) levels were normalized to expression of TATA-binding protein (17,21). The sequences of the primers used for real-time PCR were as follows: p21 sense, 5′-GGCAGACCAGCATGACAGATTTC-3′ and antisense 5′-CGGATTAGGGCTTCCTCTTGG-3′ and TATA-binding protein sense, 5′-TGCACAGGAGCCAAGATGGAA-3′ and antisense 5′-CACATCACAGCTCCCCACCA-3′. The PCR primers for ATF3, CTH, DUSP1, NAG1, programmed cell death 6, suppressor of cytokine signaling 1, TNFα-induced protein 3 and TR3 were purchased from Qiagen (Valencia, CA).
Microarray experiments
Microarray studies focused on early induced genes, and L3.6pL cells were treated with DMSO or 15 μM DIM-C-pPhOCH3 for 2 and 6 h. RNA was isolated as described for the PCR experiment and analyzed for gene expression using the Codelink Whole Genome Bioarrays (300026), and three replicates were determined for each time point and the DMSO control. The microarray data were analyzed using GeneSpring software version 7.2 (Agilent, Palo Alto, CA). The data were normalized in two steps. First, for each array, the expression value of each gene was divided by the median of all the values in that array. Second, for each gene, the expression value in each array was divided by the median value of that gene across all arrays. Genes with low quality signals were excluded for statistical analysis. One-way analysis of variance (assume equal variances) was carried out to identify differentially expressed genes. A gene was said to be differentially expressed if the Benjamini and Hochberg adjusted P-values were <0.05.
Transfection of small inhibitory RNAs
Cells (1.5 × 105 cells per well) were plated in six-well plates in DMEM/Ham’s F-12 media supplemented with 10% charcoal-stripped FBS. After 16 h, the cells were transfected with 100 nM of each small inhibitory RNAs duplex for 7 h using LipofectAMINE 2000 reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol. The medium was then changed to DMEM/Ham’s F-12 medium containing 10% charcoal-stripped FBS and incubated for indicated time. After incubation, cells were collected for western blot analysis and quantitative real-time PCR assay.
Xenograft studies in athymic mice
Male athymic nude mice (Foxn1nu, ages 7–8 weeks) were purchased from Harlan (Indianapolis, IN). The mice were housed and maintained in laminar flow cabinets under specific pathogen-free conditions. A xenograft was established by subcutaneous injection of in vitro cultured L3.6pL cells (1 × 107 cells/150 μl) into the flanks of individual mice. Tumors were allowed to grow for 7 days until tumors were palpable. Mice were then randomized into two groups of five mice per group and dosed daily by oral gavage with either corn oil or 25 mg/kg/day DIM-C-pPHOCH3 for 21 days. The mice were weighed and tumor size was measured twice a week with calipers to permit calculation of tumor volumes, V = L × W2/2, where L and W were length and width. Final body, organ and tumor weights were determined at the end of the dosing regimen (on day 21), and both organ and tumor blocks were obtained for hematoxylin and eosin staining and histopathological analysis.
Immunohistochemical analysis and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling assay
Immunohistochemical staining was performed on paraffin-embedded specimens by using standard avidin–biotin complex (ABC) method described previously (17). After deparaffinization, tissue sections were subjected to antigen retrieval with 0.1% pepsin in 0.01 N HCl at room temperature for 10 min followed by treatment with 0.1% H2O2 to block endogenous peroxidase activity. Sections were incubated with the normal rabbit IgG or rabbit polyclonal anti-cleaved caspase-3 antibody (1:100) at 4°C overnight after blocking with normal goat serum at room temperature for 1 h. After washing in PBS, sections were incubated with biotinylated goat anti-rabbit IgG at room temperature for 30 min. Staining Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine (Biogenex Laboratories, San Ramon, CA) as the chromagen was used as described (17) following manufacturer’s protocol. The sections were counterstained with hematoxylin and dehydrated and mounted. There was no specific staining when secondary antibody was used alone as a negative control. Transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) staining was carried out using DeadEnd Fluorometric TUNEL System (Promega, Madison, WI) according to the manufacturer’s protocol as described previously (21).
Statistical analysis
The results are expressed as means ± standard deviations and differences between means for two groups were determined by unpaired Student’s t-test. A P value of <0.05 was considered statistically significant.
Results
Activation of TR3 inhibits growth and induces apoptosis
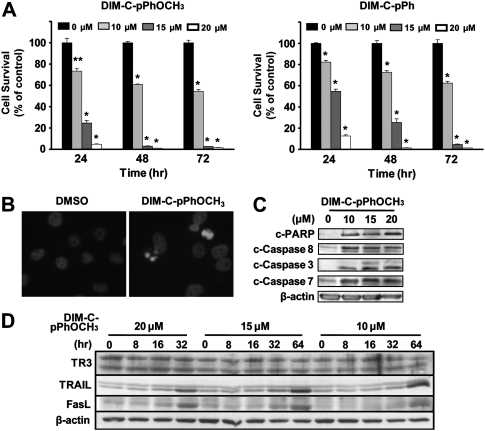
Treatment of L3.6pL pancreatic cancer cell lines with DMSO (solvent control), DIM-C-pPhOCH3 or DIM-C-pPh (10, 15 and 20 μM) for 24, 48 and 72 h resulted in a significant decrease in cell growth at all time points (Figure 1A). These data were comparable with previous results using Panc28 and MiaPaca-2 pancreatic cancer cells and in subsequent studies, DIM-C-pPhOCH3 is used as a prototypical activator of TR3. The growth inhibitory effects of DIM-C-pPhOCH3 were accompanied by induction of apoptosis as evidenced by increased 4′,6-diamidino-2-phenylindole staining (Figure 1B) and induction of cleaved PARP and cleaved (activated) caspases 3, 7 and 8 (Figure 1C). After treatment of L3.6pL cells for 48 h, the concentration-dependent effects of DIM-C-pPhOCH3 on cleaved PARP and activated caspases were observed at concentrations of 10, 15 and 20 μM. There was a time- and concentration-dependent induction of TRAIL and FasL in L3.6pL cells (Figure 1D). At the low concentration (10 μM), DIM-C-pPhOCH3 induced TRAIL and FasL after treatment for 32–64 h, whereas 20 μM DIM-C-pPhOCH3 induced these proteins within 16–32 h.
Fig. 1.
TR3-active C-DIMs inhibit growth and induce apoptosis in human pancreatic cancer cells. (A) Growth inhibition. L3.6pL cells were treated with different concentrations of DIM-C-pPhOCH3 and DIM-C-pPh for 3 days, and the number of cells in each well was counted on days 1, 2 and 3. Results are presented as means ± standard deviations for three replicate determinations for each treatment group. *P < 0.005 versus DMSO; **P < 0.001 versus DMSO for each time point. (B) 4′,6-Diamidino-2-phenylindole staining. Cells were treated with either DMSO or 15 μM of DIM-C-pPhOCH3 for 24 h and stained for 4′,6-diamidino-2-phenylindole as described in the Materials and Methods. Induction of apoptosis (C) and TRAIL/FasL (D). Cells were treated with either DMSO or different concentrations of DIM-C-pPhOCH3 for 48 h (C) or different time periods as indicated (D). Whole cell lysates were analyzed by western blot analysis as described in the Materials and Methods. β-Actin was used as a loading control. Similar results were observed in duplicate experiments.
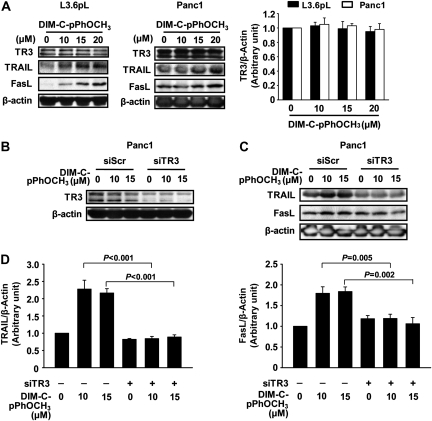
Figure 2A confirms the concentration-dependent induction of both TRAIL and FasL protein expression by DIM-C-pPhOCH3 in L3.6pL and Panc1 cells, whereas levels of TR3 protein were unchanged. Panc1 cells were treated with DIM-C-pPhOCH3 and transfected with siScr (non-specific control) or siTR3 to knockdown TR3 (Figure 2B); loss of TR3 did not affect basal expression of TRAIL or FasL proteins but significantly inhibited induction of these proteins by DIM-C-pPhOCH3 (Figure 2C and D), confirming that their induction is TR3-dependent as previously observed for TRAIL in Panc28 cells (16).
Fig. 2.
TR3-dependent induction of TRAIL and Fas-L by DIM-C-pPhOCH3 in human pancreatic cancer cells. (A) Induction of TRAIL and FasL. L3.6pL and Panc1 cells were treated with DMSO or different concentrations of DIM-C-pPhOCH3 for 48 h and whole cell lysates were analyzed by western blots as described in the Materials and Methods. (B and C) RNA interference studies. Panc1 cells were transfected with either siScr or siTR3, and treated with different concentrations of DIM-C-pPhOCH3 for 48 h. Whole cell lysates were analyzed by western blot analysis, and β-actin was used as a loading control. (D) Quantitation of TRAIL and FasL protein expression. The intensity of protein expression was quantitated using ImageJ software (National Institutes of Health, Bethesda, MD) and the results are presented as means ± standard deviations for three replicate determinations for each treatment group. The levels of significance between induction and TR3 knockdown are indicated.
DIM-C-pPhOCH3-induced gene expression: microarray analysis
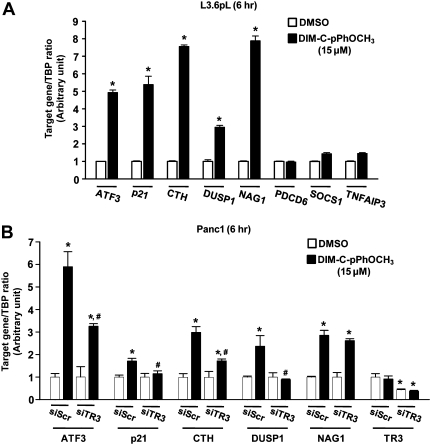
The effects of DIM-C-pPhOCH3 on expression of genes in pancreatic cancer cells was investigated by treating L3.6pL cells with 15 μM DIM-C-pPhOCH3 for 2 and 6 h and analyzing induction of mRNAs using an Amersham Biosciences CodeLink whole human genome array as described previously (17,19). Supplementary Table 1, available at Carcinogenesis Online summarizes genes associated with metabolism/homeostasis, signal transduction, transcription, stress, transport, immune responses and miscellaneous responses that were induced >2-fold. These include ATF3, p21, CTH, DUSP1, growth differentiation factor 15 (GDF15/NAG1), programmed cell death 6, suppressor of cytokine signaling 1 and tumor necrosis factor α-induced protein 3 (Table I). Real-time PCR was used to confirm induction of these genes in L3.6pL cells (Figure 3A), and we observed that ATF3, p21, CTH, DUSP1 and NAG1 were significantly induced after treatment with DIM-C-pPhOCH3 for 6 h. Three genes identified in the microarray experiment (programmed cell death 6, suppressor of cytokine signaling 1 and TNF-AIP3) were minimally induced by DIM-C-pPhOCH3 and were not further investigated. DIM-C-pPhOCH3 induced ATF3, p21, CTH, DUSP1 and NAG1 in Panc1 (Figure 3B) cells as observed in L3.6pL cells (Figure 3A), although the relative magnitude of the induction responses was variable. In Panc1 cells transfected with siTR3 and treated with DIM-C-pPhOCH3, there was a significant decrease in induced ATF3, p21, CTH, DUSP1 and TR3 (positive control) mRNA level, whereas no significant effects were observed for NAG1 mRNA levels indicating that induction of this gene was TR3 independent. TR3 knockdown was more efficient in Panc1 cells; the results suggest that induction of DUSP1 and p21 by DIM-C-pPhOCH3 were fully TR3-dependent, whereas induction of ATF3 and CTH was only partially TR3 dependent, and these observations were consistent with previous studies on C-DIM compounds (28,29).
Table I.
Induction of growth inhibitory and proapoptotic genes by DIM-C-pPhOCH3 (15 μM) in L3.6pL cells using the Amersham Biosciences CodeLink human whole genome array
| Description | Genbank | Relative expression |
|
| 2 h/DMSO | 6 h/DMSO | ||
| Activating transcription factor 3; ATF3 | NM_001674 | 8.11 | 2.04 |
| Cyclin-dependent kinase inhibitor 1A (p21, Cip1); CDKN1A | NM_000389 | 4.10 | 4.79 |
| Cystathionase (cystathionine gamma-lyase); CTH | NM_001902 | 1.97 | 5.08 |
| Dual specificity phosphatase 1; DUSP1 | NM_004417 | 4.66 | 4.13 |
| Growth differentiation factor 15 (NAG1); GDF15 | NM_004864 | 1.83 | 3.71 |
| Programmed cell death 6; PDCD6 | NM_013232 | 3.26 | 2.58 |
| Suppressor of cytokine signaling 1; SOCS1 | NM_003745 | 4.01 | 3.38 |
| Tumor necrosis factor, alpha-induced protein 3; TNFAIP3 | NM_006290 | 3.09 | 2.17 |
Fig. 3.
Induction of growth inhibitory and proapoptotic genes by DIM-C-pPhOCH3 in human pancreatic cancer cells. (A) Induction of genes in L3.6pL cells. L3.6pL cells were treated with DMSO or 15 μM of DIM-C-pPhOCH3 for 6 h, and target gene and TATA-binding protein (an internal control) mRNA expression levels were determined by real-time PCR. Results are presented as means ± standard deviations for three replicate determinations for each treatment group. *P < 0.05 versus DMSO. (B) Role of TR3 in DIM-C-pPhOCH3-induced gene expression. Panc1 cells were transfected with either siScr or siTR3, and treated with 15 μM of DIM-C-pPhOCH3 for 6 h and gene expression was determined by real-time PCR as indicated above in (A). *P < 0.05 versus DMSO in siScr group; #P < 0.05 versus DIM-C-pPhOCH3 in siScr group.
Induction of TRAIL by DIM-C-pPhOCH3 is TR3 and ATF3 dependent
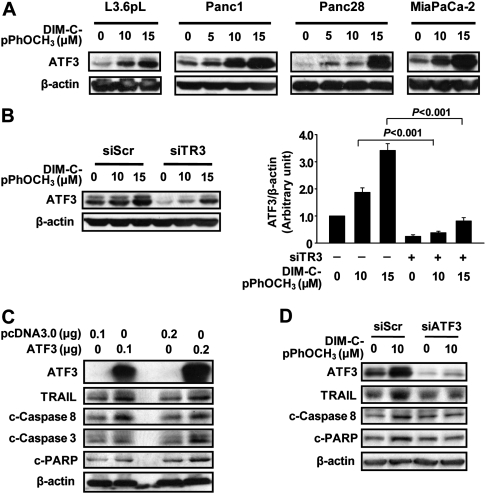
ATF3 is induced by DIM-C-pPhOCH3 in colon and pancreatic cancer cells; however, in contrast to results in Panc1 cells (Figure 3B), TR3 knockdown significantly enhanced ATF3 mRNA levels in RKO colon cancer cells (17). Therefore, the role of TR3 in regulating ATF3 mRNA and protein expression in pancreatic cancer cells was further investigated. Figure 4A shows that ATF3 protein is induced by DIM-C-pPhOCH3 in L3.6pL, Panc1, Panc28 and MiaPaca-2 pancreatic cancer cell lines. The role of TR3 in mediating DIM-C-pPhOCH3-dependent induction of ATF3 protein was determined by knockdown of TR3 by RNA interference in Panc1 cells and the results indicate that loss of TR3 significantly decreased basal and inducible ATF3 protein expression (Figure 4B). However, ATF3 was also induced (partially) after TR3 knockdown and this was consistent with induction of ATF3 by DIM-C-pPhOCH3 and other C-DIMs via a TR3-independent pathway (28,29). Similar results were also observed in L3.6pL cells (supplementary Figure 1 is available at Carcinogenesis Online). The proapoptotic effects of ATF3 were further investigated in Panc1 cells transfected with an ATF3 expression plasmid (Figure 4C). Overexpression of ATF3 induced cleaved PARP, activation of caspases 8 and 3 (cleaved) and induction of TRAIL. Results in Figure 4D show that DIM-C-pPhOCH3-induced TRAIL, cleaved caspase 8 and PARP were decreased in cells transfected with a small inhibitory RNA for ATF3 (siATF3). These results demonstrate the proapoptotic activity of ATF3 and are consistent with a report by Turchi et al. (30), showing that ATF3 expression is proapoptotic and regulates expression of TRAIL.
Fig. 4.
TR3-dependent induction of ATF3 by DIM-C-pPhOCH3 in human pancreatic cancer cells. (A) Induction of ATF3 by DIM-C-pPhOCH3 in pancreatic cancer cells. Various human pancreatic cancer cell lines were treated with DIM-C-pPhOCH3 for 6 h and whole cell lysates were analyzed by western blots as described in the Materials and Methods. (B) RNA interference. Panc1 cells were transfected with either siScr or siTR3 and treated with different concentrations of DIM-C-pPhCH3 for 6 h and whole cell lysates were analyzed by western blots as described in the Materials and Methods. The intensity of protein expression was quantitated using ImageJ software (National Institutes of Health). The experiment was repeated three times, and the results are presented as means ± standard deviations. Statistical significances between induced and knockdown are indicated. (C) ATF3 overexpression induces apoptosis and TRAIL. Panc1 cells were transfected with ATF3 expression vector for 5 h using LipofectAMINE 2000 reagent. Forty-eight hours after transfection, whole cell lysates were analyzed by western blots as described in the Materials and Methods. (D) ATF3 knockdown blocks induction of TRAIL. Panc1 cells were transfected with siCtl (non-specific oligonucleotides) or siATF3 and treated with DMSO or DIM-C-pPhOCH3, and after 48 h, whole cell lysates were obtained and analyzed by western blots as described in the Materials and Methods.
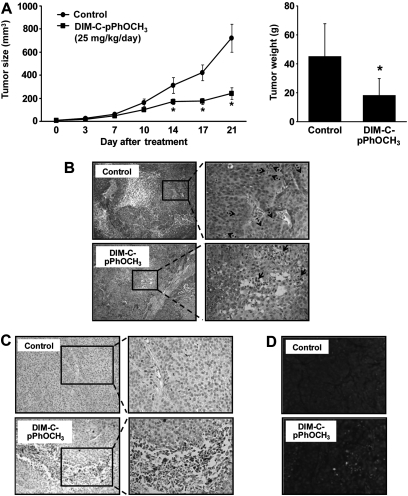
DIM-C-pPhOCH3 inhibits pancreatic tumor growth and induces apoptosis
The effect of DIM-C-pPhOCH3 (25 mg/kg/d) on tumor growth and induction of apoptosis was examined in athymic nude mice-bearing L3.6pL cells as xenografts. DIM-C-pPhOCH3 inhibited tumor volumes and tumor weights (Figure 5A) and, at this dose, there were no apparent changes in organ or body weights or organ histopathology. Hematoxylin and eosin staining showed enhanced mitotic cells in the control tumor, whereas apoptotic cells were enhanced in tumors from animals treated with DIM-C-pPhOCH3 (Figure 5B). We also observed increased cleaved caspase-3 (Figure 5C) and TUNEL staining (Figure 5D) in tumors from treated versus control animals, demonstrating that DIM-C-pPhOCH3 induced proapoptotic activity in both in vivo and in vitro models for pancreatic cancer.
Fig. 5.
DIM-C-pPhOCH3 inhibits tumor growth in a human pancreatic tumor mouse xenograft model. (A) Inhibition of tumor growth. Male athymic nude mice-bearing L3.6pL cell xenografts were treated with DIM-C-pPhOCH3 (25 mg/kg/day), and tumor volumes and weight were determined. (B) Hematoxylin and eosin staining. Images were collected at ×100 (left panels) and ×400 (right panels) maginification. Mitotic cells (dotted arrow) and apoptotic cells or area (solid arrow) are indicated. (C) Immunostaining for cleaved caspase-3. Immunohistochemical staining was performed on tumor sections as described in the Materials and Methods. Images were collected at ×200 (left panels) and ×400 (right panels) maginification. (D) TUNEL staining. TUNEL assay was used to detect apoptotic cells and performed on tumor sections. Propidium iodide stains both apoptotic and non-apoptotic cells red. Fluorescein-12-dUTP incorporation results in localized green fluorescence only within the nucleus of apoptotic cells. Fluorescent images were collected at high (×400) magnification as outlined in the Materials and methods.
Discussion
TR3 (NR4A1) was initially identified along with Nurr1 (NR4A2) and Nor1 (NR4A3) as an immediate early gene induced by nerve growth factor in PC12 cells (31). The precise physiological roles for TR3 are not fully understood; however, there is evidence that TR3 regulates muscle lipolysis and glucose metabolism (32,33) and plays a protective role in arthritis (34) and atherogenesis (35). In addition, several studies demonstrate a role for TR3 in drug-induced apoptosis in cancer cell lines (22–27,36–39). For example, a number of apoptosis-inducing agents including cadmium, phorbol ester, retinoids, acetylshikonin and related analogs, n-butylenephthalide and cytosporone B induce nuclear translocation of TR3 to the mitochondria or cytosol and interaction of TR3 with bcl-2 results in formation of a proapoptotic complex. There are some differences between studies regarding the induced nuclear translocation of TR3 to the mitochondria or cytosol, and this may be due to the chemical agent, cell context or other unknown factors. DIM-C-pPhOCH3 activates nuclear TR3 in pancreatic, colon and bladder cancer cells, and the effects of this C-DIM analog include inhibition of cancer cell and tumor growth (xenograft mouse model) (16–20).
Microarrays were used to determine DIM-C-pPhOCH3-induced gene expression in colon and bladder cancer cells (17,19) and this approach was also used to investigate DIM-C-pPhOCH3-induced gene expression in pancreatic cancer cells. Analysis of DIM-C-pPhOCH3-induced gene expression using CodeLink Whole Genome Bioarrays (30026) and GeneSpring software demonstrated that several pathways were activated in L3.6pL cells (supplementary Table 1 is available at Carcinogenesis Online). DIM-C-pPhOCH3 induced genes associated with cellular metabolism and homeostasis, signal transduction, transcription, stress, transport, immune responses and apoptosis, and given the importance of the latter pathway for an anticancer drug, we further investigated the role of TR3 in mediating induction of proapoptotic genes. Induction of TRAIL by DIM-C-pPhOCH3 appears to be an indirect response and not observed until after treatment for 24 h (Figure 1D) and induction of TRAIL was not detected in the microarray experiments due to the short (2 and 6 h) treatment times. However, RNA interference studies (siTR3) confirmed that induction of TRAIL and FasL by DIM-C-pPhOCH3 was TR3 dependent (Figure 2B–D), and this was consistent with studies on induction of TRAIL by DIM-C-pPhOCH3 in other cancer cell lines (16,17,19).
DIM-C-pPhOCH3 also activates several other growth inhibitory and proapoptotic genes in pancreatic cancer cells (Table I) and many of these same genes are also induced in bladder and colon cancer cells (17,19). Based on results of the microarray studies, we initially confirmed induction responses in L3.6pL cells (Table I) and real-time PCR showed that only five genes were consistently induced (Figure 3A), and similar results were observed in Panc1 cells (Figure 3B). Previous studies showed that induction of p21 by DIM-C-pPhOCH3 was TR3 dependent in Panc1 cells (18) and induction of CTH, ATF3 and DUSP1 but not NAG1 is also TR3 dependent in Panc1 cells (Figure 3B). Some but not all of these same genes were also induced in bladder (CTH, p21 and NAG1) (19) and colon (CTH and ATF3) (17) cancer cells. One of the most striking differences between pancreatic and colon cancer cells was the effect of RNA interference (siTR3) on the induction of ATF3 mRNA expression by DIM-C-pPhOCH3. Loss of TR3 by RNA interference in Panc1 cells decreased induction of ATF3 mRNA (Figure 3B), whereas in RKO cells, inducibility was increased (17). Therefore, the role of TR3 in mediating induction of ATF3 in pancreatic cancer cells was further investigated.
Figure 4A–B confirm that ATF3 was induced by DIM-C-pPhOCH3 in several pancreatic cancer cell lines and the induction response was attenuated in Panc1 and L3.6pL cells transfected with siTR3 (Figure 4B and supplementary Figure 1 is available at Carcinogenesis Onlineure). ATF3 is a stress-induced gene and a member of the ATF/CREB transcription factor family that regulates expression of several genes including those involved in the cell cycle and apoptosis (30,40–42). The proapoptotic function of ATF3 was recently investigated by both induction and knockdown and the results demonstrated that ATF3 expression decreased cancer cell growth, activated caspase 3 and apoptosis and also regulated expression of TRAIL (30). Since induction of TRAIL by DIM-C-pPhOCH3 is a delayed and possibly indirect response (Figure 1D), we further investigated the role of ATF3 in mediating induction of TRAIL. Overexpression of ATF3 in Panc1 cells enhanced expression of cleaved caspases 3 and 8 and also increased levels of TRAIL protein (Figure 4C) and knockdown of ATF3 by RNA interference inhibited induction of TRAIL by DIM-C-pPhOCH3 (Figure 4D) This indicates that in pancreatic cancer cells, activation of nuclear TR3 by DIM-C-pPhOCH3 induces ATF3 and ATF3-dependent genes/responses including TRAIL which play a role in the growth inhibitory/proapoptotic effects of this compound. However, C-DIM compounds including DIM-C-pPhOCH3 also activate endoplasmic reticulum stress in pancreatic and colon cancer cells and N-terminal jun kinase-dependent induction of death receptor 5 (DR5), and this TR3-independent pathway may also contribute to induction of ATF3 and activation of the extrinsic apoptotic pathway (28,29). Moreover, supplementary Figure 2A and B, available at Carcinogenesis Online confirms that DIM-C-pPhOCH3 induces phospho-JNK and other kinases in pancreatic cancer cells and C-DIM-dependent activation of kinases is important for the receptor-independent anticancer activities of these compounds (28,29,43). Thus, DIM-C-pPhOCH3 induces apoptosis (extrinsic pathway) in cancer cells through nuclear TR3-dependent and -independent (kinase activation/endoplasmic reticulum stress) pathways, which contrasts to the TR3-dependent effects of other apoptosis-inducing agents, which require nuclear export of TR3 (22–27,36–39). TR3 is somewhat unique among NRs since many different classes of mechanism-based anticancer agents induce TR3-dependent apoptosis by several pathways. For example, DIM-C-pPhOCH3 and DIM-C-pPhOH activate and deactivate nuclear TR3, respectively, in transactivation assays and this is accompanied by modulation of different genes and induction of apoptosis (16,17,21). In contrast, cytosporone B and related compounds directly bind TR3 and activate both nuclear TR3 and export of nuclear TR3; however, induction of apoptosis by these compounds is due, in part, to nuclear TR3-dependent transcriptional repression of the antiapoptotic brain and reproductive organ-expressed protein (38,39). The observed ligand-dependent differences with respect to activation/deactivation of TR3 suggest that these may be due, in part, to their activity as selective receptor modulators in which the modulation may involve direct binding to the receptor (cytosporone B) or indirect activation/deactivation of TR3 by the C-DIM compounds. The mechanisms associated with the differences in modulation of TR3 are currently being investigated.
In summary, this study identifies several genes induced by DIM-C-pPhOCH3 in pancreatic cancer cells including TR3-dependent growth inhibitory/proapoptotic p21, ATF3, DUSP1, TRAIL and CTH genes and shows that induction of TRAIL was also ATF3 dependent. These responses are consistent with the effects of DIM-C-pPhOCH3 on pancreatic cancer cell and tumor growth and the induction of apoptosis in cells (Figure 1) and tumors (Figure 5) in a xenograft model. At the dose used in this and other studies, DIM-C-pPhOCH3 did not affect organ or body weights and did not exhibit evidence for toxicity (16,17). TR3 is overexpressed in human pancreatic tumors (21), and C-DIMs that activate (this study) or deactivate TR3 (21) induce apoptosis through different pathways and inhibit pancreatic cancer cell and tumor growth, demonstrating that the orphan NR TR3 is a unique target for pancreatic cancer chemotherapy. Current studies are investigating the clinical potential of drugs that target TR3 alone and in combination with other agents for treating pancreatic cancer.
Supplementary material
Supplementary Table 1 and Figures 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (R01CA124998); Texas A&M AgriLife.
Supplementary Material
Acknowledgments
Conflict of Interest Statement: S.S. holds patents on the use of C-DIMs as anticancer agents.
Glossary
Abbreviations
- ATF3
activating transcription factor
- C-DIM
1,1-bis(3′-indolyl)-1-(p-substituted phenyl)methane
- DMEM
Dulbecco’s modified Eagle’s medium
- DIM-C-pPhOCH3
1,1-bis(3′-indolyl)-1-(p-methoxyphenyl)methane
- DIM-C-pPhOH
1,1-bis-(3′-indolyl)-1-(p-hydroxyphenyl)methane
- DMSO
dimethyl sulfoxide
- DUSP1
dual specificity phosphatase 1
- FasL
Fas ligand
- FBS
fetal bovine serum
- mRNA
messenger RNA
- NAG1
non-steroidal anti-inflammatory drug-activated gene 1
- NR
nuclear receptor
- PCR
polymerase chain reaction
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- TUNEL
transferase-mediated deoxyuridine triphosphate nick end labeling
References
- 1.Jemal A, et al. Cancer statistics, 2010. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Li D. Molecular epidemiology of pancreatic cancer. Cancer J. 2001;7:259–265. [PubMed] [Google Scholar]
- 3.Gold EB, et al. Epidemiology of and risk factors for pancreatic cancer. Surg. Oncol. Clin. N. Am. 1998;7:67–91. [PubMed] [Google Scholar]
- 4.Hruban RH. Pancreatic cancer: from genes to patient care. J. Gastrointest. Surg. 2001;5:583–587. doi: 10.1016/s1091-255x(01)80099-8. [DOI] [PubMed] [Google Scholar]
- 5.Bardeesy N, et al. Pancreatic cancer biology and genetics. Nat. Rev. Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 6.Han H, et al. Identification of differentially expressed genes in pancreatic cancer cells using cDNA microarray. Cancer Res. 2002;62:2890–2896. [PubMed] [Google Scholar]
- 7.Jaffee EM, et al. Focus on pancreas cancer. Cancer Cell. 2002;2:25–28. doi: 10.1016/s1535-6108(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 8.Haller DG. Chemotherapy for advanced pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2003;56:16–23. doi: 10.1016/s0360-3016(03)00448-6. [DOI] [PubMed] [Google Scholar]
- 9.McKenna S, et al. The medical management of pancreatic cancer: a review. Oncologist. 2003;8:149–160. doi: 10.1634/theoncologist.8-2-149. [DOI] [PubMed] [Google Scholar]
- 10.Safe S, et al. Cancer chemotherapy with indole-3-carbinol, bis(3'-indolyl)methane and synthetic analogs. Cancer Lett. 2008;269:326–338. doi: 10.1016/j.canlet.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, et al. miR-146a suppresses invasion of pancreatic cancer cells. Cancer Res. 2010;70:1486–1495. doi: 10.1158/0008-5472.CAN-09-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, et al. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin C, et al. A new class of peroxisome proliferator-activated receptor γ (PPARγ) agonists that inhibit growth of breast cancer cells: 1,1-bis(3'-indolyl)-1-(p-substitutedphenyl)methanes. Mol. Cancer Ther. 2004;3:247–259. [PubMed] [Google Scholar]
- 14.Hong J, et al. Peroxisome proliferator-activated receptor γ-dependent activation of p.21 in Panc-28 pancreatic cancer cells involves Sp1 and Sp4 proteins. Endocrinology. 2004;145:5774–5785. doi: 10.1210/en.2004-0686. [DOI] [PubMed] [Google Scholar]
- 15.Kassouf W, et al. Inhibition of bladder tumor growth by 1,1-bis(3'-indolyl)-1-(p-substitutedphenyl)methanes: a new class of peroxisome proliferator-activated receptor γ agonists. Cancer Res. 2006;66:412–418. doi: 10.1158/0008-5472.CAN-05-2755. [DOI] [PubMed] [Google Scholar]
- 16.Chintharlapalli S, et al. Activation of Nur77 by selected 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J. Biol. Chem. 2005;280:24903–24914. doi: 10.1074/jbc.M500107200. [DOI] [PubMed] [Google Scholar]
- 17.Cho SD, et al. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and independent pathways. Cancer Res. 2007;67:674–683. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]
- 18.Lee SO, et al. p21 Expression is induced by activation of nuclear nerve growth factor-induced Bα (NGFI-Bα, Nur77) in pancreatic cancer cells. Mol. Cancer Res. 2009;7:1169–1178. doi: 10.1158/1541-7786.MCR-08-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho SD, et al. Activation of nerve growth factor-induced Bα by methylene-substituted diindolylmethanes in bladder cancer cells induces apoptosis and inhibits tumor growth. Mol. Pharmacol. 2010;77:396–404. doi: 10.1124/mol.109.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inamoto T, et al. 1,1-Bis(3'-indolyl)-1-(p-chlorophenyl)methane activates the orphan nuclear receptor Nurr1 and inhibits bladder carcinogenesis. Mol. Cancer Ther. 2008;7:3825–3833. doi: 10.1158/1535-7163.MCT-08-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SO, et al. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res. 2010;70:6824–6836. doi: 10.1158/0008-5472.CAN-10-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, et al. Molecular determinants of AHPN (CD437)-induced growth arrest and apoptosis in human lung cancer cell lines. Mol. Cell. Biol. 1998;18:4719–4731. doi: 10.1128/mcb.18.8.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mu X, et al. TR3 orphan nuclear receptor mediates apoptosis through up-regulating E2F1 in human prostate cancer LNCaP cells. J. Biol. Chem. 2003;278:42840–42845. doi: 10.1074/jbc.M305594200. [DOI] [PubMed] [Google Scholar]
- 24.Lin B, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 25.Wilson AJ, et al. TR3/Nur77 in colon cancer cell apoptosis. Cancer Res. 2003;63:5401–5407. [PubMed] [Google Scholar]
- 26.Wu Q, et al. Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis. 2002;23:1583–1592. doi: 10.1093/carcin/23.10.1583. [DOI] [PubMed] [Google Scholar]
- 27.Kolluri SK, et al. A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell. 2008;14:285–298. doi: 10.1016/j.ccr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lei P, et al. 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes inhibit colon cancer cell and tumor growth through activation of c-Jun N-terminal kinase. Carcinogenesis. 2008;29:1139–1147. doi: 10.1093/carcin/bgn103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei P, et al. Structure-dependent activation of endoplasmic reticulum stress-mediated apoptosis in pancreatic cancer by 1,1-bis(3'-indoly)-1-(p-substituted phenyl)methanes. Mol. Cancer Ther. 2008;7:3363–3372. doi: 10.1158/1535-7163.MCT-08-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turchi L, et al. Hif-2alpha mediates UV-induced apoptosis through a novel ATF3-dependent death pathway. Cell Death Differ. 2008;15:1472–1480. doi: 10.1038/cdd.2008.74. [DOI] [PubMed] [Google Scholar]
- 31.Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988;1:183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell MA, et al. Nur77 regulates lipolysis in skeletal muscle cells. Evidence for cross-talk between the beta-adrenergic and an orphan nuclear hormone receptor pathway. J. Biol. Chem. 2005;280:12573–12584. doi: 10.1074/jbc.M409580200. [DOI] [PubMed] [Google Scholar]
- 33.Chao LC, et al. Nur77 coordinately regulates expression of genes linked to glucose metabolism in skeletal muscle. Mol. Endocrinol. 2007;21:2152–2163. doi: 10.1210/me.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Silva S, et al. Reduction of the incidence and severity of collagen-induced arthritis by constitutive Nur77 expression in the T cell lineage. Arthritis Rheum. 2005;52:333–338. doi: 10.1002/art.20736. [DOI] [PubMed] [Google Scholar]
- 35.Pires NM, et al. Activation of nuclear receptor Nur77 by 6-mercaptopurine protects against neointima formation. Circulation. 2007;115:493–500. doi: 10.1161/CIRCULATIONAHA.106.626838. [DOI] [PubMed] [Google Scholar]
- 36.Shin HJ, et al. Induction of orphan nuclear receptor Nur77 gene expression and its role in cadmium-induced apoptosis in lung. Carcinogenesis. 2004;25:1467–1475. doi: 10.1093/carcin/bgh135. [DOI] [PubMed] [Google Scholar]
- 37.Chen YL, et al. The induction of orphan nuclear receptor Nur77 expression by n-butylenephthalide as pharmaceuticals on hepatocellular carcinoma cell therapy. Mol. Pharmacol. 2008;74:1046–1058. doi: 10.1124/mol.107.044800. [DOI] [PubMed] [Google Scholar]
- 38.Zhan Y, et al. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat. Chem. Biol. 2008;4:548–556. doi: 10.1038/nchembio.106. [DOI] [PubMed] [Google Scholar]
- 39.Liu JJ, et al. A unique pharmacophore for activation of the nuclear orphan receptor Nur77 in vivo and in vitro. Cancer Res. 2010;70:3628–3637. doi: 10.1158/0008-5472.CAN-09-3160. [DOI] [PubMed] [Google Scholar]
- 40.Lu D, et al. Activating transcription factor 3, a stress-inducible gene, suppresses Ras-stimulated tumorigenesis. J. Biol. Chem. 2006;281:10473–10481. doi: 10.1074/jbc.M509278200. [DOI] [PubMed] [Google Scholar]
- 41.Yan C, et al. Activating transcription factor 3, a stress sensor, activates p53 by blocking its ubiquitination. EMBO J. 2005;24:2425–2435. doi: 10.1038/sj.emboj.7600712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson MR, et al. ATF3 transcription factor and its emerging roles in immunity and cancer. J. Mol. Med. 2009;87:1053–1060. doi: 10.1007/s00109-009-0520-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chintharlapalli S, et al. 1,1-Bis(3'-indolyl)-1-(p-substitutedphenyl)methanes inhibit growth, induce apoptosis, and decrease the androgen receptor in LNCaP prostate cancer cells through PPARγ-independent pathways. Mol. Pharmacol. 2007;71:558–569. doi: 10.1124/mol.106.028696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.