Abstract
Gene transfer technologies offer the prospect of enhancing bone regeneration by delivering osteogenic gene products locally to osseous defects. In most cases the gene product will be a protein, which will be synthesized endogenously within and around the lesion in a sustained fashion. It will have undergone authentic post-translational processing and lack the alterations that occur when recombinant proteins are synthesized in bioreactors and stored. Several different ex vivo and in vivo gene delivery strategies have been developed for this purpose, using viral and non-viral vectors. Proof of principle has been established in small animal models using a variety of different transgenes, including those encoding morphogens, growth factors, angiogenic factors, and transcription factors. A small number of studies demonstrate efficacy in large animal models. Developing these promising findings into clinical trials will be a long process, constrained by economic, regulatory and practical considerations. Nevertheless, the overall climate for gene therapy is improving, permitting optimism that applications in bone regeneration will eventually become available.
Keywords: vector, adenovirus, adeno-associated virus, animal model, osteoprogenitor cell, osteoblast, gene activated matrix, allograft revitalization, clinical trial
Introduction
Thanks to a considerable volume of research into the embryology of osteogenesis and the biology of fracture repair in adult organisms, we possess a large database on the ways in which nature forms bone 16, 37. This provides the opportunity to use such information to improve bone healing in the clinic. As the success of autograft bone demonstrates 49, new bone can be formed from pre-existing osteoblasts. However, most future strategies for improving bone regeneration will rely on the use progenitor cells 19. These require, at a minimum, a source of these progenitors and morphogenetic stimuli to promote their differentiation into osteoblasts or, for endochondral ossification, chondrocytes. Additional requirements, including suitable scaffolds and appropriate mechanical environments, are beyond the scope of this review but have been dealt with extensively by other authors 64, 68.
Gene transfer is a technology that allows the targeted, in vivo synthesis of useful gene products, both RNA and protein, in a controlled fashion. Because many important bone morphogenetic signals are proteins, transfer of their cognate genes can serve as the basis for an osteogenic reparative response. Unlike proteins delivered in recombinant form, those synthesized endogenously will have undergone authentic post-translational modification and be free from the altered molecules that can reduce activity and provoke immune responses. This article discusses the various strategies for developing these concepts into clinically useful gene therapy\ies for bone regeneration and reviews progress towards this end. Additional recent reviews of this area can be found in references 20, 48, 10, 52.
Gene therapy basics
Vectors
Gene therapy requires the transfer of therapeutic genes, or more usually their complementary DNAs (cDNAs), into suitable cells. Vectors are used for this purpose. Because viruses transfer their own genetic material very efficiently to the cells they infect, they serve as the basis for many vectors 28. For most purposes, the viral genome is modified to remove sequences that contribute to virulence, replication and other confounding properties. The therapeutic cDNA (transgene) is then cloned into the genetic space created by these modifications to form a recombinant virus that retains its infectivity and its ability to transfer genes to cells, but is non-replicating, much safer and less virulent than the original virus. Gene transfer using viral vectors is called transduction.
A number of different viruses have been modified in this way. Prominent viral vectors that have been used in human clinical trials are listed in Table 1. As noted in this Table, different vectors vary in their carrying capacity, immunogenicity, ease of manufacture, whether or not the target cells need to be dividing and their ability to integrate viral DNA into the host cell genome. Integration into the host cell genome is one way to prolong expression of the transgene, and is therefore useful for treating chronic genetic diseases such as X-linked severe combined immunodeficiency disease (SCID) 27. Because successful bone regeneration will not require such long-term transgene expression, most research has used vectors which do not integrate their DNA and which are spontaneously cleared by the body. Among these, recombinant adenovirus vectors have been the most widely used. They are quite easy to construct and produce at high titers, they are highly infectious in many types of cells and usually express at high levels in vivo for a period of several weeks, after which they are cleared by cell turnover or the immune system. In many instances, this profile of gene expression is likely to match nicely the needs of bone regeneration. Concerns when using adenovirus vectors include its antigenicity and the fact that most of the population has circulating antibodies against adenovirus serotype 5, the serotype most often used by adenoviral vectors. Immune recognition of the virus can inhibit gene transfer.
Table 1.
Salient properties of the main viral vectors used for human gene therapy
| Parent virus | Key properties of wild-type virus | Advantages | Disadvantages | Comment |
|---|---|---|---|---|
| Adenovirus | Double stranded DNA genome, ~ 35Kb | Straightforward production of recombinant vectors at high titers | Inflammatory and antigenic | Various generations with increasingly deleted genomes “Gutted” vectors have no viral coding sequences and large carrying capacity but are difficult to produce. |
| Non-enveloped | Transduces non-dividing cells | Tropism can be modified by altering coat proteins | ||
| Over 50 serotypes | Wide choice of serotypes | |||
| ~ 100nm in size | ||||
| Genome remains episomal in infected cells | ||||
| Herpes simplex virus | Double stranded DNA genome, ~ 150 Kb | Transduces non-dividing cells | Complex genome -Difficult to produce recombinant virus | HSV 1 and 2 most widely used as vectors. Herpes family includes Epstein Barr virus, CMV, etc |
| Enveloped | Very efficient transduction of dividing and non-dividing cells | Cytotoxicity | ||
| ~ 200nm in size | Has a natural latency in neurons | |||
| Genome remains episomal in infected cells | Very large carrying capacity | |||
| Adeno-associated virus | Single-stranded DNA genome, 4.8 Kb | Perceived to be safe (w.t. virus causes no known disease) | Difficult to produce | W.t. virus cannot replicate without helper virus |
| Non-enveloped | Transduces non-dividing cells | Carrying capacity is insufficient for certain applications | W.t. virus integrates in a site-specific manner; recombinant virus remains as a stable, concatameric plasmid | |
| Growing number of serotypes identified | Thought to have low immunogenicity, but this is being re-evaluated | Transduction efficiency sometimes low | Limitations of single stranded genome now overcome by development of double copy (self complementary) DNA viruses | |
| ~20 nm in size | ||||
| Oncoretrovirus | RNA genome ~ 8–10 Kb | Straightforward production of recombinant vectors at moderate titers | Require host cell division | Usually used ex vivo |
| Enveloped | Pseudotyped vectors have wide host range | Risk of insertional mutagenesis | 2 genomes per virion, reverse transcribed into DNA | |
| ~100nm in size | ||||
| Lentivirus | RNA genome ~ 8–10 Kb | Straightforward production of recombinant vectors at moderate titers | Risk of insertional mutagenesis, but non-integrating vectors being developed | 2 genomes per virion, reverse transcribed into DNA |
| Enveloped | Pseudotyped vectors have wide host range and are often very efficient | |||
| ~100nm in size | Transduces non-dividing cells |
Reproduced from reference [21] with permission
Because of residual safety concerns when using viral vectors, as well as their cost and complexity, there is sustained interest in using non-viral vectors to deliver therapeutic DNA 51. Gene transfer using non-viral vectors is called transfection. This may be accomplished with naked DNA, but it is more usual to facilitate cellular uptake of the DNA by associating it with a carrier, such as a liposome or other polymer, or by using a physical stimulus, such as sonication or an electric pulse (electroporation). In general terms, non-viral vectors tend to be less effective than viral vectors, both in terms of the level and duration of transgene expression.
Strategies
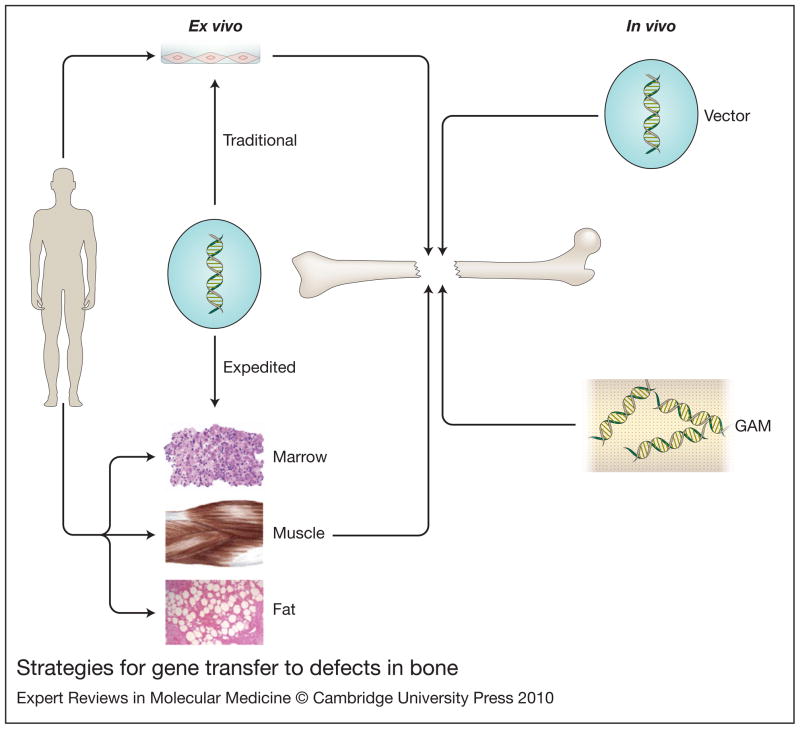
Cells can be genetically modified in situ or by their removal, genetic modification and reimplantation. The former process, also known as in vivo gene therapy, obviates the need to recover and manipulate cells, which greatly simplifies the process. However, not all vectors are suitable for in vivo gene delivery and the introduction of extraneous genetic material directly into the body raises considerable safety issues. Genetic modification of cells outside the body, known as ex vivo gene therapy, obviates many of these safety concerns but is cumbersome and expensive, especially when using autologous cells that need to be cultured in vitro. Based upon these principles, four strategies have emerged for promoting bone regeneration by gene transfer (figure 1). Two of them are ex vivo (traditional and expedited) and two are in vivo (direct injection and gene-activated matrix (GAM))20.
Figure 1. Strategies for gene transfer to defects in bone.
There are two general strategies: in vivo (right hand side) and ex vivo (left hand side). For in vivo gene delivery, the vector is introduced directly into the site of the osseous lesion, either as a free suspension (top, right hand side) or incorporated into a gene activated matrix (GAM) (bottom, right hand side). For ex vivo delivery, vectors are not introduced directly into the defect. Instead they are used to genetically modify cells, which are subsequently implanted. Traditional ex vivo methods (top, left hand side) usually involve the establishment of cell cultures, which are genetically modified in vitro. The modified cells are then introduced into the lesion, often after seeding onto an appropriate scaffold. Expedited ex vivo methods (bottom, left hand side) avoid the need for cell culture by genetically modifying tissues such as marrow, muscle and fat, intraoperatively and inserting them into the defect during a single operative session.
Reproduced from reference [20] with permission.
Choice of transgenes
Table 2 lists the transgenes that have been used in pre-clinical studies of gene therapy for bone healing. Not surprisingly, cDNAs encoding osteogenic bone morphogenetic proteins (BMPs) have been the most widely used. BMP-2 and BMP-7 have the additional attraction that their recombinant proteins are already in clinical use. This greatly aids the translatability of a gene therapy based on these molecules. As well as growth factors that enhance osteoblast differentiation and matrix deposition, there is interest in using factors, such as vascular endothelial growth factor (VEGF), that promote angiogenesis 14. This reflects the increasing recognition of the vital need for a blood supply when forming bone. In this context it is interesting that, although most research has focused on forming new bone by stimulating direct osteogenesis of precursor cells, there is a growing appreciation of the advantages of the endochondral route to bone formation 58. One attraction of this pathway is the spontaneous secretion of angiogenic factors by the cartilage as it hypertrophies and prepares to ossify. This means that the endogenous biology of the system relieves the clinician of having to manipulate the system exogenously to ensure adequate blood supply to the healing bone. This is important, because large osseous lesions are likely to be highly hypoxic, a circumstance that strongly favors the chondrogenic differentiation of progenitor cells 14.
Table 2.
Transgenes used in experimental models of bone healing by gene transfer
| Gene | Representative reference |
|---|---|
| BMP-2 | 42 |
| BMP-4 | 56 |
| BMP-6 | 31 |
| BMP-7 | 8 |
| BMP-9 | 29 |
| BMP-2 plus BMP-7 | 36 |
| TGF-β plus BMP-4 | 41 |
| IGF-1 | 59 |
| VEGF | 53 |
| VEGF plus BMP-4 | 53 |
| VEGF plus RANKL | 33 |
| PDGF | 11 |
| FGF-2 | 26 |
| LMP-1 | 69 |
| LMP-3 | 39 |
| ca Alk-2 | 35 |
| PTH 1–34 | 24 |
| PTH 1–34 plus BMP-4 | 24 |
| COX-2 | 57 |
| Nell-1 | 44 |
| Osterix | 67 |
| Runx2 | 74 |
BMP: Bone Morphogenetic Protein, TGF: Transforming Growth Factor, IGF-1: Insulin-like Growth Factor-1, VEGF: Vascular Endothelial Growth Factor, PDGF: Platelet-derived growth factor, FGF: Fibroblast Growth Factor, RANKL: Receptor Activator of NF-κB Ligand, LMP: LIM Mineralization Protein, Ca Alk-2: Constitutively active Alk-2 receptor, PTH: Parathyroid Hormone, COX-2: Cyclooxygenase 2, Nell-1: Nel-like molecule 1
As well as morphogens and growth factors, there is literature on the use of cDNAs encoding transcription factors, such as Runx2 and Osterix, that are associated with osteogenesis (Table 2). Because these are intracellular proteins, as are the osteogenic LIM mineralization (LMP) proteins, they are difficult to deliver by other means. The same is true for the constitutively active form of the BMP receptor Alk-2, which requires endogenous synthesis to insert itself correctly into the cell membrane.
Rather than promoting osteogenesis directly, an additional strategy is to reduce the synthesis of BMP inhibitors using a technology known as RNA interference. Encouraging results have been published when chordin 38 or noggin 71 are reduced in this manner. In future, it may be worthwhile to investigate the effects of transgenes that reduce inflammation or inhibit osteoclast activity.
It seems likely that a combination of different transgene products would be more osteogenic than any single factor delivered alone. While there is experimental evidence that this is indeed the case 36, 41, 53, 75, the delivery of multiple transgenes would greatly complicate the regulatory issues surrounding clinical translation.
Experimental findings
Traditional ex vivo
Lieberman and colleagues were the first to use this approach 42, 43. Using a rat model, they removed bone marrow, expanded the stromal cell population by monolayer culture, and introduced a cDNA encoding bone morphogenetic protein-2 (BMP-2) using an adenovirus vector (Ad.BMP-2). The transduced cells, secreting high levels of BMP-2, were seeded onto a collagenous scaffold and placed into critical sized defects in the femora of rats. This procedure healed the defects within 12 weeks, with evidence of superior new bone quality as compared to the use of recombinant (r) BMP-2. This group of investigators has subsequently demonstrated the efficacy of this method for achieving spinal fusion in a rat model 54. In later work, they used a lentivirus for delivery of BMP-2 cDNA for both long bone healing 70 and spinal fusion 46, 47. This vector enabled the in vivo expression of BMP-2 for much longer than the adenovirus vector and produced bone with greater mechanical strength. Gazit et al 25, in contrast, were able to heal osseous defects in mice effectively using transfected cells transiently expressing low levels of BMP-2.
Subsequent investigators have confirmed the utility of this approach with Ad.BMP-2 and osteoprogenitor cells derived from fat 55, periosteum 8, muscle 40 and other such sites. However, it is unclear whether osteoprogenitor cells are necessary for this method to work efficiently, and success has been achieved using fibroblasts obtained from skin 32 and gingiva 60. Moreover, data from experiments in regenerative medicine using so-called mesenchymal stem cells (MSCs) suggest that the MSCs do not survive long in the defect and do not form part of the regenerated tissue. Instead, they may serve to secrete morphogens that stimulate endogenous regeneration by host cells 30. This matter remains the subject of considerable research.
MSCs are of additional interest because they may have the ability to home to sites of osseous injury 59 which would greatly simplify delivery, especially when there are multiple sites needing repair. There is also the possibility that MSCs can be successfully allografted 13. If so, the establishment of genetically modified, universal donor MSCs would greatly reduce the cost and complexity of ex vivo gene delivery to bone. However, Tsuchida et al 66 were unable to heal osseous defects in rats with allograft marrow cells expressing BMP-2 in the absence of immunosuppression. Nevertheless, Yi et al 73 reported success in healing fractures in osteoporotic rats using xenogeneic, irradiated, transduced chondrocytes expressing BMP-2.
Because the bones of small animals used by laboratory researchers tend to heal well, it is important and necessary to perform experiments in large animals. The traditional ex vivo approach using Ad.BMP-2 has given highly encouraging results in horses 32, goats15, 65, 72 and pigs 12.
Expedited ex vivo
Because traditional ex vivo approaches are cumbersome and very expensive, there is interest in developing expedited methods that can be achieved in the operating room 23. The aim is to biopsy tissue, genetically modify the cells and return them to an osseous lesion within a single operative period. There are two examples of this approach.
Using a rabbit spinal fusion model model, Viggeswarapu et al69 withdrew blood, isolated “buffy coat” cells, transduced them with a recombinant adenovirus carrying cDNA encoding LMP-1, and implanted them within a single operation. All animals went on to successful spinal fusion. In a second approach known as “facilitated endogenous repair”23, critical sized defects in the femora of rats were healed using genetically modified muscle and fat 22. These tissues were selected because they contain osteoprogenitor cells, can be readily biopsied and have intrinsic scaffolding properties. They were transduced with Ad.BMP-2 and inserted into the osseous lesions. Healing of the critical sized defects was uniform and rapid 22.
Further study of bone regeneration using gene-activated muscle suggested that the implant rapidly undergoes chondrogenesis, leading to efficient healing by endochondral ossification 22. At least some of the newly formed lining osteoblasts were of donor origin. The speed and efficiency of this method probably reflects the fact that the implantation of fat or muscle expressing BMP-2 provides simultaneously progenitor cells, a morphogenetic signal and an osteoconductive scaffold. Unlike the case when Ad.BMP-2 was injected directly into the rat, abbreviated ex vivo delivery using fat and muscle did not increase the levels of circulating anti-adenovirus antibody 22. Clotted bone marrow provides an additional vehicle for this approach to tissue regeneration 50.
One advantage of ex vivo approaches, whether expedited or not, is the provision of cells to the lesion. This will be of major importance when the soft tissue surrounding a defect has been compromised through irradiation, injury or disease.
In vivo delivery
The most direct way to deliver genes to osseous lesions is to inject the vector directly; in many cases, this could be achieved percutaneously 3,5. In most instances adenovirus has been used for this purpose, but success has been reported using retrovirus vectors 56, 57, 63 suggesting that there is sufficient cell division in the area of experimental defects to support retroviral transduction. Most investigators have used BMP-2 as the transgene, but success has been reported with BMPs -2, -4, -6, -7 and -9, cyclooxygenase-2 and LMP-1. In the rat segmental defect model, healing in response to the intralesional injection of Ad.BMP-2 was improved by delaying the injection until 10 days after creation of the defect 4. This may reflect the time needed for osteoprogenitor to cells to enter the lesion, as well as the time of optimum expression of the coxsackievirus adenovirus receptor (CAR) 34. Bone formation in response to Ad.BMP-2 was dose-dependent 6, but as high doses of adenovirus are inflammatory this needs to be carefully titrated. The inflammatory response to adenovirus in the rat may explain why the mechanical strength of the healed bone was only about 25% of normal after eight weeks 5. Studies in rabbits suggested very limited transduction of cells occurred beyond the osseous lesion into which the recombinant adenovirus was injected 2, indicating that adverse systemic sequelae are unlikely.
Results for large animla models have been mixed. Both Ad.BMP-2 and Ad.BMP-6 have been used successfully to heal experimentally created osseous defects in horses 31, 32. However, responses in sheep models were muted, possibly because of immune responses by the sheep to adenovirus and human BMP-217, 18.
Gene activated matrices (GAMs)
In their original formulation GAMs consist of a collagenous scaffold impregnated with plasmid DNA encoding osteogenic products 24. When the GAM is inserted into an osseous lesion, host cells enter the scaffold, become transfected by the DNA and secrete the osteogenic gene product, leading to repair. Because DNA is chemically stable, GAMs have the potential to become “off-the-shelf” products – a major practical advantage.
Although promising initial results were published using rat 24 and dog 7 models, the original plasmid-based technology did not prove efficient enough for further development. However, scaffolds containing viral vectors have proved much more promising and a GAM containing adenovirus carrying platelet-derived growth factor (PDGF) cDNA is in clinical trials for healing diabetic skin ulcers (www.t-r-co.com). The same GAM has shown promise in rat model of periodontal bone loss 11. There is evidence that a collagenous scaffold can reduce the immune response to adenovirus 62.
An intriguing extension of the GAM concept uses allograft bone as the scaffold and recombinant adeno-associated virus (AAV) as the vector. This technology was primarily developed to heal large segmental defects that would normally receive a structural allograft. Because these allografts do not remodel, they integrate poorly with the host bone and have a high failure rate. Schwarz’s group has developed a “revitalized” allograft coated with AAV. In their first experiments they used two AAV vectors, one of which encoded receptor activator of NF-κB ligand (RANKL) to promote osteoclastogenesis and the other encoded VEGF to promote angiogenesis 33. Allograft coated with these two vectors was inserted into segmental defects in mouse femora. Cells were locally transduced by the AAV vectors, leading to invasion of the allograft by osteoclasts and its replacement with host bone. Similar data 35 were subsequently obtained using as the transgene a cDNA encoding a constitutively active form of the Alk-2 type I receptor for BMP which bears the same mutation as found in patients with the disease fibrodysplasia ossificans progressiva61. Because AAV is quite stable, especially when freeze-dried onto a solid support, this construct could also serve as an “off-the-shelf” product.
Progress towards clinical trials
Getting gene therapy protocols into human clinical trials is a lengthy, expensive and frustrating process, especially for conditions that are not lethal 21. Clinical trials require Investigational New Drug (IND) permission from the FDA. Before gaining approval, the efficacy of any potential gene therapy for bone regeneration would need to be confirmed in large animals, such as sheep, goats, pigs or horses. Safety is of especial concern for any gene therapy and safety testing requires extensive and exacting pharmacology and toxicology studies, including biodistribution analysis of the transferred genomes, conducted under Good Laboratory Practice (GLP) conditions. Production of the relatively large amounts of clinical grade vector needed for trials is another complicated and expensive requirement. Outcome measures are presently inadequate for clinical trial purposes and novel, non-invasive techniques are being developed to determine bone quality and mechanical strength.
Gene therapy for bone regeneration has applications in veterinary, as well as human, medicine and the regulatory route is less burdensome. Success in veterinary applications would facilitate their adoption into human clinical use.
All things considered, it is likely that gene-based modalities for regenerating bone will enter clinical trials within the next 5–10 years. As noted, a GAM containing adenovirus encoding PDGF is already in the clinic for wound healing and has shown promise in stimulating periodontal bone formation. Moreover a number of additional genetic strategies have already proved effective in large animal models. Moving such technologies into the clinic requires considerable sums of money that are unlikely to be found in academia. Other sources of funding are thus required, and future development may depend to a large degree on the level of enthusiasm generated within industry for gene-based products. Recent clinical successes of gene therapy for the treatment of several diseases, including X-linked SCID27, Leber’s congenital amaurosis 1, 45, and lipoprotein lipase deficiency 9 should be helpful in this regard.
Acknowledgments
The author’s work in this area has been supported by The Orthopaedic Trauma Association, NIH grant R01 AR 050243 from NIAMS and the AO Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 2.Baltzer AW, Lattermann C, Whalen JD, et al. A gene therapy approach to accelerating bone healing. Evaluation of gene expression in a New Zealand white rabbit model. Knee Surg Sports Traumatol Arthrosc. 1999;7:197–202. doi: 10.1007/s001670050147. [DOI] [PubMed] [Google Scholar]
- 3.Baltzer AW, Lattermann C, Whalen JD, et al. Genetic enhancement of fracture repair: healing of an experimental segmental defect by adenoviral transfer of the BMP-2 gene. Gene Ther. 2000;7:734–9. doi: 10.1038/sj.gt.3301166. [DOI] [PubMed] [Google Scholar]
- 4.Betz OB, Betz VM, Nazarian A, et al. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther. 2007;14:1039–44. doi: 10.1038/sj.gt.3302956. [DOI] [PubMed] [Google Scholar]
- 5.Betz OB, Betz VM, Nazarian A, et al. Direct percutaneous gene delivery to enhance healing of segmental bone defects. J Bone Joint Surg Am. 2006;88:355–65. doi: 10.2106/JBJS.E.00464. [DOI] [PubMed] [Google Scholar]
- 6.Betz VM, Betz OB, Glatt V, et al. Healing of segmental bone defects by direct percutaneous gene delivery: effect of vector dose. Hum Gene Ther. 2007;18:907–15. doi: 10.1089/hum.2007.077. [DOI] [PubMed] [Google Scholar]
- 7.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–9. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 8.Breitbart AS, Grande DA, Mason JM, et al. Gene-enhanced tissue engineering: applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg. 1999;42:488–95. [PubMed] [Google Scholar]
- 9.Burnett JR, Hooper AJ. Alipogene tiparvovec, an adeno-associated virus encoding the Ser(447)X variant of the human lipoprotein lipase gene for the treatment of patients with lipoprotein lipase deficiency. Curr Opin Mol Ther. 2009;11:681–91. [PubMed] [Google Scholar]
- 10.Carofino BC, Lieberman JR. Gene therapy applications for fracture-healing. J Bone Joint Surg Am. 2008;90(Suppl 1):99–110. doi: 10.2106/JBJS.G.01546. [DOI] [PubMed] [Google Scholar]
- 11.Chang PC, Cirelli JA, Jin Q, et al. Adenovirus encoding human platelet-derived growth factor-B delivered to alveolar bone defects exhibits safety and biodistribution profiles favorable for clinical use. Hum Gene Ther. 2009;20:486–96. doi: 10.1089/hum.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang SC, Lin TM, Chung HY, et al. Large-scale bicortical skull bone regeneration using ex vivo replication-defective adenoviral-mediated bone morphogenetic protein-2 gene-transferred bone marrow stromal cells and composite biomaterials. Neurosurgery. 2009;65:75–81. doi: 10.1227/01.NEU.0000345947.33730.91. discussion -3. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Tredget EE, Liu C, Wu Y. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PLoS One. 2009;4:e7119. doi: 10.1371/journal.pone.0007119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai J, Rabie AB. VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res. 2007;86:937–50. doi: 10.1177/154405910708601006. [DOI] [PubMed] [Google Scholar]
- 15.Dai KR, Xu XL, Tang TT, et al. Repairing of goat tibial bone defects with BMP-2 gene-modified tissue-engineered bone. Calcif Tissue Int. 2005;77:55–61. doi: 10.1007/s00223-004-0095-z. [DOI] [PubMed] [Google Scholar]
- 16.Deschaseaux F, Sensebe L, Heymann D. Mechanisms of bone repair and regeneration. Trends Mol Med. 2009;15:417–29. doi: 10.1016/j.molmed.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Egermann M, Baltzer AW, Adamaszek S, et al. Direct adenoviral transfer of bone morphogenetic protein-2 cDNA enhances fracture healing in osteoporotic sheep. Hum Gene Ther. 2006;17:507–17. doi: 10.1089/hum.2006.17.507. [DOI] [PubMed] [Google Scholar]
- 18.Egermann M, Lill CA, Griesbeck K, et al. Effect of BMP-2 gene transfer on bone healing in sheep. Gene Ther. 2006;13:1290–9. doi: 10.1038/sj.gt.3302785. [DOI] [PubMed] [Google Scholar]
- 19.El Tamer MK, Reis RL. Progenitor and stem cells for bone and cartilage regeneration. J Tissue Eng Regen Med. 2009;3:327–37. doi: 10.1002/term.173. [DOI] [PubMed] [Google Scholar]
- 20.Evans CH. Gene therapy for bone healing. Expert Rev Mol Med. 2010 June;12:e18. doi: 10.1017/S1462399410001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans CH, Ghivizzani SC, Robbins PD. Getting arthritis gene therapy into the clinic. Nature Rev Rheumatol. 2010 doi: 10.1038/nrrheum.2010.193. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans CH, Liu FJ, Glatt V, et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater. 2009;18:96–111. doi: 10.22203/ecm.v018a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans CH, Palmer GD, Pascher A, et al. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Eng. 2007;13:1987–93. doi: 10.1089/ten.2006.0302. [DOI] [PubMed] [Google Scholar]
- 24.Fang J, Zhu YY, Smiley E, et al. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci U S A. 1996;93:5753–8. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gazit D, Turgeman G, Kelley P, et al. Engineered pluripotent mesenchymal cells integrate and differentiate in regenerating bone: a novel cell-mediated gene therapy. J Gene Med. 1999;1:121–33. doi: 10.1002/(SICI)1521-2254(199903/04)1:2<121::AID-JGM26>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 26.Guo X, Zheng Q, Kulbatski I, et al. Bone regeneration with active angiogenesis by basic fibroblast growth factor gene transfected mesenchymal stem cells seeded on porous beta-TCP ceramic scaffolds. Biomed Mater. 2006;1:93–9. doi: 10.1088/1748-6041/1/3/001. [DOI] [PubMed] [Google Scholar]
- 27.Hacein-Bey-Abina S, Hauer J, Lim A, et al. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2010;363:355–64. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heilbronn R, Weger S. Viral vectors for gene transfer: current status of gene therapeutics. Handb Exp Pharmacol. 2010:143–70. doi: 10.1007/978-3-642-00477-3_5. [DOI] [PubMed] [Google Scholar]
- 29.Helm GA, Alden TD, Beres EJ, et al. Use of bone morphogenetic protein-9 gene therapy to induce spinal arthrodesis in the rodent. J Neurosurg. 2000;92:191–6. doi: 10.3171/spi.2000.92.2.0191. [DOI] [PubMed] [Google Scholar]
- 30.Horwitz EM, Dominici M. How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy. 2008;10:771–4. doi: 10.1080/14653240802618085. [DOI] [PubMed] [Google Scholar]
- 31.Ishihara A, Shields KM, Litsky AS, et al. Osteogenic gene regulation and relative acceleration of healing by adenoviral-mediated transfer of human BMP-2 or -6 in equine osteotomy and ostectomy models. J Orthop Res. 2008;26:764–71. doi: 10.1002/jor.20585. [DOI] [PubMed] [Google Scholar]
- 32.Ishihara A, Zekas LJ, Litsky AS, et al. Dermal fibroblast-mediated BMP2 therapy to accelerate bone healing in an equine osteotomy model. J Orthop Res. 2010;28:403–11. doi: 10.1002/jor.20978. [DOI] [PubMed] [Google Scholar]
- 33.Ito H, Koefoed M, Tiyapatanaputi P, et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med. 2005;11:291–7. doi: 10.1038/nm1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito T, Tokunaga K, Maruyama H, et al. Coxsackievirus and adenovirus receptor (CAR)-positive immature osteoblasts as targets of adenovirus-mediated gene transfer for fracture healing. Gene Ther. 2003;10:1623–8. doi: 10.1038/sj.gt.3302060. [DOI] [PubMed] [Google Scholar]
- 35.Koefoed M, Ito H, Gromov K, et al. Biological effects of rAAV-caAlk2 coating on structural allograft healing. Mol Ther. 2005;12:212–8. doi: 10.1016/j.ymthe.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Koh JT, Zhao Z, Wang Z, et al. Combinatorial gene therapy with BMP2/7 enhances cranial bone regeneration. J Dent Res. 2008;87:845–9. doi: 10.1177/154405910808700906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwong FN, Harris MB. Recent developments in the biology of fracture repair. J Am Acad Orthop Surg. 2008;16:619–25. doi: 10.5435/00124635-200811000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Kwong FN, Richardson SM, Evans CH. Chordin knockdown enhances the osteogenic differentiation of human mesenchymal stem cells. Arthritis Res Ther. 2008;10:R65. doi: 10.1186/ar2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lattanzi W, Parrilla C, Fetoni A, et al. Ex vivo-transduced autologous skin fibroblasts expressing human Lim mineralization protein-3 efficiently form new bone in animal models. Gene Ther. 2008;15:1330–43. doi: 10.1038/gt.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JY, Musgrave D, Pelinkovic D, et al. Effect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. J Bone Joint Surg Am. 2001;83-A:1032–9. doi: 10.2106/00004623-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Li BC, Zhang JJ, Xu C, et al. Treatment of rabbit femoral defect by firearm with BMP-4 gene combined with TGF-beta1. J Trauma. 2009;66:450–6. doi: 10.1097/TA.0b013e3181848cd6. [DOI] [PubMed] [Google Scholar]
- 42.Lieberman JR, Daluiski A, Stevenson S, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999;81:905–17. doi: 10.2106/00004623-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Lieberman JR, Le LQ, Wu L, et al. Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res. 1998;16:330–9. doi: 10.1002/jor.1100160309. [DOI] [PubMed] [Google Scholar]
- 44.Lu SS, Zhang X, Soo C, et al. The osteoinductive properties of Nell-1 in a rat spinal fusion model. Spine J. 2007;7:50–60. doi: 10.1016/j.spinee.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 45.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyazaki M, Sugiyama O, Tow B, et al. The effects of lentiviral gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J Spinal Disord Tech. 2008;21:372–9. doi: 10.1097/BSD.0b013e31814cf51d. [DOI] [PubMed] [Google Scholar]
- 47.Miyazaki M, Sugiyama O, Zou J, et al. Comparison of lentiviral and adenoviral gene therapy for spinal fusion in rats. Spine (Phila Pa 1976) 2008;33:1410–7. doi: 10.1097/BRS.0b013e3181761003. [DOI] [PubMed] [Google Scholar]
- 48.Nauth A, Miclau T, 3rd, Li R, Schemitsch EH. Gene therapy for fracture healing. J Orthop Trauma. 24(Suppl 1):S17–24. doi: 10.1097/BOT.0b013e3181cec6fb. [DOI] [PubMed] [Google Scholar]
- 49.Pape HC, Evans A, Kobbe P. Autologous bone graft: properties and techniques. J Orthop Trauma. 24(Suppl 1):S36–40. doi: 10.1097/BOT.0b013e3181cec4a1. [DOI] [PubMed] [Google Scholar]
- 50.Pascher A, Palmer GD, Steinert A, et al. Gene delivery to cartilage defects using coagulated bone marrow aspirate. Gene Ther. 2004;11:133–41. doi: 10.1038/sj.gt.3302155. [DOI] [PubMed] [Google Scholar]
- 51.Pathak A, Patnaik S, Gupta KC. Recent trends in non-viral vector-mediated gene delivery. Biotechnol J. 2009;4:1559–72. doi: 10.1002/biot.200900161. [DOI] [PubMed] [Google Scholar]
- 52.Pelled G, Ben-Arav A, Hock C, et al. Direct gene therapy for bone regeneration: gene delivery, animal models, and outcome measures. Tissue Eng Part B Rev. 16:13–20. doi: 10.1089/ten.teb.2009.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng H, Wright V, Usas A, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751–9. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peterson B, Iglesias R, Zhang J, et al. Genetically modified human derived bone marrow cells for posterolateral lumbar spine fusion in athymic rats: beyond conventional autologous bone grafting. Spine (Phila Pa 1976) 2005;30:283–9. doi: 10.1097/01.brs.0000152380.71248.fe. discussion 9–90. [DOI] [PubMed] [Google Scholar]
- 55.Peterson B, Zhang J, Iglesias R, et al. Healing of critically sized femoral defects, using genetically modified mesenchymal stem cells from human adipose tissue. Tissue Eng. 2005;11:120–9. doi: 10.1089/ten.2005.11.120. [DOI] [PubMed] [Google Scholar]
- 56.Rundle CH, Miyakoshi N, Kasukawa Y, et al. In vivo bone formation in fracture repair induced by direct retroviral-based gene therapy with bone morphogenetic protein-4. Bone. 2003;32:591–601. doi: 10.1016/s8756-3282(03)00096-6. [DOI] [PubMed] [Google Scholar]
- 57.Rundle CH, Strong DD, Chen ST, et al. Retroviral-based gene therapy with cyclooxygenase-2 promotes the union of bony callus tissues and accelerates fracture healing in the rat. J Gene Med. 2008;10:229–41. doi: 10.1002/jgm.1148. [DOI] [PubMed] [Google Scholar]
- 58.Scotti C, Tonnarelli B, Papadimitropoulos A, et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A. 2010;107:7251–6. doi: 10.1073/pnas.1000302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen FH, Visger JM, Balian G, et al. Systemically administered mesenchymal stromal cells transduced with insulin-like growth factor-I localize to a fracture site and potentiate healing. J Orthop Trauma. 2002;16:651–9. doi: 10.1097/00005131-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Shin JH, Kim KH, Kim SH, et al. Ex vivo bone morphogenetic protein-2 gene delivery using gingival fibroblasts promotes bone regeneration in rats. J Clin Periodontol. 2010;37:305–11. doi: 10.1111/j.1600-051X.2009.01522.x. [DOI] [PubMed] [Google Scholar]
- 61.Shore EM, Xu M, Feldman GJ, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–7. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 62.Sonobe J, Okubo Y, Kaihara S, et al. Osteoinduction by bone morphogenetic protein 2-expressing adenoviral vector: application of biomaterial to mask the host immune response. Hum Gene Ther. 2004;15:659–68. doi: 10.1089/1043034041361208. [DOI] [PubMed] [Google Scholar]
- 63.Strohbach CA, Rundle CH, Wergedal JE, et al. LMP-1 retroviral gene therapy influences osteoblast differentiation and fracture repair: a preliminary study. Calcif Tissue Int. 2008;83:202–11. doi: 10.1007/s00223-008-9163-0. [DOI] [PubMed] [Google Scholar]
- 64.Sundelacruz S, Kaplan DL. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin Cell Dev Biol. 2009;20:646–55. doi: 10.1016/j.semcdb.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang TT, Lu B, Yue B, et al. Treatment of osteonecrosis of the femoral head with hBMP-2-gene-modified tissue-engineered bone in goats. J Bone Joint Surg Br. 2007;89:127–9. doi: 10.1302/0301-620X.89B1.18350. [DOI] [PubMed] [Google Scholar]
- 66.Tsuchida H, Hashimoto J, Crawford E, et al. Engineered allogeneic mesenchymal stem cells repair femoral segmental defect in rats. J Orthop Res. 2003;21:44–53. doi: 10.1016/S0736-0266(02)00108-0. [DOI] [PubMed] [Google Scholar]
- 67.Tu Q, Valverde P, Li S, et al. Osterix overexpression in mesenchymal stem cells stimulates healing of critical-sized defects in murine calvarial bone. Tissue Eng. 2007;13:2431–40. doi: 10.1089/ten.2006.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ulstrup AK. Biomechanical concepts of fracture healing in weight-bearing long bones. Acta Orthop Belg. 2008;74:291–302. [PubMed] [Google Scholar]
- 69.Viggeswarapu M, Boden SD, Liu Y, et al. Adenoviral delivery of LIM mineralization protein-1 induces new-bone formation in vitro and in vivo. J Bone Joint Surg Am. 2001;83-A:364–76. doi: 10.2106/00004623-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Virk MS, Conduah A, Park SH, et al. Influence of short-term adenoviral vector and prolonged lentiviral vector mediated bone morphogenetic protein-2 expression on the quality of bone repair in a rat femoral defect model. Bone. 2008;42:921–31. doi: 10.1016/j.bone.2007.12.216. [DOI] [PubMed] [Google Scholar]
- 71.Wan DC, Pomerantz JH, Brunet LJ, et al. Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem. 2007;282:26450–9. doi: 10.1074/jbc.M703282200. [DOI] [PubMed] [Google Scholar]
- 72.Xu XL, Tang T, Dai K, et al. Immune response and effect of adenovirus-mediated human BMP-2 gene transfer on the repair of segmental tibial bone defects in goats. Acta Orthop. 2005;76:637–46. doi: 10.1080/17453670510041709. [DOI] [PubMed] [Google Scholar]
- 73.Yi Y, Choi KB, Lim CL, et al. Irradiated human chondrocytes expressing bone morphogenetic protein 2 promote healing of osteoporotic bone fracture in rats. Tissue Eng Part A. 2009;15:2853–63. doi: 10.1089/ten.TEA.2008.0578. [DOI] [PubMed] [Google Scholar]
- 74.Zhao Z, Wang Z, Ge C, et al. Healing cranial defects with AdRunx2-transduced marrow stromal cells. J Dent Res. 2007;86:1207–11. doi: 10.1177/154405910708601213. [DOI] [PubMed] [Google Scholar]
- 75.Zhu W, Rawlins BA, Boachie-Adjei O, et al. Combined bone morphogenetic protein-2 and -7 gene transfer enhances osteoblastic differentiation and spine fusion in a rodent model. J Bone Miner Res. 2004;19:2021–32. doi: 10.1359/JBMR.040821. [DOI] [PubMed] [Google Scholar]