Abstract
Background Lipoprotein(a) [Lp(a)] is an established risk factor for coronary disease and stroke, but mechanisms underlying this association are unknown. We examined the association of Lp(a) with early atherosclerosis by using conventional epidemiologic analysis and a Mendelian randomization analysis. The latter utilized genetic variants that are associated with Lp(a) to estimate causal effect.
Methods A prospective population-based cohort study of 939 men and 1141 women was conducted. Lp(a) was measured repeatedly at mean ages 17 and 38 years. Measurements of carotid intima-media thickness (IMT) and brachial flow-mediated dilation (FMD) at mean ages 32 and 38 years were used to determine the level and 6-year progression of subclinical atherosclerosis. Lp(a)-related genetic variant, rs783147, was identified by a genome wide association analysis (P = 3.1 × 10−58), and a genetic score was constructed based on 10 Lp(a)-related variants. Mendelian randomization test was performed using a two-stage instrumental variables analysis.
Results rs783147 and the genetic score were strong instruments for nonconfounded Lp(a) levels (F-statistics 269.6 and 446.0 in the first-stage instrumental variable analysis). However, Lp(a) levels were not associated with the levels of or change in IMT or FMD in any of the conventional and instrumental variables tests. The null finding was observed both with rs783147 and the genetic score as instruments and remained unchanged after adjustment for clinical characteristics, such as age, sex, HDL and LDL cholesterol, ApoB, systolic and diastolic blood pressure, diabetes and smoking.
Conclusions Data from conventional and Mendelian randomization analyses provide no support for early atherogenic effects of increased Lp(a) levels.
Keywords: Lipoprotein(a), atherosclerosis, risk factors, single-nucleotide polymorphism, Mendelian randomization
Introduction
Lipoprotein(a), or Lp(a), is composed of a low-density lipoprotein (LDL)-like particle covalently bound to a glycoprotein, apolipoprotein(a).1 Lp(a) contains cholesterol, cholesteroesters, phospholipids, triglycerides and apolipoprotein B (ApoB).1 The physiological function and cardiovascular effects of Lp(a) are poorly understood, but several epidemiological studies have shown high Lp(a) concentration to be associated with an increased risk of coronary disease and stroke.2,3 Furthermore, recent evidence from single-nucleotide polymorphisms (SNPs) affecting Lp(a) levels suggests that the association with coronary disease may be causal.4–8
Atherosclerosis is a major component of coronary disease and stroke aetiology, but whether the effect of Lp(a) on these vascular endpoints is mediated through accelerated atherosclerosis remains unclear. The few available studies published on this issue have failed to demonstrate a robust link between Lp(a) and indicators of atherosclerosis, such as intima-media thickness (IMT) and brachial flow-mediated arterial dilation (FMD).9–15 Confounding may have contributed to inconsistencies in epidemiologic evidence. A Mendelian randomization approach exploits genetic variants related to hypothesized risk factors to improve causal inference when using observational data.16 However, to our knowledge, no Mendelian randomization analyses using LPA SNPs as instruments for non-confounded Lp(a) levels are available for these atherosclerosis outcomes.
In this study, we therefore use both conventional and Mendelian randomization analyses to examine whether Lp(a) concentrations and Lp(a)-related genetic variants are associated with levels and progression of early atherosclerosis in a large population-based cohort.17
Methods
Study population and genotyping
The study population and methods have been described previously.18,19 Briefly, the subjects were 939 white men and 1141 white women with data on LPA-genotype and atherosclerosis from the population-based Young Finns cohort study. The subjects were genotyped with custom-made Illumina 670K chip according to manufacturer's protocols. Genotypes were culled from intensity data using Illuminus software.20 In genome wide analysis, we identified several variants near the known LPA locus at chromosome 6q26–27 that were strongly associated with Lp(a) levels. We chose rs783147 (chromosome 6: 161 057 980) in the LPA gene as the relevant genetic marker for this study because it had the strongest association with Lp(a) levels (P = 3.1 × 10−58). For a subsidiary analysis, we selected 20 SNPs with a strong association with Lp(a) levels (the 20 lead SNPs in the genome wide analysis; see Table 1 in supplementary data available at IJE online).
Table 1.
Characteristics of study participants
| Characteristica | N | Statisticb |
|---|---|---|
| Age, years | 2080 | 37.7 (5.0) |
| Sex, % | ||
| Male | 939 | 45.1 |
| Female | 1141 | 54.9 |
| LPA SNP (rs783147) | ||
| AA (CC) | 595 | 28.6 |
| AB (CT) | 1044 | 50.2 |
| BB (TT) | 441 | 21.2 |
| Lp(a), mg/dlc | 1670 | 7.5 (3.3–18.5) |
| Lp(a) in 1986, mg/dlc | 1593 | 4.5 (1.5–14.3) |
| Carotid IMT, mm | 2014 | 0.626 (0.096) |
| Carotid IMT in 2001, mm | 2080 | 0.581 (0.092) |
| ΔIMT (2001 to 2007), mm | 1664 | 0.046 (0.084) |
| FMD, % | 1934 | 7.98 (4.38) |
| FMD in 2001, % | 1654 | 8.77 (4.57) |
| ΔFMD (2001 to 2007), % | 1534 | 0.86 (5.40) |
| HDL-C, mmol/l | 2001 | 1.34 (0.33) |
| LDL-C, mmol/l | 1973 | 3.07 (0.78) |
| ApoA1, g/l | 2010 | 1.60 (0.26) |
| ApoB, g/l | 2010 | 1.02 (0.26) |
| Systolic blood pressure, mmHg | 2009 | 121 (14) |
| Diastolic blood pressure, mmHg | 2008 | 76 (11) |
| BMI, kg/m2 | 1982 | 26.0 (4.7) |
| Diabetes mellitus, % | ||
| No | 1965 | 98.5 |
| Yes | 31 | 1.5 |
| Current smoking | ||
| No | 1630 | 81.6 |
| Yes | 368 | 18.4 |
aMeasured in 2007 unless otherwise stated.
bStatistics are mean (SD) for continuous variables or percentage for categorical variables unless otherwise stated.
cMedian (inter-quartile range).
Assessment of Lp(a)
Lp(a) was measured in serum stored at –70°C. In 2007, we used immunoturbidimetric method after <6 months’ storage (Lp(a)-HA reagent, Wako Chemicals GmbH, Germany). The detection limit for this method is 1.0 mg/dl and the antibody used in the reagent is reactive to the various apolipoprotein(a) isoforms.21 In 1986, Lp(a) was assessed by radioimmunoassay (detection limit 3.0 mg/dl), according to the same principles as described for an immunoenzymatic assay,22 using research kits from Pharmacia Diagnostics, Uppsala, Sweden.
Indicators of atherosclerosis
We performed ultrasound studies in 2001 and 2007 using Sequoia 512 ultrasound mainframes (Acuson, CA, USA) with 13.0-MHz linear array transducers to assess carotid IMT and brachial FMD.18,19 Carotid IMT, used as a surrogate marker for atherosclerosis, correlates with cardiovascular risk factors19 and the severity of coronary atherosclerosis23 and is a predictor of future vascular events, such as stroke and myocardial infarction.24 We measured carotid IMT on the posterior (far) wall of the left carotid artery. At least four measurements were taken ∼10 mm proximal to the bifurcation to derive mean carotid IMT. At this region of the common carotid artery, none of the subjects had plaques. The digitally stored scans were manually analysed by the same reader in 2001 and 2007 blinded to subjects’ details. The between-visit coefficient of variation (CV) of IMT measurements was 6.4%, and the intra-observer CV was 3.4%.
Brachial FMD is a correlate of coronary endothelial function25 and there is some evidence to suggest an association between FMD and subsequent progression of carotid IMT,26 consistent with the model that endothelial function becomes abnormal before the structural changes in intima-media occur. We measured the left brachial artery diameter at rest and during reactive hyperaemia. Increased flow was induced by inflation of a pneumatic tourniquet placed around the forearm to a pressure of 250 mmHg for 4.5 min, followed by a release. Three measurements of arterial diameter were performed at end-diastole at a fixed distance from an anatomic marker at rest and 40, 60 and 80 s after cuff release. The vessel diameter after reactive hyperaemia was expressed as the percentage relative to resting diameter. The greatest value between 40 and 80 s was used to derive FMD. The 3-month between-visit CV was 3.2% for diameter measurements and 26.0% for FMD. All FMD measurements were performed by one experienced reader.
Assessment of risk factors
Other clinical characteristics were assessed in 2007 using standard procedures: HDL and LDL cholesterol (from Friedewald formula), apolipoproteins A1 (ApoA1) and ApoB, systolic and diastolic blood pressure, body mass index (BMI), smoking status and prevalent diabetes mellitus.
Statistical analysis
We used the PLINK toolset (http://pngu.mgh.harvard.edu/purcell/plink/)27 to identify SNPs associated with Lp(a) levels and performed all other statistical analyses with Stata, version 10 (Stata Institute, Texas, USA). In order to maximize statistical power, each analysis was based on the maximum number of subjects. Lp(a) values below the detection limit were assigned a value of 0.5 mg/dl in 2007 (4.6%) and 1.5 mg/dl in 1986 (36.0%). Lp(a) levels were loge-transformed before the analysis to reduce skewness. Age- and sex-adjusted associations of rs783147 and Lp(a), as exposures, with levels of and change in IMT and FMD, as outcome measures, were studied using linear regression analysis. Multivariable models of the association between Lp(a) and these outcomes were additionally adjusted for HDL and LDL cholesterol, ApoB, systolic and diastolic blood pressure, diabetes and smoking. We corrected LDL cholesterol and ApoB levels for the Lp(a) contribution prior to their inclusion in the analysis.28 Thus, total lipoprotein(a) mass was multiplied by 0.3, and this value was subtracted from LDL cholesterol values. Similarly, total lipoprotein(a) mass multiplied by 0.16 was subtracted from ApoB values.
In addition to conventional linear regression analysis, we performed an instrumental variables regression analysis to examine the association of Lp(a) with IMT and FMD in a Mendelian randomization study.16,29 Mendelian randomization is used to estimate the magnitude of a causal effect of a risk factor on an outcome by using genetic variants as instrumental variables. Because the genotype, being determined at conception, necessarily precedes the onset of atherosclerosis, any association of the genetic variant with IMT and FMD should be unaffected by reverse causation bias arising from the potential effects of atherosclerosis on Lp(a) concentration. Furthermore, the random assortment of alleles at the time of gamete formation leads to population distributions of genetic variants that are generally independent of the environmental exposures that commonly confound epidemiological risk factor–disease associations.
The first stage of the instrumental variable analysis tested the association between the genetic variant rs783147 (the instrument) and Lp(a). F statistics were used to evaluate the strength of the instrument, with values greater than 10 taken as evidence against weak instruments.30,31
The second stage tested the association between Lp(a) (as instrumented by rs783147) with IMT and FMD. We compared results from the instrumental variable estimates of this association to those from standard linear regression using the Durbin form of the Durbin-Wu-Hausman statistic.32 To test the robustness of the instrumental variable estimates, we repeated the analysis by using a simple additive genetic risk score based on 10 and 20 SNPs as instruments for Lp(a) levels.
Results
Clinical characteristics of the study population are presented in Table 1. The genotype frequencies in rs783147 were in Hardy–Weinberg equilibrium (P > 0.6), thus supporting the integrity of our genetic data. Minor allele frequency (MAF) was 0.47. After adjustment for age and sex, rs783147 was modestly associated with ApoB (regression coefficient B per A allele = 0.017, P = 0.04) and HDL cholesterol (B = −0.019, P = 0.05) but unrelated to LDL cholesterol (B = 0.036, P = 0.14), ApoA1 (B = –0.007, P = 0.38), systolic blood pressure (B = –0.675, P = 0.11), diastolic blood pressure (B = –0.405, P = 0.24), BMI (B = 0.064, P = 0.67), diabetes (B = 0.002, P = 0.67) and current smoking (B = –0.007, P = 0.58).
Mean carotid IMT was 0.64 [standard deviation (SD) 0.11] mm in men and 0.61 (SD 0.08) mm in women. In an age- and sex-adjusted analysis, HDL and LDL cholesterol, ApoA1 and ApoB, systolic and diastolic blood pressure, BMI and diabetes were all associated with IMT. Mean FMD was 7.6% (SD 3.8) in men and 9.9% (SD 4.9) in women. Only ApoB and BMI were associated with FMD. SNP rs783147 was not associated with IMT or FMD (Table 2).
Table 2.
Age- and sex-adjusted association of clinical characteristics and LPA polymorphism rs783147 with carotid IMT and brachial flow-mediated dilation
| IMT, mm |
FMD, % |
|||||
|---|---|---|---|---|---|---|
| Characteristica | N | Regression coefficient (SE) | P | N | Regression coefficient (SE) | P |
| HDL-C, mmol/l | 2001 | −0.038 (0.006) | <0.0001 | 1986 | −0.125 (0.320) | 0.70 |
| LDL-C, mmol/l | 1973 | 0.009 (0.003) | 0.001 | 1958 | 0.207 (0.134) | 0.12 |
| ApoA1, g/l | 2010 | −0.033 (0.008) | <0.0001 | 1995 | 0.236 (0.406) | 0.56 |
| ApoB, g/l | 2010 | 0.053 (0.008) | <0.0001 | 1995 | 1.129 (0.402) | 0.005 |
| Systolic blood pressure, mmHg | 2009 | 0.001 (0.0001) | <0.0001 | 1994 | −0.000 (0.007) | 0.95 |
| Diastolic blood pressure, mmHg | 2008 | 0.001 (0.0002) | <0.0001 | 1993 | 0.007 (0.009) | 0.44 |
| BMI, kg/m2 | 1982 | 0.005 (0.0004) | <0.0001 | 1967 | 0.098 (0.021) | <0.0001 |
| Diabetes mellitus, 0 = no, 1 = yes | 1996 | 0.038 (0.016) | 0.02 | 1981 | 0.072 (0.802) | 0.93 |
| Current smoking, 0 = no, 1 = yes | 1998 | 0.006 (0.005) | 0.25 | 1983 | −0.238 (0.259) | 0.34 |
| LPA SNP, per allele | 2014 | −0.000 (0.003) | 0.93 | 1999 | 0.115 (0.141) | 0.42 |
aAll variables were measured in 2007.
Conventional regression analysis
As expected, logLp(a) was associated with LDL cholesterol [β = 0.095, standard error (SE) = 0.016, P < 0.0001] and ApoB (β = 0.014, SE = 0.005, P = 0.009). However, both age- and sex-adjusted and multivariable adjusted conventional regression analyses provided null findings for all associations of logLp(a) with IMT (Table 3) and FMD (Table 4). As residual confounding in conventional regression analyses can lead to an underestimation of true associations, we continued to examine the association between Lp(a) and indicators of atherosclerosis by using Mendelian randomization.
Table 3.
Association of Lp(a) with carotid IMT according to standard regression analysis and LPA polymorphism rs783147 instrumented regression analysis
| Exposure and analytic approach | Outcome | N | Regression coefficient (SE) | P |
|---|---|---|---|---|
| LogLp(a) lipoprotein in 1986, mg/dl | IMT in 2001, mm | |||
| Standard regression, age and sex adjusted | 1593 | 0.003 (0.002) | 0.09 | |
| Instrumental variables regression, age and sex adjusted | 1593 | −0.000 (0.006) | 0.96 | |
| LogLp(a) lipoprotein in 1986, mg/dl | IMT in 2007, mm | |||
| Standard regression, age and sex adjusted | 1540 | 0.001 (0.002) | 0.46 | |
| Instrumental variables regression, age and sex adjusted | 1540 | −0.000 (0.007) | 0.95 | |
| LogLp(a) lipoprotein in 1986, mg/dl | ΔIMT (2001–07), mm | |||
| Standard regression, age and sex adjusted | 1308 | −0.000 (0.002) | 0.99 | |
| Instrumental variables regression, age and sex adjusted | 1308 | −0.008 (0.007) | 0.26 | |
| LogLp(a) lipoprotein in 2007, mg/dl | IMT in 2007, mm | |||
| Standard regression, age and sex adjusted | 2010 | 0.001 (0.002) | 0.68 | |
| Standard regression, multivariable adjusteda | 1918 | 0.000 (0.002) | 0.82 | |
| Instrumental variables regression, age and sex adjusted | 2010 | −0.000 (0.002) | 0.91 | |
| Instrumental variables regression, multivariable adjusteda | 1918 | −0.000 (0.005) | 0.95 |
aAdjusted for age, sex, HDL and LDL cholesterol, ApoB, systolic and diastolic blood pressure, diabetes and smoking. Correction for the cholesterol content of Lp(a) particles was made by subtracting estimated Lp(a) cholesterol values from the levels of LDL cholesterol and ApoB values prior to inclusion in the analysis.
Table 4.
Association of Lp(a) with brachial FMD according to standard regression analysis and LPA polymorphism rs783147 instrumented regression analysis
| Exposure and analytic approach | Outcome | N | Regression coefficient (SE) | P |
|---|---|---|---|---|
| LogLp(a) lipoprotein in 1986, mg/dl | FMD in 2001, % | |||
| Standard regression, age and sex adjusted | 1492 | 0.086 (0.092) | 0.35 | |
| Instrumental variables regression, age and sex adjusted | 1492 | 0.229 (0.329) | 0.49 | |
| LogLp(a) lipoprotein in 1986, mg/dl | FMD in 2007, % | |||
| Standard regression, age and sex adjusted | 1530 | −0.148 (0.097) | 0.13 | |
| Instrumental variables regression, age and sex adjusted | 1530 | 0.115 (0.335) | 0.73 | |
| LogLp(a) lipoprotein in 1986, mg/dl | ΔFMD (2001–07), % units | |||
| Standard regression, age and sex adjusted | 1215 | −0.227 (0.131) | 0.08 | |
| Instrumental variables regression, age and sex adjusted | 1215 | −0.077 (0.466) | 0.87 | |
| LogLp(a) lipoprotein in 2007, mg/dl | FMD in 2007, % | |||
| Standard regression, age and sex adjusted | 1995 | −0.051 (0.082) | 0.53 | |
| Standard regression, multivariable adjusteda | 1903 | −0.082 (0.082) | 0.33 | |
| Instrumental variables regression, age and sex adjusted | 1995 | 0.178 (0.237) | 0.45 | |
| Instrumental variables regression, multivariable adjusteda | 1903 | 0.224 (0.241) | 0.35 |
aAdjusted for age, sex, HDL and LDL cholesterol, ApoB, systolic and diastolic blood pressure, diabetes and smoking. Correction for the cholesterol content of Lp(a) particles was made by subtracting estimated Lp(a) cholesterol values from the levels of LDL cholesterol and ApoB values prior to inclusion in the analysis.
Instrumental variables analysis using rs783147 as an instrument
There was a strong association between rs783147 and Lp(a) levels, with median (IQR) values being 3.3 (1.5–5.3), 7.3 (3.9–16.7) and 13.1 (5.8–28.1) mg/dl for those with 0, 1 and 2 effect alleles respectively. SNP rs783147 explained 11.7% of the variance in logLp(a). F-values of 269.6 and 37.59 in the first-stage regression analysis suggest that rs783147 is a strong instrument for Lp(a) levels in 2007 and in 1986.
The second-stage instrumental variables analysis, using rs783147 as an instrument for deconfounded Lp(a) levels, found no evidence of an association of Lp(a) with IMT or FMD (Tables 3 and 4). Instrumental-variable estimates of the association of Lp(a) with IMT and FMD did not differ from those obtained from conventional regression analyses (P > 0.31 in age- and sex-adjusted models)
Instrumental variables analysis using genetic score as an instrument
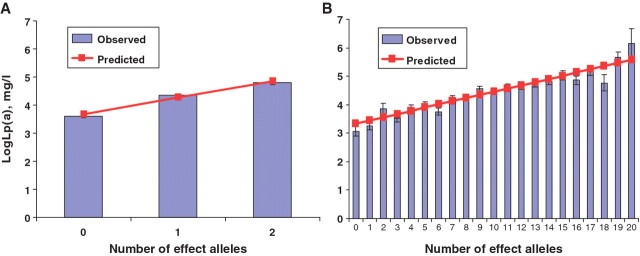
A genetic score based on 10 SNPs (an additive model) explained 17.5% of the variance in logLp(a). F-values of 446.0 and 72.4 from the first-stage regression suggest that the genetic score was a stronger instrument for Lp(a) in 2007 and 1986 than rs783147 alone. Figure 1 illustrates this with predicted means (linear regression) and observed (genotype specific) means of logLp(a) in 2007 by number of effect alleles in rs783147 (Figure 1A) and the number of effect alleles in the genetic score (Figure 1B).
Figure 1.
Associations of rs783147 (A) and genetic score from 10 SNPs (B) with logarithmically transformed lipoprotein(a) levels
In agreement with the rs783147-based analyses, instrumental variables regression with the genetic score provided no support for an association of Lp(a) with IMT or FMD (Table 2 in supplementary data available at IJE online). No further analyses were performed using the first 20 SNPs as they did not provide a stronger genetic instrument [F = 353.98 and 57.10 for Lp(a) in 2007 and 1986, respectively] than the 10-SNP score.
Discussion
This population-based cohort study exploiting both phenotypical and genotype data found no association between Lp(a) and early atherosclerosis, as indicated by carotid IMT and brachial flow-mediated dilation. We are not aware of previous Mendelian randomization data on this issue, but our conventional analyses are in line with prior small-scale studies employing only a single measurement of Lp(a) and atherosclerosis.9–15 In the Atherosclerosis Risk in Communities (ARIC) study, Lp(a) was associated with IMT, but only in subgroups, such as white men and diabetic black women.15
Some limitations to this study and the Mendelian randomization method may contribute to false null findings. First, measurement error reduces opportunities to detect an association and is considered to be greater for Lp(a) levels determined based on frozen rather than fresh samples33 and assays sensitive to apolipoprotein(a) isoforms.34 In this study, Lp(a) was measured in 2007 using apolipoprotein(a) isoform independent assays and we found a strong association between LPA genetic variants (which are not affected by this source of error) and Lp(a), supporting the accuracy of our measurement.
Second, population stratification, pleiotropic effects of the genetic variant, and an association of the genetic instrument with other genetic variants related to the outcome can potentially bias Mendelian randomization analysis.16,35 These are not likely explanations for our findings given that our cohort included only White Europeans and there was no strong evidence of associations between the LPA SNPs and potential confounding factors. Developmental canalization (i.e. genetic variants inducing compensatory phenomena that mask the effect on the outcome) is a source of bias in Mendelian randomization, but seems an implausible explanation for our findings, because it would mean that the canalization masks the effect on atherosclerosis but not on vascular disease endpoints such as myocardial infarction and stroke.4–8
Third, as influences of genetic variants on risk factors are typically small, Mendelian randomization estimates of associations tend to be less precise (although they are also assumed to be less biased) than estimates from conventional regression analysis. In this study, a genome wide association analysis allowed identification of strong instruments for modelling Lp(a) levels. Indeed, F-values of >400 in the first-stage regression are exceptionally high, being 5–40 times higher than those reported in several previous Mendelian randomization studies with positive findings for other risk factors.36–38 Nevertheless, as indicated by large standard errors of the estimates, the present study is not well powered to detect small effects of Lp(a) on atherosclerosis; meta-analyses that pool our data with findings from future Mendelian randomization studies are needed to detect such effects.
The validity of the present data is supported by the convergent results across different analyses, repeated measurements of Lp(a) (with two different methods), and more than one validated indicator for atherosclerosis. A recent meta-analysis of 36 prospective studies found Lp(a) to be an independent, albeit modest, predictor of coronary disease and stroke mortality.2 Large-scale studies have also confirmed an association of genetic variants influencing levels of Lpa(a) with these diseases.4–8 However, subclinical atherosclerosis does not equate future cardiovascular event risk,39 and in vitro and animal studies have reported that Lp(a) may in particular promote thrombosis and inflammation,40,41 i.e. factors that are associated with coronary events and stroke but do not necessarily causally contribute to subclinical atherosclerosis in early adulthood.
In conclusion, all the tests performed in this study suggest that higher Lp(a) concentration does not promote early atherosclerotic changes in a clinically meaningful way among young adults. These findings need to be replicated in other independent cohorts. Our evidence suggests that future research should also be directed to other potential pathological mechanisms, such as prothrombotic and proinflammatory effects and progression of atherosclerosis in later life.
Supplementary data
Supplementary data are available at IJE online.
Funding
This study was supported financially by the Academy of Finland (grants 117797, 126925 and 121584); the Social Insurance Institution of Finland; government grants to the Tampere, Kuopio and Turku University Hospitals; the Finnish Foundation of Cardiovascular Research; the Turku University Foundation; Turku University; the Juho Vainio Foundation; the Lydia Maria Julin Foundation; the Finnish Medical Foundation; and the Research Foundation of Orion Corp. Genotyping was supported by the Wellcome Trust, UK. Mika Kivimaki is supported by the National Heart, Lung, and Blood Institute (grant HL36310) and the National Institute on Aging, US National Institutes of Health (grant AG034454), the Academy of Finland (grant 132944) and the BUPA Foundation, UK.
Conflict of interest: None declared.
KEY MESSAGES.
High lipoprotein(a) [Lp(a)] concentration is associated with an increased risk of coronary disease and stroke. Whether these effects are mediated through accelerated atherosclerosis remains unclear.
In a prospective study of 2080 Finns, Lp(a) concentration was not associated with the levels of, or change in, carotid intima-media thickness (IMT) or brachial flow-mediated dilation (FMD) in adulthood.
Single-nucleotide polymorphism rs783147 provided a strong instrument for Lp(a) levels. In spite of this, Mendelian randomization analyses provided null findings for all associations of Lp(a) with carotid IMT or FMD.
These data suggest that Lp(a) concentration does not contribute to early atherosclerotic changes in a clinically meaningful way.
Supplementary Material
Acknowledgements
Ville Aalto and Irina Lisinen are acknowledged for skilful data management and/or statistical analysis.
References
- 1.Utermann G, Lipoprotein(a) In: The Metabolic and Molecular Bases of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. New York: McGraw-Hill; 2006. pp. 2753–87. [Google Scholar]
- 2.Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamstrup PR. Lipoprotein(a) and ischemic heart disease – a causal association? A review. Atherosclerosis. 2010;211:15–23. doi: 10.1016/j.atherosclerosis.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 4.Luke MM, Kane JP, Liu DM, et al. A polymorphism in the protease-like domain of apolipoprotein(a) is associated with severe coronary artery disease. Arterioscler Thromb Vasc Biol. 2007;27:2030–36. doi: 10.1161/ATVBAHA.107.141291. [DOI] [PubMed] [Google Scholar]
- 5.Shiffman D, Kane JP, Louie JZ, et al. Analysis of 17,576 potentially functional SNPs in three case-control studies of mycardial infarction. PLoS ONE. 2008;3:e2895. doi: 10.1371/journal.pone.0002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiffman D, O'Meara ES, Bare LA, et al. Association of gene variants with incident myocardial infarction in the Cardiovascular Health study. Arterioscler Thromb Vasc Biol. 2008;28:173–79. doi: 10.1161/ATVBAHA.107.153981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–39. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 8.Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–28. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 9.Schlaich MP, John S, Langenfeld MR, Lackner KJ, Schmitz G, Schmieder RE. Does lipoprotein(a) impair endothelial function? J Am Coll Cardiol. 1998;31:359–65. doi: 10.1016/s0735-1097(97)00497-x. [DOI] [PubMed] [Google Scholar]
- 10.Raitakari OT, Adams MR, Celermajer DS. Effect of lp(a) on the early functional and structural changes of atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:990–95. doi: 10.1161/01.atv.19.4.990. [DOI] [PubMed] [Google Scholar]
- 11.El-Gendi SS, Bakeet MY, El-Hamed EA, Ibrahim FK, Ahmed R. The value of lipoprotein(a), homocysteine, and doppler of carotid and femoral arteries in assessment of atherosclerosis in asymptomatic cardiovascular risk patients. J Cardiol. 2008;52:202–11. doi: 10.1016/j.jjcc.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Grebe MT, Schoene E, Schaefer CA, et al. Elevated lipoprotein(a) does not promote early atherosclerotic changes of the carotid arteries in young, healthy adults. Atherosclerosis. 2007;190:194–98. doi: 10.1016/j.atherosclerosis.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Sramek A, Reiber JH, Baak-Pablo R, Sturk A, Rosendaal FR. Lipoprotein(a) and ultrasonographically determined early atherosclerotic changes in the carotid and femoral artery. J Thromb Haemost. 2003;1:374–79. doi: 10.1046/j.1538-7836.2003.00064.x. [DOI] [PubMed] [Google Scholar]
- 14.Denti L, Marchini L, Pasolini G, Baffoni MT, Ablondi F, Valenti G. Lipoprotein lp(a) and cerebrovascular disease in the elderly: Correlations with the severity of extracranial carotid atherosclerosis assessed by ultrasonography. Acta Biomed Ateneo Parmense. 1995;66:175–83. [PubMed] [Google Scholar]
- 15.Schreiner PJ, Heiss G, Tyroler HA, Morrisett JD, Davis CE, Smith R. Race and gender differences in the association of lp(a) with carotid artery wall thickness. The Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 1996;16:471–78. doi: 10.1161/01.atv.16.3.471. [DOI] [PubMed] [Google Scholar]
- 16.Davey Smith G, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]
- 17.Raitakari OT, Juonala M, Rönnemaa T, et al. Cohort profile: the cardiovascular risk in young finns study. Int J Epidemiol. 2008;37:1220–26. doi: 10.1093/ije/dym225. [DOI] [PubMed] [Google Scholar]
- 18.Koskinen J, Magnussen CG, Taittonen L, et al. Arterial structure and function after recovery from the metabolic syndrome. The cardiovascular risk in young finns study. Circulation. 2010;121:392–400. doi: 10.1161/CIRCULATIONAHA.109.894584. [DOI] [PubMed] [Google Scholar]
- 19.Raitakari OT, Juonala M, Kähönen M, et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the cardiovascular risk in young finns study. JAMA. 2003;290:2277–83. doi: 10.1001/jama.290.17.2277. [DOI] [PubMed] [Google Scholar]
- 20.Teo YY, Inouye M, Small KS, et al. A genotype calling algorithm for the Illumina BeadArray platform. Bioinformatics. 2007;23:2741–46. doi: 10.1093/bioinformatics/btm443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WAKO. Lp(a) Specificity. Diagnostics technical information 960520. Germany: Wako Chemicals GmbH; [Google Scholar]
- 22.Dahlen G, Holmlund E, Jansson G. 1986. A solid phase two-site immunoenzymetric assay for apolipoprotein[a] Scand J Clin Lab Invest. 1986;46(Suppl. 185):155. [Google Scholar]
- 23.Burke GL, Evans GW, Riley WA, et al. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 1995;26:86–91. doi: 10.1161/01.str.26.3.386. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz MV, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circ. 2007;115:459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 25.Teragawa H, Ueda K, Matsuda K, et al. Relationship between endothelial function in the coronary and brachial arteries. Clin Cardiol. 2005;28:460–66. doi: 10.1002/clc.4960281004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halcox JPJ, Donald AE, Ellins E, et al. Endothelial function predicts progression of carotid intima-media thickness. Circulation. 2009;119:1005–12. doi: 10.1161/CIRCULATIONAHA.108.765701. [DOI] [PubMed] [Google Scholar]
- 27.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Human Gen. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamstrup PR, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117:176–84. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 29.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–63. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 30.Stock JH, Wright JH, Yogo M. A survey of weak instruments and weak identification in generalized method of moments. J Business Econ Stat. 2002;20:518–29. [Google Scholar]
- 31.Staiger D, Stock JH. Instrumental variables regression with weak instruments. Econometrica: J Econometric Soc. 1997;65:557–86. [Google Scholar]
- 32.Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000;29:722–29. doi: 10.1093/ije/29.4.722. [DOI] [PubMed] [Google Scholar]
- 33.Kronenberg F, Trenkwalder E, Dieplinger H, Utermann G. Lipoprotein(a) in stored plasma samples and the ravages of time: why epidemiological studies might fail. Arterioscler Thromb Vasc Biol. 1996;16:1568–72. doi: 10.1161/01.atv.16.12.1568. [DOI] [PubMed] [Google Scholar]
- 34.Marcovina SM, Koschinsky ML, Albers JJ, Skarlatos S. Report of the national heart, lung, and blood institute workshop on lipoprotein(a) and cardiovascular disease: recent advances and future directions. Clin Chem. 2003;49:1785–96. doi: 10.1373/clinchem.2003.023689. [DOI] [PubMed] [Google Scholar]
- 35.Nitch D, Molokhia M, Smeeth L, DeStavola BL, Whittaker JC, Leon DA. Limits to causal inference based on Mendelian randomization: a comparison with randomized controlled trials. Am J Epidemiol. 2006;163:397–403. doi: 10.1093/aje/kwj062. [DOI] [PubMed] [Google Scholar]
- 36.Kivimäki M, Davey Smith M, Timpson NJ, et al. Lifetime BMI and later atherosclerosis risk in young adults: Examining causal links using Mendelian randomisation in the Cardiovascular Risk in Young Finns Study. Eur Heart J. 2008;29:2552–60. doi: 10.1093/eurheartj/ehn252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bochud M, Marquant F, Marques-Vidal PM, et al. Association between C-reactive protein and adiposity in women. J Clin Endocrinol Metab. 2009;94:3969–77. doi: 10.1210/jc.2008-2428. [DOI] [PubMed] [Google Scholar]
- 38.Timpson NJ, Harbord R, Davey Smith G, Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. Does greater adiposity increase blood pressure and hypertension risk?: Mendelian randomization using the FTO/MC4R genotype. Hypertension. 2009;54:84–90. doi: 10.1161/HYPERTENSIONAHA.109.130005. [DOI] [PubMed] [Google Scholar]
- 39.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: Results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 40.Boffa MB, Marcovina SM, Koschinsky ML. Lipoprotein(a) as a risk factor for atherosclerosis and thrombosis: mechanistic insights from animal models. Clin Biochem. 2004;37:333–43. doi: 10.1016/j.clinbiochem.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Poon M, Zhang X, Dunsky KG, Taubman MB, Harpel PC. Apolipoprotein(a) induces monocyte chemotactic activity in human vascular endothelial cells. Circulation. 1997;96:2514–19. doi: 10.1161/01.cir.96.8.2514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.