How did the study come about?
The Therapeutic Research, Education and AIDS Training (TREAT) Asia Pediatric human immunodeficiency virus (HIV) Observational Database (TApHOD) is a collaborative cohort of HIV-infected children in the Asia–Pacific region. TApHOD was established in 2007 in response to a need for better information about treatment and care of HIV-infected children living in this region. Its aim is to examine the natural history of HIV disease, monitor antiretroviral therapy (ART) and prophylactic treatments, monitor toxicities related to ART and develop capacity for standardized and systematic data collection that will lead to improved management of children living with HIV.
In Asia and the Pacific, approximately 140 000 children are living with HIV; the number has risen dramatically in recent years.1 In 2004, an estimated 37 000 children died of AIDS-related illnesses in South and Southeast Asia and 51 000 became infected with HIV.2 Over the past few years, the use of ART has been shown to slow disease progression and lower mortality in children.3–6 Despite the few reports of efficacy of ART in HIV-infected children in Thailand, China, Cambodia and India,7–15 there are limited data on the natural history of HIV, ART practices and clinical outcomes of these Asian children infected with HIV.
In 2001, The Foundation for AIDS Research, amfAR, in consultation with physicians, researchers and community groups across Asia and Pacific, formed a multinational network called TREAT Asia. The mission of the collaboration is to build capacity and promote safe and effective HIV/AIDS treatment and care (the members of the The TREAT Asia Paediatric HIV Network 2008 is given in Appendix 1; Asterisks, dagger and double dagger indicates TApHOD Steering Committee member, Current Steering Committee chair and co-chair). The network now encompasses 22 adult and 22 paediatric sites. In 2006, the paediatric programme of TREAT Asia launched TApHOD to gather regional paediatric data from clinical and research centres. TApHOD is a member cohort of the International Epidemiologic Databases for the Evaluation of AIDS (IeDEA).16
The organizational structure of TApHOD includes: a project management/administrative team, a data management/biostatistics team and a steering committee consisting of representatives from the former two groups and from all the participating sites (principal investigators). The project management centre is located at TREAT Asia’s Bangkok office and is responsible for the administrative and operational functions of the study. The data management centre, located at the National Centre in HIV Epidemiology and Clinical Research (NCHECR), University of New South Wales in Sydney, coordinates data collection and management and provides support of analytic activities at various stages of the research project.
The TApHOD study is funded by amfAR, the Austrian AIDS Life Association and the US National Institutes of Health, through the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Allergy and Infectious Diseases as part of IeDEA.
What does the study cover?
Currently, there is limited information available regarding HIV disease progression, ART regimens being used, treatment outcomes and mortality in children living with HIV in many countries in the Asia–Pacific region. Such data are important for improving paediatric HIV medical care. The objectives of the TApHOD study, therefore, are to:
examine HIV natural history, including relationship between access to ART and paediatric disease progression;
develop capacity for systematic and standardized paediatric HIV clinical data collection in countries of the Asia–Pacific region;
monitor antiretroviral (ARV) and prophylactic treatments as related to demographics and markers of HIV disease stage in paediatrics;
monitor toxicity to ART in children; and
assist in evaluation of new paediatric HIV treatments in the Asia–Pacific region.
Who are in the sample?
TApHOD is a multi-site, open observational cohort study of infants and children living with HIV in the Asia–Pacific. The study enrolls:
children aged ≤18 years who are conclusively diagnosed with HIV using age-appropriate diagnostic tests: positive HIV virologic test [DNA polymerase chain reaction (PCR), RNA PCR or ultrasensitive p24 antigen assay (Up24)] at any age, or positive antibody testing at age ≥18 months; and
children with severe HIV disease (presumptive diagnosis): HIV antibody-positive at any age (or recent HIV-related maternal death or advanced HIV disease in mother) and CD4 <25%.
A child is excluded or removed from the study if he/she previously was included based on presumptive diagnostic criteria and then has a negative HIV test at ≥18 months of age.
The study participants are recruited from TREAT Asia clinical sites following approval from local governing ethics committees or institutional review boards (IRBs). The first site received IRB approval for the TApHOD protocol in December 2007. Any data collected after a site obtained ethics approval are considered prospective. For patients included in the prospective follow-up, all retrospective data are collected from the date of first entry into the clinic. The date of the earliest enrolment determines the commencement of retrospective data for each site. Sites are also required to provide data for those who died and lost to follow-up during the retrospective period.
Participating sites were selected from major paediatric HIV clinical centres in resource-limited countries in Asia based on their capacity to provide clinical care to children with HIV. Some of these sites are tertiary care referral hospitals and some are primary care centres. Children are referred to these sites from local and district hospitals, medical clinics and maternal–child health clinics, although referrals from other services do occur. Many of the children have their first point of HIV testing/clinical contact directly at the sites.
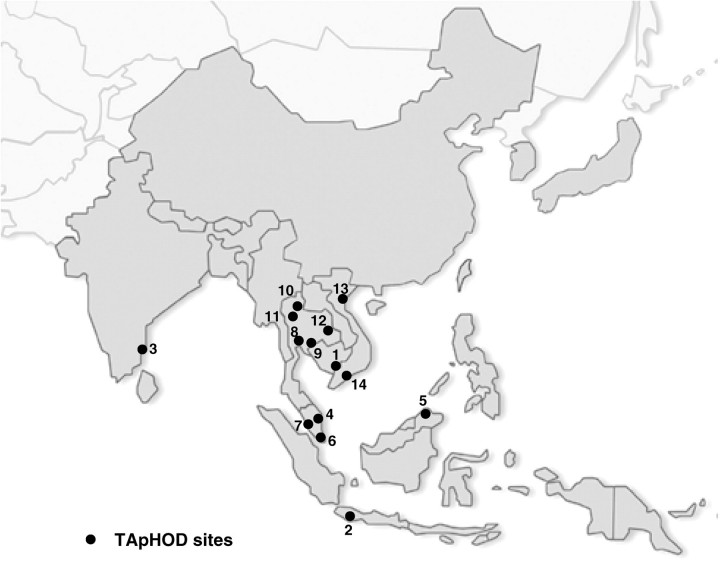
Currently, 14 sites in six countries (Figure 1) have agreed to participate and 12 sites transferred their data to the data management centre for aggregation. Each clinical site will enroll and continuously follow all HIV-infected children receiving care at the facility. Since data are anonymously forwarded to the data coordinating centre, informed consent is not a requirement, except if required by a site’s local ethics committee.
Figure 1.
Geographical location of the participating sites in TApHOD
How often have patients been followed up?
The cohort participants are followed as to the standard of care for paediatric HIV infection at the clinical site attended. The data contributed by each site are determined by ongoing patient care and, therefore, the timing of clinic visits and scope of data collection are determined by the nature of the health-care services provided.
Characteristics of participating sites
In March 2008 each participating site completed a site survey to assess its geographic and demographic attributes, clinical care and diagnostic capacity and access to ART. Selected characteristics of the participating sites are summarized in Table 1. The participating sites are predominantly public, university-based clinics and hospitals located in urban areas. ART access was first implemented in Thailand (1994–2002) and Malaysia (1995–97). Other participating sites implemented ART programmes between 1998 and 2005. At the time of survey, 2765 HIV-infected children were on ART in these sites with an average of 198 children per site [median 168; inter-quartile range (IQR) 103–228]. CD4+ cell counts are used routinely in all sites (Table 2). All sites reported access to HIV RNA testing, although three sites reported that access was limited. The sites in India and Indonesia charge a fee for CD4+ cell count and HIV RNA tests. Paediatric ART is free of charge at all sites except for India.
Table 1.
Profile of participating sites
| Country, city, clinic | Location type | Site type | Public clinic? | Year of ART introduction | Children on ART | Use of generic drugs? | ARV payment |
|---|---|---|---|---|---|---|---|
| Cambodia | |||||||
| 1 Phnom Penh, National Centre for HIV/AIDS, Dermatology and STI, National Pediatric Hospital | Urban | University- based clinic | Yes | 2002 | 656 | Yes | Site/funder |
| Indonesia | |||||||
| 2 Jakarta, Cipto Mangunkusumo Hospital | Urban | University- based clinic | Yes | 2002 | 170 | No | Site/funder |
| India | |||||||
| 3 Chennai, YRG care | Urban | Non-university clinic | No | 1998 | 125 | Yes | Site and patient |
| Malaysia | |||||||
| 4 Kota Bharu, Hospital Raja Perempuan Zainab II | Mixed | Non-university clinic | Yes | 1997 | 68 | Yes | Site/funder |
| 5 Kota Kinabalu, Hospital Likas | Mixed | Non-university clinic | Yes | 2001 | 24 | Yes | Site/funder |
| 6 Kuala Lumpur, Hospital Kuala Lumpur | Urban | Non-university clinic | Yes | 1995 | 96 | Yes | Site/funder |
| 7 Penang, Penang Hospital | Urban | Government clinic | Yes | 1996 | 17 | Yes | Site/funder |
| Thailand | |||||||
| 8 Bangkok, HIV-NAT | Urban | University- based clinic | Yes | 2000 | 153 | Yes | Site/funder |
| 9 Bangkok, Siriraj Hospital | Urban | University- based clinic | Yes | 1994 | 231 | Yes | Site/funder |
| 10 Chiang Rai, Chiang Rai Regional Hospital | Urban | Government clinic | Yes | 2002 | 381 | Yes | Site/funder |
| 11 Chiang Mai, Chiang Mai University | Urban | University- based clinic | Yes | 2002 | 220 | Yes | Site/funder |
| 12 Khon Kaen, Khon Kaen University | Mixed | University- based clinic | Yes | 1995 | 212 | Yes | Site/funder |
| Vietnam | |||||||
| 13 Hanoi, National Hospital of Pediatrics | Urban | University- based clinic | Yes | 2005 | 166 | No | Site/funder |
| 14 Ho Chi Minth City, Children’s Hospital No.1 | Urban | University- based clinic | Yes | 2005 | 246 | No | Site/funder |
Table 2.
Participating sites current access to diagnostic testing
| Country, city, clinic | Access to RNA test | Access to DNA test | Viral load frequency | Access to CD4+ count | CD4+ count frequency | Access to CD4+ percentage | Access to Lymphocyte count | CD4+payment | Viral load payment |
|---|---|---|---|---|---|---|---|---|---|
| Cambodia | |||||||||
| Phnom Penh, National Centre for HIV/AIDS, Dermatology and STD, National Pediatric Hospital | Sometimes | Always | Every 6–12 months | Always | Every 6–12 months | Always | Always | Site/funder | Site/funder |
| Indonesia | |||||||||
| Jakarta, Cipto Mangunkusumo Hospital | Sometimes | No | Not routinely done | Always | Every 6–12 months | Always | Always | Site and patient | Patient |
| India | |||||||||
| Chennai, YRG care | Always | Always | Not routinely done | Always | Every 3–6 months | Always | Always | Site and patient | Patient |
| Malaysia | |||||||||
| Kota Bharu, Hospital Raja Perempuan Zainab II | Always | Always | Every 6–12 months | Always | Every 3–6 months | Always | Always | Site/funder | Site/funder |
| Kota Kinabalu, Hospital Likas | Always | Always | Every 3–6 months | Always | Every 3–6 months | Always | Always | Site/funder | Site/funder |
| Kuala Lumpur, Hospital Kuala Lumpur | Always | Always | Every 3–6 months | Always | Every 3–6 months | Always | Always | Site/funder | Site/funder |
| Penang, Penang Hospital | Always | Always | Every 3–6 months | Always | Every 3–6 months | No | Always | Site/funder | Site/funder |
| Thailand | |||||||||
| Bangkok, HIV-NAT | Always | Always | Every 6–12 months | Always | Every 3–6 months | Always | Always | Site/funder | Site/funder |
| Bangkok, Siriraj Hospital | Always | Always | Every 6–12 months | Always | Every 3–6 months | Always | Always | Site/funder | Site/funder |
| Chiang Mai, Chiang Mai University | Always | Always | Every 6–12 months | Always | Every 3–6 months | Always | Always | Site/funder | Site/funder |
| Chiang Rai, Chiang Rai Regional Hospital | Always | Always | Every 12–24 months | Always | Every 6–12 months | Always | Always | Site/funder | Site/funder |
| Khon Kaen, Khon Kaen University | Always | Always | Every 12–24 months | Always | Every 3–6 months | Always | Always | Site/funder | Site/funder |
| Vietnam | |||||||||
| Hanoi, National Hospital of Pediatrics | Sometimes | Sometimes | Not routinely done | Always | Every 3–6 months | Always | Always | Site/funder | Site/funder |
| Ho Chi Minth City, Children’s Hospital No.1 | Always | Always | Only to assess treatment failure | Always | Every 3–6 months | Always | Always | Site/funder | Site/funder |
Always (Diagnostic test always available if requested by clinicians); Sometimes (25–50% of children can access this test).
Criteria for starting ART changed with time. Currently all sites use three age bands for initiation of treatment: infants aged <12 months, children aged 1–3 years and children and adolescents aged >3 years. They initiate therapy:
in children with AIDS or significant symptoms (WHO stage 3, 4), regardless of CD4 percentage/count or plasma HIV RNA level; and
in children who have met the age-related CD4 threshold for initiating treatment (CD4 < 25% for children aged <1 year, <20% for children 1–3 years and <15% for children >3 years), regardless of symptoms or plasma HIV RNA level.
All sites use non-nucleoside reverse transcriptase inhibitor (NNRTI) containing regimens as first-line therapy and protease inhibitor- (PI) based regimens as the second-line (Table 3).
Table 3.
Type of first- and second-line regimens currently used by the participating sites
| Country, city, clinic | First-line regimen | Second-line regimen |
|---|---|---|
| Cambodia | ||
| Phnom Penh, National Centre for HIV/AIDS, Dermatology and STD, National Pediatric Hospital | AZT/d4T + 3TC + NVP/EFV | ABC + ddI + LPV/r |
| Indonesia | ||
| Jakarta, Cipto Mangunkusumo Hospital | AZT/d4T + 3TC + NVP/EFV | TDF + ddI + LPV/r |
| India | ||
| Chennai, YRG care | AZT + 3TC + NVP/EFV | TDF/ABC + 3TC/FTC + LPV/r |
| Malaysia | ||
| Kota Bharu, Hospital Raja Perempuan Zainab II | AZT + 3TC + NVP/EFV | d4T/ddI + LPV/r |
| Kota Kinabalu, Hospital Likas | ||
| Kuala Lumpur, Hospital Kuala Lumpur | AZT/d4T + 3TC + NVP or AZT/ddi + NVP/EFV | 2NRTI + LPV/r |
| Penang, Penang Hospital | AZT/d4T + 3TC + NVP | 2NRTI + LPV/r |
| Thailand | ||
| Bangkok, HIVNAT | AZT/d4T + 3TC + NVP/EFV PI-based for infants exposed to NVP | NRTI + boosted PI/double boosted PI depends on drug resistance |
| Bangkok, Siriraj Hospital | AZT/d4T + 3TC + NVP/EFV | PI-based regimen (LPV/r) |
| Chiang Mai, Chiang Mai University | AZT/d4T + 3TC + NVP/EFV | 2NRTI + LPV/r or double boosted PI |
| Chiang Rai, Chiang Rai Regional Hospital | AZT/d4T + 3TC + NVP/EFV | 2NRTI + LPV/r |
| Khon Kaen, Khon Kaen University | AZT + 3TC + EFV if < 3 years: AZT + 3TC + NVP | 3TC + EFV + LVR/r |
| Vietnam | ||
| Hanoi, National Hospital of Pediatrics | AZT/d4T + 3TC + NVP/EFV | ABC + ddI + LPV/r/NFV |
| Ho Chi Minth City, Children’s Hospital No.1 | AZT/d4T + 3TC + NVP/EFV | ABC + ddI + LPV/r/NFV |
AZT: aidovudine; d4T: stavudine; 3TC: lamivudine; NVP: nevirapine; EFV: efavirenz; DDI: didanosine; ABC: abacavir; TDF: tenofovir; LPV/r: lopinavir\ritonavir low dose; NRTI: nucleoside reverse transcriptase inhibitor; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor.
Patient characteristics
The TApHOD database currently includes information on 2280 children who enrolled during a period for which we also have complete data on those who died and lost to follow-up. The earliest visit to the clinic was in 1991. Of 2280 children, 549 (24.1%) were lost to follow-up. Table 4 shows the characteristics of the study participants at the time of first clinic visit. Children range from newborns to adolescents aged 17 years; the majority were of school age (5–13 years). There are 1122 (49.2%) females. Perinatal exposure was the dominant mode (>90%) of HIV transmission. CD4+ cell percentage at baseline (first clinic visit) was available for 1169 children. Approximately 61% of these children were severely immunosuppressed, based on CD4+ cell percentage (<15%) at baseline. WHO clinical stage was available for 1241 children. Of these children, 797 (64.2%) were WHO stage III and IV at baseline before initiation of ART. There were some significant differences between patients lost to follow-up and those remaining in the cohort. Those lost to follow-up were less likely to use ARV treatment (49.9 vs 85.4%, P < 0.0001), less likely to have an AIDS-defining illness when they first entered care (19.3% vs 27.7%, P = 0.008), more likely to receive prophylactic treatment (52.8 vs 41.0, P < 0.0001), more likely to experience maternal or infant ARV intervention and more likely to belong to a particular ethnic group.
Table 4.
Demographic characteristics of 2280 TApHOD patients at the time of first clinic visita
| Demography | Retained in the programme (n = 1731) | Lost to follow-up (n = 549)b | P-valuec |
|---|---|---|---|
| Age (years) | 0.197 | ||
| <1 | 252 (14.6) | 78 (14.2) | |
| 1–4 | 505 (29.2) | 184 (33.5) | |
| 5–9 | 663 (38.3) | 208 (37.9) | |
| 10–14 | 296 (17.1) | 74 (13.5) | |
| ≥15 | 15 (0.9) | 5 (0.9) | |
| Median age, years (IQR) | 5.8 (2.4–8.9) | 5.3 (2.2–8.3) | |
| Sex | 0.197 | ||
| Female | 865 (50.0) | 257 (46.8) | |
| Male | 866 (50.0) | 292 (53.2) | |
| Ethnicity | <0.0001 | ||
| Thai | 1123 (64.9) | 283 (51.6) | |
| Indian | 197 (11.4) | 203 (37.0) | |
| Indonesian | 132 (7.6) | 16 (2.9) | |
| Malay | 115 (6.6) | 12 (2.2) | |
| Khmer | 86 (5.0) | 15 (2.7) | |
| Chinese | 22 (1.3) | 3 (0.6) | |
| Other/unknown | 56 (3.2) | 17 (3.1) | |
| Mode of exposure | 0.047 | ||
| Perinatal | 1628 (94.1) | 470 (85.6) | |
| Blood products | 19 (1.1) | 15 (2.7) | |
| Sexual intercourse/ abuse | 9 (0.5) | 2 (0.4) | |
| Other/unknown | 75 (4.3) | 62 (11.3) | |
| Infant ARV exposure | 0.004 | ||
| Yes | 184 (10.6) | 31 (5.7) | |
| No | 968 (55.9) | 86 (15.7) | |
| Unknown | 579 (33.5) | 432 (78.7) | |
| Maternal ARV exposure | 0.015 | ||
| Yes | 156 (9.0) | 26 (4.7) | |
| No | 1000 (57.8) | 94 (17.1) | |
| Unknown | 575 (33.2) | 429 (78.1) | |
| CD4+cell percent | 0.449 | ||
| <10 | 429 (24.8) | 125 (22.8) | |
| 10–14 | 130 (7.5) | 33 (6.0) | |
| 15–24 | 208 (12.0) | 75 (13.7) | |
| ≥25 | 130 (7.5) | 39 (7.1) | |
| Unknown | 834 (48.2) | 277 (50.5) | |
| Median %CD + (IQR) | 10.0 (3.0–20.0) | 11 (4.0–19.9) | |
| WHO clinical stagingd | 0.008 | ||
| Stage I/II | 357 (20.6) | 87 (15.8) | |
| Stage III | 326 (18.8) | 152 (27.7) | |
| Stage IV | 262 (15.1) | 57 (10.4) | |
| Unknown | 786 (45.4) | 253 (46.1) | |
| Severe anaemiae | 0.641 | ||
| Yes | 61 (3.5) | 19 (3.5) | |
| No | 951 (54.9) | 261 (47.5) | |
| Unknown | 719 (41.5) | 269 (49.0) | |
| Mean haemoglobin, Hb (SD) | 10.4 (1.9) | 10.5 (1.9) | |
| ARTf | <0.0001 | ||
| Mono or dual therapy | 182 (10.5) | 90 (16.4) | |
| HAART-NNRTI | 1219 (70.4) | 163 (29.7) | |
| HAART-NNRTI\PI | 7 (0.4) | 0 | |
| HAART-NRTI | 3 (0.2) | 1 (0.2) | |
| HAART-PI | 67 (3.9) | 20 (3.6) | |
| No ART | 253 (14.6) | 275 (50.1) | |
| Prophylactic treatments | <0.0001 | ||
| Yes | 709 (41.0) | 290 (52.8) | |
| No | 1022 (59.0) | 259 (47.2) | |
Values are numbers (percentages) unless stated otherwise.
Percentages may not add up to 100% due to rounding.
aFor CD4+ the closest value within the 3 months before the date of enrolment is used. For haemoglobin and prophylactic treatment the closest value within the 3 months before and one month after enrolment in the clinic are used.
bIncludes those who have been transferred to other clinics or temporary involved in another study.
cExcluding the unknown categories.
dMaximum stage before enrolment is used.
eSevere anaemia was defined according to US guidelines: Hb <10 g/dL for children <21 days; Hb < 8 g/dL for children between 22 and 35 days; Hb < 7 g/dL for children between 36 and 56 days; Hb < 7.5 g/dL for children ≥57 days.
fRefers to the first ART regimen.
SD: standard deviation.
What has been measured?
All of the participating sites collect demographic, treatment, laboratory and clinical data as part of their routine clinical care of HIV patients (Table 5). Because there are no scheduled visits for the purpose of this study, the frequency of clinic visits and laboratory testing depends on the clinic’s standard practice patterns. The participating sites are requested to extract variables from their existing clinic databases or their medical records, and to put them into a Microsoft Access database, which has been designed for TApHOD. These data are then forwarded electronically to the NCHECR for aggregation. Aggregated data are updated in March and September each year. Tailored computer programmes are used to assure data consistency and accuracy following each transfer. All potential errors are addressed by the site personnel and data are then updated prior to the next transfer.
Table 5.
Data collected for TApHOD
| Category | Variables |
|---|---|
| Demographic, background information | Sex, date of birth, date of first clinic visit, date of the most recent contact, ethnicity, HIV exposure category, maternal and infant ARV exposure for PMTCT, date lost to follow-up, date and cause of death |
| HIV diagnostic testing | DNA PCR, RNA PCR, Up24, ELISA <18 months, ELISA ≥18 months, other antibody test (and date), clinical presumptive diagnosis |
| Family history | HIV status of biological mother and father, vital status of biological mother and father, primary caretaker, orphan status, disclosure to child and date, residential status, school attendance and school grade |
| Serology—hepatitis and syphilis | Hepatitis B vaccination, HBV surface antigen, HBV e antigen, HBV core antibody, HCV antibody, syphilis RPR, syphilis VDRL, syphilis TPHA, syphilis other tests |
| Opportunistic infection | Date of diagnosis, CDC category, WHO staging, WHO event, diagnosis, outcome, immune reconstitution syndrome |
| CD4+/HIV testing | Test date, CD4+ count, CD4+ percentage, CD8+ count, CD8+ percentage, total lymphocyte count, viral load (HIV RNA) |
| Other laboratory and physical examination | Test date, height, weight, haemoglobin, haematocrit, platelet count, ALT (SGPT), AST (SGOT), alkaline phosphatase, bilirubin, cholesterol, HDL, glucose, triglycerides, creatinine |
| ART | ART treatment and date started and stopped, dosage, frequency, formulation, generic or patent drug, part of a fixed dose combination, reason ARV stopped |
| Prophylaxis | Prophylactic treatment and start and stop date, primary or secondary |
| Adverse events | Adverse events, onset date and stop date, hopitalization for adverse events, admission and discharge date, discharge diagnosis |
ELISA: enzyme-linked immunosorbent assay; HBV: hepatitis B virus; HCV: hepatitis C virus; CDC: US Centres for Disease Control; RPR: rapid plasma regain; VDRL: venereal disease research laboratory; TPHA: treponema pallidum hemagglutination assay; ALT: alanine aminotransferase also called SGPT: serum glutamic-pyruvic transferase; AST: aspartate transaminase also called SGOT: serum glutamic oxaloacetic transaminase; HDL: high-density lipoprotein; PMTCT: preventing mother-to-child transmission.
In addition, on an annual basis, as a quality assurance measure, the TApHOD coordinator at the NCHECR randomly selects 10% of patients from each site and retrieves their data from the previous 12 months. The site coordinator checks these data against the medical record or clinic database for accuracy and identification of errors. If >10% of the total data points are in error, the site is asked to conduct an additional 10% data check. If the proportion of error remains >10%, all data at the site are checked for accuracy.
We also assessed the quality of the transferred data by computing the percentage of missing key variables (age, sex, clinical stage of HIV infection, CD4+ cell percent and year of ART initiation). The median (IQR) percentage of missing data at the time of first clinic visit was 59% (IQR 33–68%) per site for CD4+ cell percent and 44% (IQR 30–64%) per site for clinical staging. The number of missing values declined with increasing time after enrolment; 29% (IQR 16–45%) for CD4+ cell percent and 5% (IQR 0–14%) for clinical staging at the time of last visit. There were no missing values for age, sex and year of ARV start.
What has been found?
The focus of the project is currently on collecting and combining data for research questions identified by the Steering Committee. The first formal data transfer took place in March 2008. Three manuscripts are in progress to address scientific questions that focus on ART outcomes including survival, shift in opportunistic infections and the experience of adolescents in Asian HIV care and treatment programmes. TApHOD aims to develop scientific collaboration with other paediatric cohorts involved in the IeDEA such as The Paediatric Antiretroviral Treatment Programmes in Lower-Income Countries (KIDS-ART-LINC).17 By merging and analysing data from different regions we will be able to address new hypothesis and provide the evidence needed for appropriate management of HIV children.
What is attrition like?
Loss to follow-up in our study is defined as the proportion of children who did not attend the clinic during the subsequent year after their last visit. We are in the early stage of data collection and it is not possible for us to estimate attrition. However, we examined the proportion of children lost to follow-up among the 2280 children who enrolled in the participating sites for HIV care and are included in the TApHOD database. The median duration of follow-up was 3.0 years (IQR 1.1–4.9). A total of 549 (24.1%) children were identified as lost to follow-up. The median percent of lost to follow-up across different sites is thus 19.1% (IQR 10.7–24.1%; range 2.3–51.4%) or 7.3 (6.7–7.9) per 100 child. The reasons for lost to follow-up were not collected systematically for children and reported only for 153 of them. The reported reasons include: transfer to adult or another ART programme (22.6%), enrolled temporarily in a clinical trial or other studies (4.6%), moved to a new country (0.7%) and unknown (72.1%). Loss to follow-up is important and might bias results if it is associated with mortality or other outcomes. Currently there is no procedure in our participating clinics for tracing patients lost to follow-up in order to ascertain their vital status. A goal for TApHOD is to reduce the number of patients lost to follow-up by strengthening referral systems and regular exchange of information between different clinics, together with patient education and regular updates of contact details.
What are the main strengths and weaknesses?
TApHOD is the first collaborative cohort describing HIV care for infants and children in resource-limited settings in the Asia–Pacific region. This study will provide an important resource to help develop optimal management strategies for HIV-infected children in the region. The main strength of the study is the inclusion of multiple sites from different countries across the region. Four of the participating clinics are located in countries with high Human Development Index (HDI range ≥0.750) and 10 are from countries with a medium HDI (range 0.500–0.749). This makes our study population relatively heterogeneous with respect to demographics, health status, education and social factors. Other strengths include a prospective study design, collection of detailed demographic and clinical data at baseline as well as throughout the follow-up period, a high level of quality control, and a common protocol and manual of operation for defining components of data collection and to maximize consistency of data over time. TApHOD can make unique contributions to the understanding of the epidemiology of paediatric HIV nationally, regionally and globally.
The primary limitation of this study is that participating study sites are mainly university-based clinics and/or referral centres, and the extent to which the findings of this collaboration can be generalized to other more primary-care-focused clinical centres cannot be fully ascertained. Another limitation relates to non-standardization of retrospective data. All clinics enrolled patients before TApHOD was initiated, without a common protocol. This has the potential to introduce bias in the results if outcome or exposure of interest is verified differently between clinics or at different times within the same clinic. Moreover, retrospective data from medical record extraction may be imperfect due to the nature of routine care that may lack some documentation needed. Loss to follow-up and the difficulty of tracing our children is another concern. It is also important to note that the number of infants in our cohort is relatively small. Infants with HIV infection have higher rates of disease progression and mortality than older children18–20 even without evidence of clear immune suppression (i.e. CD4+ above WHO thresholds). Approximately 18% of our children are <18 months of age. In other words, it is probable that there is a survivor bias in our cohort that may lead to an underestimate of the mortality and disease progression in our cohort. Moreover, the wide implementation of PMTCT programme in this region has also decreased the risk of infection in infants, and resulted in lower number of infected young children.
Can I get hold of data? How can I collaborate?
TApHOD is an ongoing study. Each participating clinical site maintains ownership of data it contributes. Collected data are maintained and stored electronically at the NCHECR. Priorities for data analyses are subject of a concept sheet process. Concepts are accepted from people external to TApHOD if submitted in collaboration with one or more TApHOD site principal investigators. All concepts are subject to Steering Committee review and approval, and prioritized according to interest and resources. Under current TApHOD working procedures, raw data would not be made available to external researchers for analysis. Data summaries would be provided for approved concepts. Any question, research concepts or request on the data should be posted to Ms Joselyn Pang at TREAT in Asia’s Bangkok office (joselyn.pang@treatasia.org).
Funding
TREAT Asia is a programme of The Foundation for AIDS Research, amfAR. The TREAT Asia Pediatric HIV Observational Database is supported in part by grants from the Austrian AIDS Life Association and the US National Institutes of Health, through the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Allergy and Infectious Diseases through the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01-AI069907). The National Centre in HIV Epidemiology and Clinical Research is funded by The Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales.
Acknowledgements
The authors wish to thank all the children and the staff in the participating centres who have given their time so generously during the course of this project.
Conflict of interest: None declared.
Appendix 1
The TREAT Asia Paediatric HIV Network 2008
A Bowen, Sydney Children's; Hospital, NSW, Sydney, Australia;
K Chokephaibulkit*,† and W Prasitsuebsai, Siriraj Hospital, Mahidol University, Bangkok, Thailand;
DA Cooper, MG Law,* A Kariminia, National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, NSW, Australia;
SM Fong* and M Thien, Hospital Likas, Kota Kinabalu, Malaysia;
R Hansudewechakul*,‡ and A Khongponoi, Chiang Rai Regional Hospital, Chiang Rai, Thailand;
BV Huy and NV Lam, National Hospital of Pediatrics, Hanoi, Vietnam;
G Jourdain, PHPT, Chiangmai, Chiang Mai, Thailand;
TH Khanh and LP Kim Thoa, Children Hospital no.1, Ho Chi Minh City, Vietnam;
N Kumarasamy* and S Saghayam, YRG Centre for AIDS Research and Education, Chennai, India;
N Kurniati* and D Muktiarti, Cipto Mangunkusumo General Hospital, Jakarta, Indonesia;
P Lumbiganon* and P Tharnprisan, Khon Kaen University, KhonKaen, Thailand;
R Nallusamy* and K C Chan, Penang Hospital, Penang, Malaysia;
NK Nik Yusoff* and L C Hai, Hospital Raja Perempuan Zainab II, Kelantan, Malaysia;
LN Oanh and LK Do, Worldwide Orphans Foundation (WWO), Ho Chi Minh City, Vietnam;
T Puthanakit* and T Suwanlerk, HIV/NAT, Bangkok, Thailand;
K Razali* and NF Abdul Rahman, Pediatric Institute, Hospital Kuala Lumpur, Kuala Lumpur, Malaysia;
AH Sohn,* J Smith and J Pang, TREAT Asia/amfAR – The Foundation for AIDS Research, Bangkok, Thailand;
V Saphonn* and S Saramony, National Centre for HIV/AIDS, Dermatology and STI, Social Heatlh Clinic (NCHADs), Phnom Penh, Cambodia;
V Sirisanthana* and L Aurpibul, Chiang Mai University, Chiang Mai, Thailand;
J Tucker, New Hope for Cambodian Children, Phnom Penh, Cambodia;
U Vibol* and S Sophan, National Pediatric Hospital, Phnom Penh, Cambodia;
FJ Zhang and N Han, Beijing Ditan Hospital, Beijing, China.
*TApHOD Steering Committee Member;
†Current Steering Committee Chair;
‡co-Chair.
References
- 1.UNAIDS. Geneva: WHO; 2007. 2008 Report on the Global AIDS Epidemic. [Google Scholar]
- 2.UNICEF. Born Free From HIV: Treatment for Children. London: UNICEF; 2007. [Google Scholar]
- 3.Gortmaker SL, Hughes M, Cervia J, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–28. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 4.Doerholt K, Duong T, Tookey P, et al. Outcomes for human immunodeficiency virus-1-infected infants in the United Kingdom and Republic of Ireland in the era of effective antiretroviral therapy. Pediatr Infect Dis J. 2006;25:420–26. doi: 10.1097/01.inf.0000214994.44346.d3. [DOI] [PubMed] [Google Scholar]
- 5.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–89. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 6.Puthanakit T, Aurpibul L, Oberdorfer P, et al. Hospitalization and mortality among HIV-Infected children after receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;44:599–604. doi: 10.1086/510489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puthanakit T, Oberdorfer A, Akarathum N, et al. Efficacy of highly active antiretroviral therapy in HIV-infected children participating in Thailand's; National Access to Antiretroviral Program. Clin Infect Dis. 2005;41:100–107. doi: 10.1086/430714. [DOI] [PubMed] [Google Scholar]
- 8.Puthanakit T, Aurpibul L, Oberdorfer P, et al. Sustained immunologic and virologic efficacy after 4 years of highly active antiretroviral therapy in human immunodeficiency virus-infected children in Thailand. Pediatr Infect Dis J. 2007;26:953–56. doi: 10.1097/INF.0b013e318125720a. [DOI] [PubMed] [Google Scholar]
- 9.Janssens B, Raleigh B, Soeung S, et al. Effectiveness of highly active antiretroviral therapy in HIV-positive children: evaluation at 12 months in a routine program in Cambodia. Pediatrics. 2007;120:e1134–40. doi: 10.1542/peds.2006-3503. [DOI] [PubMed] [Google Scholar]
- 10.Fujie Z, Haberer JE, Yan Z, et al. Chinese pediatric highly active antiretroviral therapy observational cohort: A 1-year analysis of clinical, immunologic, and virologic outcomes. JAIDS. 2007;46:594–98. doi: 10.1097/QAI.0b013e318158c08e. [DOI] [PubMed] [Google Scholar]
- 11.Natu SA, Daga SR. Antiretroviral therapy in children: Indian experience. Indian Pediatr. 2007;44:339–43. [PubMed] [Google Scholar]
- 12.Kumarasamy N, Venkatesh KK, Devaleenol B, Poongulali S, Mothi SN, Solomon S. Safety, tolerability and effectiveness of generic HAART in HIV-infected children in South India. J Trop Pediatr. 2009;55:155–59. doi: 10.1093/tropej/fmn080. [DOI] [PubMed] [Google Scholar]
- 13.Lapphra K, Vanprapar N, Chearskul S, et al. Efficacy and tolerability of nevirapine- versus efavirenz-containing regimens in HIV-infected Thai children. Int J Infect Dis. 2008;12:e33–38. doi: 10.1016/j.ijid.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 14.O'B;rien DP, Sauvageot D, Olson D, et al. Treatment outcomes stratified by baseline immunological status among young children receiving nonnucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in resource-limited settings. Clin Infect Dis. 2007;44:1245–48. doi: 10.1086/513433. [DOI] [PubMed] [Google Scholar]
- 15.Puthanakit T, Aurpibul L, Sirisanthana T, Sirisanthana V. Efficacy of non-nucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy in Thai HIV-infected children aged 2 years or less. Pediatr Inf Dis J. 2009;28:246–48. doi: 10.1097/INF.0b013e31818dd72b. [DOI] [PubMed] [Google Scholar]
- 16.International Epidemiologic Databases to Evaluate AIDS (IEDEA) [(5 October 2009, date last accessed)]. http://www.iedea-hiv.org/index.cfm. [Google Scholar]
- 17.Arrive E, Kyabayinze D, Marquis B, et al. Cohort Profile: The Paediatric Antiretroviral Treatment Programmes in Lower-Income Countries (KIDS-ART-LINC) Collaboration. Int J Epidemiol. 2008;37:474–80. doi: 10.1093/ije/dym216. [DOI] [PubMed] [Google Scholar]
- 18.Diaz C, Hanson C, Cooper ER, et al. Disease progression in a cohort of infants with vertically acquired HIV infection observed from birth: the Women and Infants Transmission Study (WITS) J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:221–28. doi: 10.1097/00042560-199807010-00004. [DOI] [PubMed] [Google Scholar]
- 19.Tovo PA, de Martino M, Gabiano C, et al. Prognostic factors and survival in children with perinatal HIV-1 infection: the Italian register for HIV infections in children. Lancet. 1992;339:1249–53. doi: 10.1016/0140-6736(92)91592-v. [DOI] [PubMed] [Google Scholar]
- 20.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]