Abstract
OBJECTIVES
To investigate the relationship of fatigue severity to other clinical features in primary Sjogren’s syndrome (PSS) and to identify factors contributing to the physical and mental aspects of fatigue.
METHODS
We identified 94 subjects who met the American-European consensus criteria for the classification of PSS. Fatigue was assessed with a VAS, the Fatigue Severity Scale (FSS) and the Profile of Fatigue (ProF.) Associations with fatigue was compared using multivariate regression.
RESULTS
Abnormal fatigue defined as a FSS score of greater than or equal to 4 was present in 67% of the patients. Pain, helplessness and depression were the strongest predictors of both FSS and the somatic fatigue domain of the ProF (Prof-S), both with and without adjustment for physiologic and serologic characteristics. Depression was associated with higher levels of fatigue; however, the majority of patients with abnormal fatigue were not depressed. Anti-Ro/SSA positive patients were no more likely to report fatigue than seronegative patients. The regression models explained 62% of the variance in FSS and 78% of the variance in Prof-S. Mental fatigue was correlated with depression and helplessness, but the model predicted only 54% of the variance in mental fatigue (Prof-M.).
CONCLUSIONS
Psychosocial variables are determinants of fatigue, but only partly account for it. While fatigue is associated with depression, depression is not the primary cause of fatigue in PSS. Investigation of the pathophysiologic correlates of physical and mental aspects of fatigue is needed to guide the development of more effective interventions.
Primary Sjogren’s syndrome (PSS) is a common systemic autoimmune disorder affecting approximately 0.09% of the adult population (1). Based on American European Consensus Criteria (AECG) criteria, prevalence among women ranges from 0.1 to 0.6 percent in US, UK and Greek cohorts (2). Patients present with constitutional symptoms, as well as a wide variety of neurologic disorders and systemic involvement, in addition to complaints of oral and ocular dryness. PSS can lead to vasculitis (3) and is associate with a 40 fold increased risk of lymphoma (4). However, fatigue and pain are the most common extra-glandular symptoms (5, 6).
Abnormal fatigue is defined as enduring, generalized tiredness and can be characterized in terms of intensity, duration and effects upon daily function (7). In population-based studies, approximately 20% of healthy adults report persistent fatigue (8, 9). Among patients with autoimmune disease the prevalence of fatigue is much higher, in the range of 60–70% (10–12). Despite the impact that fatigue has on quality of life in rheumatic disorders, there is no consensus regarding the assessment of fatigue. There is very little data comparing different fatigue measures and the pathogenesis of clinically significant persistent fatigue is unknown.
In the primary care setting, chronic fatigue is strongly associated with depression. In a general medical practice, 73% of patients presenting with a complaint of chronic fatigue had a psychiatric diagnosis (13). The relationship of fatigue to depression in PSS is less clear. Despite the importance of clinically significant fatigue in the majority of patients with PSS, only a few studies have examined the relationship of fatigue to other clinical variables (5, 14–19). The relative contribution of physiologic variables, behavioral and immune mediated factors to fatigue has not been well characterized. While previous studies of PSS have demonstrated an association of fatigue with depression (5), the relationship between fatigue and depression is complex and the effect of illness severity is unclear. A key unresolved issue is whether chronic fatigue experience by patients with systemic autoimmunity is mediated primarily by a disturbance in immune function or by factors such as sleep disorder and depression which are associated with fatigue in persons with non-autoimmune disorders.
In order to better understand the determinants of fatigue in PSS, we concurrently evaluated the differential effect of behavioral, cognitive and clinical variables contributing to fatigue in PSS. We used multiple validated measures to assess fatigue and compared the factors predictive of fatigue using 3 instruments and a visual analog scale. We hypothesized that depression; pain and helplessness contribute to, but do not entirely account for, the variance in fatigue severity in patients with PSS.
PATIENTS AND METHODS
Subjects with sicca symptoms defined as: xeropthalmia (dry eyes) and xerostomia (dry mouth); were recruited from the community and from rheumatology, neurology and oral medicine clinics at the University of Minnesota. The study was approved by the Institutional Review Board, University of Minnesota Medical School, for studies involving human subjects, and informed consent was obtained from all subjects. All participants were evaluated with a detailed medical history regarding organ manifestations and family background of autoimmune disorders. Systemic connective tissue disorders other that PSS were established by history, physical exam and careful review of medical records according to ACR criteria (20–22). Uniform application of the AECG criteria for PSS (23) salivary and ocular function and minor salivary gland biopsy was performed to differentiate subjects with primary and secondary Sjogren’s syndrome from individuals with sicca symptoms who did not meet criteria for PSS. Phlebotomy was performed at the time of the clinic visit for complete blood count and differential, westergren sedimentation rate (ESR), as well as serologic tests including antinuclear antibody (ANA), rheumatoid factor (RF), and anti-double stranded DNA performed by the University of Minnesota Medical Center, Fairview Diagnostic Laboratories using standard methodologies. Anti-Ro/SSA and anti-La/SSB titers were determined by ELISA following manufacturer’s instructions (Immunovision, Springdale, Ark.).
Subjects provided information concerning the impact of fatigue on daily life, presence of depression, pain and helplessness. Global descriptions of fatigue and pain were obtained by asking the patient to indicate the degree of symptom severity on a 10-cm scale (range 0 to 100.) For the fatigue assessment we used a double anchored VAS labeled as “Fatigue is no problem” to Fatigue is a major problem.” The specific anchors for the VAS pain were “No pain” and “Pain as bad as it could be.”
Fatigue was also evaluated with validated self report questionnaires: the Fatigue Severity Scale (FSS) and the Profile of Fatigue (ProF). The FSS is a nine item instrument that focuses on the behavioral consequences of fatigue (12). Individuals rate the extent to which they agree with each statement regarding the impact of fatigue on activities of daily living according to a 7 point Likert scale where 1 = strongly disagree and 7 = strongly agree. Score range is 1 to 7. The composite score is the average of the 9 item scores. Higher scores indicate more severe fatigue. The FSS has been shown to have high sensitivity, reliability and internal consistency in the assessment of fatigue (12) and has been widely utilized to assess fatigue severity in neurologic and autoimmune disorders including Systemic Lupus Erythematosus (SLE), Multiple Sclerosis (MS) and Chronic Hepatitis C virus (HCV) infection (24–26). We used a cut off score greater than or equal to 4 to define fatigue cases based on data in the literature demonstrating that an FSS score greater than or equal to 4 reliably differentiates subjects with fatigue from controls (12, 25, 27).
The Profile of Fatigue (ProF) is a multidimensional instrument which was developed to characterize the pattern of fatigue associated with PSS (28). The ProF consists of 16 items used to evaluate 2 domains of fatigue: somatic fatigue (12 items divided into 4 facets) and mental fatigue (4 items divided into 2 facets.). Respondents are asked to rate how they felt in the past 2 weeks on a scale of 0 to 7 with 0 indicated “no problem at all” and 7 meaning “as bad as imaginable.” The facet score is obtained by adding up the item scores within the facet and dividing the sum by the number of items within the facet. The domain scores are obtained in the same way, that is by adding up the facet scores within the domain and dividing the sum by the number of facets within the domain (28). ProF domain scores range from 0 to 7 with higher scores indicating worse functioning. A cutoff score of greater than 2 for both the somatic and mental fatigue domains was used to distinguish PSS cases. The ProF has been validated in 2 studies of PSS (28, 29). The individual domains of somatic and mental fatigue obtained with the ProF may vary independently of each other and a composite score is not provided (28).
The presence of depression was assessed with the Centers for Epidemiologic Studies Depression scale (CES-D) (30). The CES-D is a 20-item questionnaire designed to evaluate depression. The score of each item ranges from 0 to 3. The overall score is the sum of all the items. Scores of greater than or equal to 16 correlate highly with the presence of depression on structured psychometric interviews.
Learned helplessness is a concept which refers to a psychological state in which individuals expect that nothing they do or can do will modify their symptoms. We used the 5 item helplessness subscale derived from the original 15 item “ Rheumatology Attitude Index” The brief RAI has been validated in multiple studies of RA and SLE and reliability of the 5 item helplessness subscale tested in several ethnic groups (31,50). Respondents were asked to rate their degree of agreement with each item using a five-point Likert scale (ranging from strongly disagree to strongly agree.) Higher scores reflect greater degrees of helplessness. Possible scores range between 5–25.
Statistical Methods
Continuous demographic variables and symptom characteristics were summarized by mean and standard deviation for normally distributed variables, while skewed variables were transformed to the log scale. For logged variables, the geometric mean was reported with the interval from 33rd to 66th percentile, corresponding to the percentiles enclosed by mean ± standard deviation for a Normal distribution. Categorical variables were summarized by number and percent. Comparison of fatigued and non-fatigued subgroups was by t-test for continuous variables and chi-square for categorical variables. Confidence intervals for Pearson correlations were estimated from 1000 bootstrap samples with the bias-corrected accelerated (BCA) estimator (32). We compared the roles of three questionnaire instruments in predicting four different fatigue ratings using linear regression models, with and without adjustment for demographic and symptom characteristics. All response and predictor variables were standardized to mean = 0 and standard deviation = 1; for logged variables, the rescaling was done after taking logs. These linear transformations do not affect inferences (p-values) and allow comparison of effect size and direction. All computations were performed using SAS Version 9.1 (SAS Version 9.1, SAS Institute (2003) Cary NC).
RESULTS
Patient Characteristics
Ninety-four subjects met American European consensus criteria (23) for the diagnosis of PSS. All 94 patients are included in the data analysis. The demographic variables, serologic status and percentage of the patients in the cohort with positive histopathology on minor salivary gland biopsy of the patient cohort are given in Table 1. Minor salivary gland biopsies were performed on 69 subjects (73%) of the cohort. Our subjects were 98% Caucasian predominantly of Northern European descent with mean age 58 years (range 29 to 79) at examination. Average disease duration from time of diagnosis was 7.9 years and average age at diagnosis was 49.5 years.
Table 1.
Demographic and symptom characteristics of 94 study participants.
| All | Not Fatigued (FSS < 4) | Fatigued (FSS ≥ 4) | P-value | |
|---|---|---|---|---|
| N = 94 | N = 31 | N = 63 | ||
| Gender: Females | 90 (96%) | 29 (94%) | 61 (97%) | .46 |
| Race: Caucasian | 92 (98%) | 30 (97%) | 62 (98%) | |
| African-American | 1 (1%) | 0 | 1 (2%) | |
| Asian | 1 (1%) | 1 (3%) | 0 | .28 |
| Education: | ||||
| High school only | 21 (22%) | 7 (23%) | 14 (22%) | |
| College or post-grad | 73 (78%) | 24 (77%) | 49 (78%) | .97 |
| Age at examination (yrs) | 58 ± 12 | 57 ± 14 | 58 ± 11 | .90 |
| Post-Menopause (87 females with known status) | 65 (74%) | 18 (67%) | 47 (78%) | .25 |
| Fatigued (FSS ≥ 4) | 63 (67%) | |||
| Fatigued (Prof-M > 2) | 45 (48%) | 6 (19%) | 39 (62%) | .0001 |
| Fatigued (Prof-S > 2) | 90 (96%) | 9 (29%) | 56 (89%) | .0001 |
| FSS** | 4.6 ± 1.6 | 2.5 ± .7 | 5.4 ± .9 | — |
| Prof-M** | 2.8 ± 1.8 | 0.9 ± 1.6 | 3.3 ± 1.7 | .0003 |
| Prof-S** | 3.5 ± 1.8 | 2.2 ± 1.6 | 4.1 ± 1.5 | .0001 |
| VAS – Fatigue (mm)** | 56 ± 33 | 29 ± 32 | 68 ± 25 | .0001 |
| VAS – Pain (mm)** | 39 ± 30 | 20 ± 26 | 48 ± 28 | .0001 |
| CES-D (Depression)** | 13.2 ± 10 | 8.4 ± 9 | 15.5 ± 9 | .0006 |
| Depressed (CES-D ≥ 16) | 30 (32%) | 4 (13%) | 26 (41%) | .0055 |
| RAI (Helplessness) | 12.5 ± 5 | 10 ± 5 | 13.6 ± 4 | .0004 |
| Biopsy positive (69 biopsied) | 51 (74%) | 13 (72%) | 38 (75%) | .85 |
| La positive | 74 (79%) | 26 (84%) | 48 (76%) | .39 |
| Ro concentration | 12 (.1–1150) | 77 (1–6000) | 5 (.1–400) | .006 |
| Ro positive | 80 (80%) | 28 (90%) | 52 (83%) | .32 |
| ANA positive | 64 (68%) | 24 (77%) | 40 (63%) | .17 |
| Absolute Lymphocytes 109/L | 1.3 (0.9–2.0) | 1.4 (.9 – 1.9) | 1.3 (.9 – 2.0) | .77 |
| RF (IU/ml) | 40 (8–200) | 71 (13 – 380) | 30 (7 – 140) | .015 |
| ESR (mm/hour) | 19 (9 – 37) | 23 (12 – 42) | 17 (8 – 34) | .049 |
| WUSF (ml/min) | 1.3 (0.6– 3.2) | 1.2 (.5 – 3.0) | 1.4 (.6 – 3.2) | .32 |
| IgG (mg/dL) | 1400 (900 – 2200) | 1630 (1020 – 2600) | 1300 (900 – 2000) | .019 |
| Schirmer’s * (mm/5 min) | 6.5 (2.8 – 15) | 5.4 (2.5 –12) | 7.1 (3.0 – 17) | .14 |
Average of the Right and Left eye.
Values are mean ± standard deviation, number (percent), or for highly skewed variables geometric mean (33rd percentile – 66th percentile). P-values for comparison between subgroups with FSS < 4 vs FSS ≥ 4
FSS = Fatigue Severity Scale, Prof-M = Profile of Fatigue - Mental Domain, Prof-S = Profile of Fatigue - Somatic Domain, VAS = Visual Analogue Scores, CES-D = Center for Epidemiologic Studies Depression Scale, RAI = Rheumatology Attitudes Index, RF = Rheumatoid Factor, ESR = Erythrocyte Sedimentation Rate, WUSF = Whole Unstimulated Salivary Flow
Prevalence and Predictors of Fatigue Severity
Fatigued patients (FSS ≥ 4) composed 67% of our cohort. Fatigued patients did not differ from non-fatigued in gender proportion, race, age at examination, or education level. Fatigued patients had lower RF, ESR, and IgG than those not fatigued. Mean scores for each of the psychometric scales is also given in Table 1, with marked differences between fatigued and non-fatigued patients on all scales.
We investigated the correlations between the psychometric variables as shown in Table 2. Each of the variables were only moderately related and the strength of the interrelationships between pain and depression; between helplessness and depression and between helplessness and pain were similar. Each variable proved to be a unique predictor of fatigue in the multivariate models shown in Tables 3 and 4.
Table 2.
Correlations between CES-D, VAS-Pain, and RAI scores.
Values are Pearson correlation coefficients, with 95% confidence interval estimated using bootstrap resampling (1000 replications).
VAS = Visual Analogue Scores, CES-D = Center for Epidemiologic Studies Depression Scale, RAI = Rheumatology Attitudes Index (Helplessness)
Table 3.
Comparison of fitted regression models for four fatigue measures, FSS, Prof-M, Prof-S and VAS-Fatigue with adjustment for clinical characteristics*
| FSS* | Prof-M* | Prof-S* | VAS-Fatigue* | |||||
|---|---|---|---|---|---|---|---|---|
| CES-D* | 21 |
|
43 | ● | 33 | ● | 17 | ○ |
| VAS-Pain* | 37 | ● | 19 | 57 | ● | 31 |
|
|
| RAI* | 41 | ● | 27 |
|
24 |
|
36 |
|
| Age at exam | − 5 | − 11 | − 20 |
|
− 17 |
|
||
| Abs Lymph (log) | − 26 |
|
− 16 | ○ | − 5 | − 23 |
|
|
| RF* (log) | − 27 |
|
− 8 | 5 | − 29 |
|
||
| ESR* (log) | 7 | 7 | 3 | 9 | ||||
| WUSF* (log) | 18 |
|
12 | 7 | 1 | |||
| IgG (log) | 24 | ○ | 11 | − 1 | 7 | |||
| Eye average (log) | 0 | − 3 | − 7 | 9 | ||||
| ANA positive | 26 | − 17 | 54 |
|
3 | |||
| Ro concentration (log) | − 19 | − 10 | − 17 | − 4 | ||||
| R-squared | 62% | 54% | 78% | 58% | ||||
Legend for statistical significance:
● p ≤ 0.001
 p ≤ 0.01
p ≤ 0.01
 p ≤ 0.05
p ≤ 0.05
○ p ≤ 0.150
Prof-S, and VAS-Fatigue, all on a standardized scale. The standardized regression coefficients show relative effect size; in the table they have been multiplied by 100. Predictors labeled (log) have been transformed to the log scale, and all predictors, transformed or untransformed, have been standardized. R-squared is the percent of variability in the fatigue measure explained by the model.
FSS = Fatigue Severity Scale, Prof-M = Profile of Fatigue - Mental Domain, Prof-S = Profile of Fatigue - Somatic Domain, VAS = Visual Analogue Scores, CES-D = Center for Epidemiologic Studies Depression Scale, RAI = Rheumatology Attitudes Index (Helplessness), RF = Rheumatoid Factor, ESR = Erythrocyte Sedimentation Rate, WUSF = Whole Unstimulated Salivary Flow
Table 4.
Comparison of fitted regression models for four fatigue measures, FSS Prof-M, Prof-S, and VAS-Fatigue without adjustment for clinical characteristics
| FSS* | Prof-M* | Prof-S* | VAS-Fatigue* | |||||
|---|---|---|---|---|---|---|---|---|
| CES-D* | 24 |
|
45 | ● | 30 | ● | 24 |
|
| VAS-Pain* | 35 | ● | 16 | ○ | 44 | ● | 34 | ● |
| RAI* | 32 |
|
24 |
|
29 | ● | 27 |
|
| R-squared | 51% | 49% | 71% | 49% | ||||
Legend for statistical significance:
● p ≤ 0.001
 p ≤ 0.01
p ≤ 0.01
 p ≤ 0.05
p ≤ 0.05
○ p ≤ 0.10
The standardized regression coefficients show relative effect size; in the table they have been multiplied by 100. R-squared is the percent of variability in the fatigue measure explained by the model.
FSS = Fatigue Severity Scale, Prof-M = Profile of Fatigue - Mental Domain, Prof-S = Profile of Fatigue - Somatic Domain, VAS = Visual Analogue Scores, CES-D = Center for Epidemiologic Studies Depression Scale, RAI=Rheumatology Attitudes Index (Helplessness)
Depression, defined as CES-D of greater than or equal to 16, was present in 32% of the patients. The relationship between fatigue and depression is illustrated in Figure 1. The mean FSS in patients with depression was 5.5 (SD=1.3). The mean FSS in the not depressed group was 4.2 (SD= 1.5). Mean FSS scores were significantly higher in the group of patients with depression 95% CI (0.7, 2.0) with a p-value <0.001. While higher levels of fatigue were correlated with depression, the majority of the fatigued patients (59%) were not depressed. (Figure 1). Interestingly, the anti-Ro/SSA negative patients were clustered in the group of fatigued-not depressed patients.
Figure 1. Scatter Plot of FSS* by CES-D*.
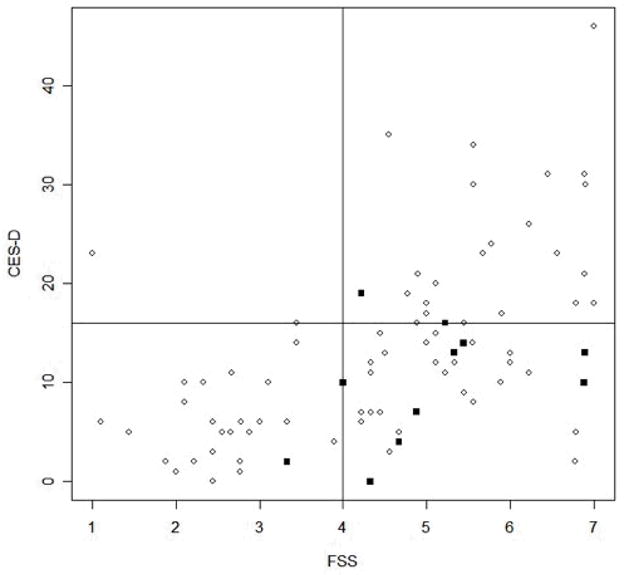
* FSS = Fatigue Severity Scale, CES-D = Center for Epidemiologic Studies Depression Scale
We compared linear regression models using FSS, VAS fatigue and the ProF somatic and mental domains as the dependent variables, adjusting for symptom characteristics. Pain, helplessness and depression were the strongest predictors of both FSS and Prof-S, the somatic fatigue domain of the ProF (Table 3). The mental domain of the ProF (Prof-M) was most strongly associated with depression, while VAS-Fatigue was associated with pain and helplessness. Absolute lymphocyte count (log) was a significant predictor of FSS fatigue and VAS-fatigue. RF (log) was a predictor of FSS and VAS-Fatigue and ANA positivity was predictive of ProF-S only. All these regression models explained well over half the variability in the response.
We compared similar regression models for FSS, VAS fatigue and the ProF somatic and mental domains as the dependent variables without adjustment (Table 4). The predictive associations were essentially unchanged. Using the VAS fatigue as the outcome variable in the model gave similar results to the FSS except with less weight on helplessness and more weight on age. Somatic fatigue is more heavily weighted on pain. Mental fatigue is more heavily weighted on depression.
DISCUSSION
The main purpose of this study was to investigate the relative contributions of disease status (sicca severity), behavioral and immunological factors to fatigue in PSS. The main finding of this study is that psychological factors are determinants of fatigue but explain only 62% of the variability in FSS. Depression is correlated with fatigue severity but is not the primary cause of fatigue in PSS. We also determined that the relationship of pain, depression and helplessness to fatigue were similar in regression models using the various fatigue measures as response variable although the fatigue scales have somewhat different properties. Neither sicca severity nor lab variables were consistently correlated with fatigue.
The mean FSS score of 4.6 in the PSS subjects is significantly different than the mean of 2.3, SD=0.7 reported in normal healthy adults (12). The mean ProF domain scores in this study of 3.43 for somatic fatigue and 2.80 for mental fatigue were similar in magnitude to ProF domain scores previous reported for PSS cohorts in the UK and Sweden suggesting the experience of fatigue is similar among PSS patient groups in different cultural contexts (28, 29).
This study confirms that psychosocial variables are strong determinants of fatigue as previously reported in PSS and in other rheumatic disorders (33). Similar relationships between fatigue, pain and psychological distress have been reported in SLE (34). In a recent study of fatigue in SLE, pain and depression predicted 42% of the variance in fatigue severity (35). In this study, while more severe fatigue was reported by patients with depression, abnormal fatigue was frequently experienced in the absence of depression, suggesting that pathways leading to fatigue and depression are independent but interrelated. Use of a multi-dimensional tool such as the ProF to explore the factors contributing to the mental and somatic aspects of fatigue as suggested by Bowman (28) could be useful to study hypotheses of fatigue pathophysiology.
This study is the first to examine the relationship of fatigue severity and the construct of helplessness in patients with PSS. The observation that helplessness is associated with fatigue in PSS suggests that, as previously observed in SLE, fatigue in PSS can be viewed from the behavioral aspect as a consequence of decreased ability to cope with chronic illness (36). Previous work in RA suggests that an individual with higher helplessness scores is more likely to “experience” greater pain, depression and functional impairment (37). Cognitive interventions designed to modulate helplessness may have efficacy in PSS as has been demonstrated previously in other rheumatic disorders (38).
Our findings are compatible with helplessness theory suggesting that patients who see themselves as unable to influence or control their condition are more susceptible to fatigue and depression. Helplessness might contribute to fatigue directly in several ways. In previous research, helplessness correlated with less effective medication use and less positive health behavior such as exercise (31). The relationship between helplessness and fatigue in our patients with PSS remained significant after taking into account the role of depression. Further study is needed to clarify to what extent there are common underlying mechanisms of fatigue and depression and to clarify the contribution of helplessness to fatigue and depression over time.
Our findings are generally consistent with those of previous studies of PSS in which laboratory variables were not correlated with fatigue (5, 18). Barendregt found no correlation between fatigue scores and ESR, disease duration or hemoglobin level in PSS patients. Tensing reported that values of ANA, anti-Ro/SSA, and IgG level in PSS correlated positively with vitality (and hence inversely with fatigue) on the Medical Outcomes Study Short form (SF-36.)(18). The work by Tensing and Berendregt taken together with our data does not support a role for inflammatory or serologic variables in the pathogenesis of fatigue. Neither IgG level nor Anti-Ro titer was predictive of fatigue in any of the multivariate models; ANA positivity was significant only for ProF-S and RF titer (log) was negatively correlated with FSS and VAS-Fatigue. This study is however, the first to examine the relationship of lymphocyte count to fatigue in PSS and the finding of a negative correlation of lymphocyte count with the FSS and the VAS-Fatigue measures in this study is intriguing.
Inconsistent results were reported in 2 previous studies which examined the relationship of lymphocyte count to fatigue in SLE (34, 39), and negative correlations between inflammatory indices and fatigue have been reported in previous studies of RA and SLE (11, 24, 33, 36). While a minority of studies of SLE have demonstrated a weak correlation of FSS with disease activity (34, 40), even patients with low disease activity or inactive disease have abnormal fatigue. Given the relationship between fatigue severity and lymphopenia that we observed at a single point in time, longitudinal data would be of interest to clarify the relationship between fatigue and immunologic disease activity in PSS.
Current understanding of the physiologic factors contributing to the perception of fatigue is limited. The effects of sleep quality and neuroendocrinologic variables have not been well studied. There is data regarding the relationship of fatigue to muscle endurance and aerobic exercise capacity in PSS. Strombeck examined the relationship between fatigue and aerobic exercise performance (41). Aerobic capacity and fatigue were negatively correlated, however it is unclear whether patients are less fit because of their fatigue or whether decreased ability to exercise plays a causal role in fatigue.
Despite differences in patient age and gender, the mean FSS score of 4.6, SD=1.6 among PSS patients in this cohort are similar in magnitude to mean FSS scores previously reported in multiple other autoimmune disease cohorts including SLE; FSS= 4.7, SD=1.5, Multiple Sclerosis (MS) FSS= 4.8, SD=1.3 and Primary Biliary Cirrhosis FSS= 4.6, SD= 1.6 (12, 42). Fatigue, depression and cognitive dysfunction are a poorly understood complex of symptoms characteristic of multiple chronic illnesses including SLE, MS, Primary Biliary Cirrhosis and HCV infection. (10, 11, 34, 40, 42–45). A similar syndrome of fatigue and neuropsychological symptoms also occurs in patients treated with interferon-alpha. In interferon-alpha mediated fatigue, the central nervous system effects are mediated by inflammatory cytokines (46). The acute administration of interferon-alpha is associated with fatigue which is followed by depression and progressive cognitive dysfunction if interferon therapy is continued (47). We have previously demonstrated that the interferon signature correlates with sicca severity and anti-Ro/SSA titer in PSS (48), hence the finding that anti-Ro does not have a significant relationship with fatigue in this study is inconsistent with the hypothesis that peripheral cytokines mediated by interferon modulate fatigue in PSS. There is evidence in animal models of autoimmune disease that suggests that elaboration of inflammatory cytokines within the central nervous system mediates behavioral and neuropsychological abnormalities (49). The animal data suggests a potential role for local cytokines generated within the central nervous system in modulating fatigue, depression and possibly mild cognitive impairment in PSS, however to date, the role of intrathecal inflammatory cytokines in PSS has not been defined.
The strengths of this study include 1) concurrent assessment of the relationship of pain, depression, helplessness and clinical variables; 2) the evaluation of a large community based sample of PSS patients; 3) comparison between multiple well validated instruments for evaluating fatigue and 4) the careful application of criteria for diagnosis of Sjogren’s in individuals with sicca symptoms. The demographic characteristics of our patient population are similar to other large community based cohorts. The ethnic distribution of the patient group reflects the ethnicity of PSS in the general population of Minnesota. Our study does have several limitations. We did not control for the effect of medications on fatigue. We used self-report instruments potentially subject to response bias to measure the subjective experience of fatigue. Given the cross sectional design of this study, the relationship between disease activity and fatigue remains somewhat unclear. Longitudinal data would be helpful to establish the relationship between the subjective experience of fatigue and potential physiologic correlates of fatigue. More research is needed to clarify the immune- neuroendocrine interactions contributing to the pathogenesis of fatigue in PSS and related autoimmune disorders.
The burden of fatigue in PSS and other autoimmune disorders is considerable. We have demonstrated that psychological factors contribute to fatigue but do not completely account for it. In the future, delineation of the biologic pathways underlying the various subjective aspects of fatigue could provide additional insight into the causes of the persistent fatigue associated with PSS. Moreover, elucidation of the neuroendocrinologic factors contributing to fatigue is likely to provide clues to the enigmatic, subtle cognitive dysfunction frequently reported by patients with PSS.
Acknowledgments
Supported by the National Institutes of Health/NIAMS/RO1 AR50782-01
The authors wish to thank the following research assistants without whose support this study could not have been done: Megan Slater, Jill Weber, Liliana Tobon, Carol Dunn, Carolyn Meyer, Amber Leiran, Anita Peterson and Nicky Te Poel. We also gratefully acknowledge the kind cooperation of the patients, without their cooperation, this study would not have been possible. This project was supported by NIH RO1-AR050782 (KLM) and a grant from the Phileona Foundation (KLM).
References
- 1.Alamanos Y, Tsifetaki N, Voulgari PV, Venetsanopoulou AI, Siozos C, AAD Epidemiology of primary Sjogren’s syndrome in north-west Greece, 1982–2003. Rheumatology. 2006;45:187–191. doi: 10.1093/rheumatology/kei107. [DOI] [PubMed] [Google Scholar]
- 2.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the Prevalence of Arthritis and other Rheumatic Conditions in the United States. Arth Rheumatism. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 3.Tsokos M, Lazarou SA, Moutsopoulos HM. Vasculitis in primary Sjogren’s syndrome: Histologic classifcation and clinical presentation. Am Journal of Clinical Pathology. 1987;88:26. doi: 10.1093/ajcp/88.1.26. [DOI] [PubMed] [Google Scholar]
- 4.Kassen SS, Thomas TL, Moutsopoulos HM, et al. Increased risk of lymphoma in sicca syndrome. Ann Internal Med. 1989;89:888. doi: 10.7326/0003-4819-89-6-888. [DOI] [PubMed] [Google Scholar]
- 5.Barendregt PJ, Visser M, Smets E, Tulen JHM, Vanden Meiracker AH, Boomsma F, et al. Fatigue in Primary Sjogren’s syndrome. Ann Rheum Dis. 1998;57:291–95. doi: 10.1136/ard.57.5.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markusse HM, Oudkerk M, Vroom TM, Breedveld FC. Primary Sjogren’s syndrome: clinical spectrum and mode of presentation based on an analysis of 50 patients selected from a department of rheumatology. Netherlands J Med. 1992;40:125–134. [PubMed] [Google Scholar]
- 7.Belza BL. Comparison of Self-Reported Fatigue in Rheumatoid Arthritis and Controls. J of Rheumatology. 1995;22:639–643. [PubMed] [Google Scholar]
- 8.Chen MK. The epidemiology of self-perceived fatigue among adults. Prev Med. 1986;15:74–81. doi: 10.1016/0091-7435(86)90037-x. [DOI] [PubMed] [Google Scholar]
- 9.Loge JH, Ekeberg O, Kaasa S. Fatigue in the general Norwegian Population: Normative data and Associations. Journal of Psychosomatic Research. 1997;45:53–65. doi: 10.1016/s0022-3999(97)00291-2. [DOI] [PubMed] [Google Scholar]
- 10.Piche T, Gelsi E, Schneider SM, Hebuterne X, Giudicelli J, Ferrua B, et al. Fatigue is associated with high circulating leptin levels in chronic hepatitis C. Gut. 2002;51:434–9. doi: 10.1136/gut.51.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omdal R, Waterloo K, Koldingsnes W, Husby G, Mellgren SI. Fatigue in patients with systemic lupus erythematosus: the psychosocial aspects. J Rheumatology. 2003;30:283–7. [PubMed] [Google Scholar]
- 12.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of Neurology. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 13.Lane T, Matthews D, Manu P. The low yield of physical examinations and laboratory investigations of patients with chronic fatigue. Am J Med Sci. 1990;299:313–18. doi: 10.1097/00000441-199005000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Gudbjornsson G, Broman JE, Hetta J, Hallgren R. Sleep Disturbances in patients with Primary Sjogren’s Syndrome. Brit J Rheumatol. 1993;32:1072–1076. doi: 10.1093/rheumatology/32.12.1072. [DOI] [PubMed] [Google Scholar]
- 15.Giles I, Isenberg D. Fatigue in primary Sjogren’s syndrome: Is there a link with the fibromyalgia syndrome? Ann Rheum Dis. 2000;59:875–878. doi: 10.1136/ard.59.11.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauch E, Volk C, Kratzsch G, Krapf H, Kornhuber HH, Laufen H, et al. Neurological and neuropsychiatric dysfunction in primary Sjogren’s syndrome. Acta Neurol Scand. 1994;89:31–35. [PubMed] [Google Scholar]
- 17.Tishler M, Barak Y, Paran D, Yaron M. Sleep disturbances, fibromyalgia and primary Sjogren’s syndrome. Clin Exp Rheumatology. 1997;15:71–4. [PubMed] [Google Scholar]
- 18.Tensing EK, Solovieva SA, Tervahartiala T, Nordstrom DC, Laine M, Niissalo S, Konttinen YT. Fatigue and health profile in sicca syndrome of Sjogren’s and non-Sjogren’s syndrome origin. Clin Exp Rheumatology. 2001;19:313–316. [PubMed] [Google Scholar]
- 19.Vriezekolk JE, Hartkamp A, Godaert GL, Bootsma H, Kruize AA, Bijlsma JW, et al. Psychological and somatic predictors of perceived and measured ocular dryness of patients with primary Sjogren’s syndrome. J Rheumatology. 2005;32:2351–5. [PubMed] [Google Scholar]
- 20.Hochberg MC, et al. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arth Rheumatism. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 21.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the multicenter committee. Arthritis and Rheumatism. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 23.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:544–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krupp LB, Larocca NG, Muir J, Steinberg AD. A Study of Fatigue in SLE. J Rheumatology. 1990;17:1450–1452. [PubMed] [Google Scholar]
- 25.Flachenecker P, Kumpfel T, Kallmann B, Gottschalk M, Grauer O, Rieckmann P, et al. Fatigue in Multiple Sclerosis: a comparison of different rating scales and correlation to clinical parameters. Multiple Sclerosis. 2002;8:523–526. doi: 10.1191/1352458502ms839oa. [DOI] [PubMed] [Google Scholar]
- 26.Kleinman L, Zodet MW, Hakim Z, Aledort J, Barker K, Krupp L, et al. Psychometric evaluation of the fatigue severity scale for use in chronic hepatitis C. Quality of Life Research. 2000;9:499–500. doi: 10.1023/a:1008960710415. [DOI] [PubMed] [Google Scholar]
- 27.Ad Hoc Committee on Systemic Lupus Erythematosus Response Criteria for Fatigue. Measurement of Fatigue in SLE: A systematic Review. Arthritis and Rheumatism. 2007;57:1348–1357. doi: 10.1002/art.23113. [DOI] [PubMed] [Google Scholar]
- 28.Bowman SJ, Booth DA, Platts RG UK Sjogren’s Interest Group. Measurement of fatigue and discomfort in primary Sjogren’s syndrome using a new questionnaire tool. Rheumatology. 2004;43:758–764. doi: 10.1093/rheumatology/keh170. [DOI] [PubMed] [Google Scholar]
- 29.Strombeck B, Theander E, Jacobsson LT. Assessment of fatigue in primary Sjogren’s Syndrome: the Swedish version of the Profile of Fatigue. Scand J Rheumatology. 2005;34:455–459. doi: 10.1080/03009740510026571. [DOI] [PubMed] [Google Scholar]
- 30.Radloff LS. The Center for Epidemiology Studies Scale for Depression: A Self-Report depression scale for research in the general population. Appl Psychol M. 1977;3:383–401. [Google Scholar]
- 31.DeVellis RF, Callahan LF. A Brief Measure of Helplessness in Rheumatic Disease: The Helplessness Subscale of the Rheumatology Attitudes Index. J Rheumatology. 1993;20:866–9. [PubMed] [Google Scholar]
- 32.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- 33.Wolfe F, Hawley DJ, Wilson K. The Prevalence and Meaning of Fatigue in Rheumatic disease. J Rheumatology. 1996;23:1407–17. [PubMed] [Google Scholar]
- 34.Zonana-Nacach A, Roseman JM, McGwin G, Friedman AW, Baethge BA, Reveille JD, et al. SLE in three ethnic groups: Factors associated with fatigue within 5 years of criteria diagnosis. Lupus. 2000;9:101–109. doi: 10.1191/096120300678828046. [DOI] [PubMed] [Google Scholar]
- 35.Jump RL, Robinson ME, Armstrong AE, Barnes EV, Kilbourn KM, Richards HB. Fatigue in systemic lupus erythematosus: contributions of disease activity, pain, depression, and perceived social support. Journal of Rheumatology. 2005;32:1699–705. [PubMed] [Google Scholar]
- 36.Wang B, Gladman DD, Urowitz M. Fatigue in Lupus is not correlated with disease activity. J Rheumatology. 1998;25:892. [PubMed] [Google Scholar]
- 37.Stein MJ, Wallston KA, Nicassio PM, Castner NM. Correlates of a Clinical Classification Schema for the Arthritis Helplessness Subscale. Arth Rheumatism. 1988;31:876–881. doi: 10.1002/art.1780310708. [DOI] [PubMed] [Google Scholar]
- 38.Parker JC, Frank RG, Beck NC, et al. Pain management in rheumatoid arthritis patients. Arth Rheumatism. 1988;31:593–601. doi: 10.1002/art.1780310503. [DOI] [PubMed] [Google Scholar]
- 39.Wysenbeek AJ, Leibovici L, Weinberger A, Guedj D. Fatigue in SLE. Prevalence and relation to disease expression. Brit J Rheumatol. 1993;32:633–5. doi: 10.1093/rheumatology/32.7.633. [DOI] [PubMed] [Google Scholar]
- 40.Tench CM, McCurdie I, White PD, D’Cruz DP. The prevalence and associations of fatigue in SLE. Rheumatology. 2000;39:1249–1254. doi: 10.1093/rheumatology/39.11.1249. [DOI] [PubMed] [Google Scholar]
- 41.Strombeck BE, Theander E, Jacobsson LT. Effects of exercise on aerobic capacity and fatigue in women with primary Sjogren’s syndrome. Rheumatology (Oxford) 2007;46:868–71. doi: 10.1093/rheumatology/kem004. [DOI] [PubMed] [Google Scholar]
- 42.Huet PM, Deslauriers J, Tran A, Faucher C, Charbonneau J. Impact of Fatigue on the Quality of Life of Patients with Primary Biliary Cirrhosis. Amer J Gastroenterology. 2000;95:760–767. doi: 10.1111/j.1572-0241.2000.01857.x. [DOI] [PubMed] [Google Scholar]
- 43.Barkhuizen A, Rosen HR, Wolf S, Flora K, Benner K, Bennett RM. Musculoskeletal pain and fatigue are associated with chronic hepatitis C: a report of 239 hepatology patients. Gastroenterology. 1999;94:1355–60. doi: 10.1111/j.1572-0241.1999.01087.x. [DOI] [PubMed] [Google Scholar]
- 44.Da Costa D, Dritsa M, Bernatsky S, Pineau C, Menard HA, Dasgupta K, et al. Dimensions of Fatigue in SLE: Relationship to Disease Activity, Behavioral and Psychosocial Factors. Arth Rheumatism Supple. 2003;48:S185. [Google Scholar]
- 45.Janardhan V, Bakshi R. Quality of Life in Patients with Multiple Sclerosis. J Neurologic Science. 2002;205:51–58. doi: 10.1016/s0022-510x(02)00312-x. [DOI] [PubMed] [Google Scholar]
- 46.Bonaccorso SPA, Marino V, Pasquini M, Biondi M, Artini M, Almerighi C, et al. Immunotherapy with interferon -alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry Res. 2001;105:45–55. doi: 10.1016/s0165-1781(01)00315-8. [DOI] [PubMed] [Google Scholar]
- 47.Malik UR, Makower DF, Wadler S. Interferon-mediated fatigue. Cancer Practice. 2001;92:1664–1668. doi: 10.1002/1097-0142(20010915)92:6+<1664::aid-cncr1494>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Emamian ES, Leon J, Rao S, Meyer CM, Leiran A, Gillespie EC, Behrens T, Segal B, Rhodus N, Moser K. Over-expression of interferon-inducible genes is highly correlated with sicca manifestations and autoantibody levels in Sjogren’s syndrome. Arthritis and Rheumatism. 2004;50:S. [Google Scholar]
- 49.Tomita M, Holman BJ, Santoro TJ. Aberrant cytokine gene expression in the hippocampus in murine systemic lupus erythematosus. Neurosci Lett. 2001;302:129–132. doi: 10.1016/s0304-3940(01)01679-2. [DOI] [PubMed] [Google Scholar]
- 50.Thumboo J, Feng P-H, Chan S-P, Boey M-L, Thio S, Fong K. A Chinese version of the Rheumatology Attitudes Index is a valid and reliable measure of learned helplessness in patients with SLE. Lupus. 2002;11:88–94. doi: 10.1191/0961203302lu156oa. [DOI] [PubMed] [Google Scholar]


