Abstract
We report the results of an fMRI investigation of the neural bases of written language comprehension (reading) and production (spelling). Both tasks were examined in the same individuals, allowing greater precision in establishing the relationship between the neural underpinnings of these two cognitive functions. Also examined was the relationship between written language substrates and those involved in face and object (house) processing. The results reveal that reading and spelling share specific left hemisphere substrates in the mid-fusiform gyrus and in the inferior frontal gyrus/junction. Furthermore, the results indicate that the left mid-fusiform substrates are specifically involved in lexical orthographic processing. We also find that written language and face processing exhibit largely complementary activation patterns in both the fusiform and the inferior frontal/junction areas, with left and right lateralization, respectively. In sum, these results provide perhaps the strongest evidence to date of components that are shared by written language comprehension (reading) and production (spelling), and they further our understanding of the role of literacy within the larger repertoire of cognitive operations and their neural substrates.
INTRODUCTION
Among the central questions in written language research is whether reading and spelling share representations and processes. This issue is of interest for a number of reasons. The question is, of course, central to our understanding of the cognitive and neural machinery that supports literacy. Furthermore, because written language involves both comprehension (reading) and production (spelling), it constitutes a domain in which to investigate the general question of the relationship between recognition and production, perception, and action. Finally, because written language is a relatively recent human invention, the identification of its neural bases and an understanding of how these relate to those of evolutionarily older domains such as visual object recognition and face processing further our knowledge of how human cortex may develop expertise in novel cognitive domains.
In this article, we report the results of an fMRI investigation of the neural bases of spelling and reading. Unlike any previous neuroimaging studies of written language processing, in this investigation both reading and spelling were examined in the same individuals, allowing greater precision in establishing the relationship between these functions. Also examined was the relationship of written language substrates to those involved in face and object (house) processing.
Reading and Spelling: Independent or Shared Processes?
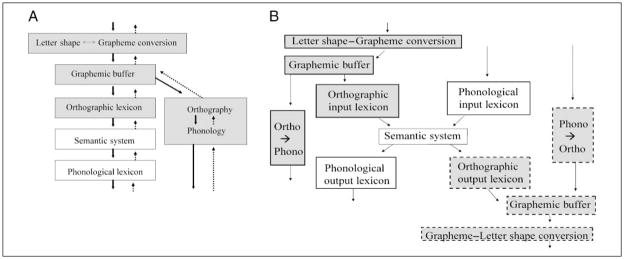
Both spelling and reading require various long-term and working memory mechanisms that operate over letters and word spellings. These are responsible for the translation between letters and words and their corresponding sounds, and for words, there is the additional mapping between word spellings and meanings. It is generally assumed that the knowledge of word meanings (lexical semantics) and word sounds (the phonological lexicon) is not specific to reading or spelling as they form an essential part of the spoken language system. The debate regarding the relationship between reading and spelling, therefore, concerns the status of the orthographic components. One possibility is that all orthographic mechanisms are shared by spelling and reading (the shared components architecture; see Figure 1A). Another is that none is shared and that spelling and reading are independent production and comprehension systems (the independent components architecture; see Figure 1B). A third position is that some mechanisms (e.g., orthographic working memory) are shared whereas others are not.
Figure 1.
Hypotheses concerning the relationship between reading and spelling. (A) Shared components architecture in which reading and spelling share all orthographic processes and representations. Shaded boxes represent orthographic components, and white boxes represent components shared by written and spoken language systems. Solid arrows indicate access for reading, and dashed arrows show access for spelling. (B) Independent components architecture in which reading and spelling share only nonorthographic components that are also shared with the spoken language system. Solid box outlines represent components used in reading, and dashed box outlines represent components used in spelling.
Ortho = orthography; Phono = phonology (figures adapted from Hillis & Rapp, 2004).
It might seem to be a relatively straightforward matter to adjudicate between these hypotheses; however, this has not been the case despite the fact that both behavioral and neural evidence have been brought to bear on these questions. The majority of the evidence has come from individuals with acquired dysgraphia/dyslexia as a result of neural injury, with only a few studies involving neurologically intact adults. Furthermore, although there have been a large number of neuroimaging studies of reading in neurologically intact adults, there have been a very small number that have considered any aspect of spelling and none that have included both reading and spelling.
Behavioral Evidence
The association of acquired deficits of reading and spelling in the same individual would seem to favor the shared components account, whereas the dissociation of reading and spelling deficits (such that in specific individuals, one skill is intact whereas the other is impaired) would seem to favor the independent components account. Both patterns of performance have been reported subsequent to brain injury (for reviews, see Hillis & Rapp, 2004; Tainturier & Rapp, 2001). The interpretative difficulty lies in that under an independent components architecture, associated deficits could be the result of coincidental damage to independent components that are instantiated in adjacent neural substrates. In turn, under a shared components architecture, dissociations can be interpreted as arising from deficits affecting modality-specific access to shared components (rather than from deficits affecting the components themselves) (Allport & Funnell, 1981). For these reasons, the mere association or dissociation of reading and spelling impairments is insufficient to resolve this question. Therefore, observations of fine-grained similarities or differences in specific errors types, rates, and distributions provide more compelling evidence of shared or independent components.
One type of evidence indicating that a single set of lexical orthographic representations is used in both reading and spelling are reports of individuals with acquired deficits who exhibit a high degree of consistency in the specific words that give rise to errors in both reading and spelling (Behrmann & Bub, 1992; Friedman & Hadley, 1992; Coltheart & Funnell, 1987). Similarly, there is evidence of word-specific treatment generalization effects in acquired deficits, with training in reading generalizing to spelling for trained but not untrained words (e.g., Hillis, 1993; but see Weekes & Coltheart, 1996).
A small number of behavioral studies with neurologically intact participants have also taken the approach of investigating word-specific performance similarities/differences in spelling and reading. Using individually tailored word lists, Holmes and Carruthers (1998) and also Burt and Tate (2002) showed that individuals are slower and/or less accurate in performing lexical decision or visual spelling accuracy judgments for the specific words that they cannot spell correctly. Also consistent with a shared representations account, Monsell (1987) found significant repetition priming from the task of spelling (without visual feedback) to a subsequent reading task.
Although the relatively sparse behavioral evidence would seem to generally favor a shared components view, arguments can be raised against this conclusion. First, there is the concern that the evidence is largely correlational, leaving open the possibility that an uncontrolled factor is responsible for the observed relationship across the modalities. In addition, and specifically with regard to the studies with neurologically intact participants, it is difficult to rule out the possibility that episodic memory traces acquired during the experimental condition evaluating one modality are available and influence performance in the other modality. It is also important to note that research to date has largely focused on the question of a shared versus independent lexical orthographic component, largely ignoring the relationship between reading and spelling as concerns other orthographic components. With regard to the cognitive neuropsychological reports that have evaluated these other components, we find both some striking associations (e.g., Tainturier & Rapp, 2003; Caramazza, Capasso, & Miceli, 1996; Rapp & Caramazza, 1989) as well as dissociations (Rapp & Caramazza, 1997; Beauvois & Dérouesné, 1981) between reading and spelling. Clearly, the evidence is not yet sufficiently clear or consistent so as to support definitive conclusions.
Neural Substrates of Reading and Spelling
Given some of the difficulties of interpretation of the behavioral evidence, data concerning the neural substrates that support the specific processing components of reading and spelling may be especially useful.
Lesion-deficit Studies
Impairments in reading and/or spelling have been most commonly associated in both chronic and acute stroke with tissue dysfunction (lesion or hypoperfusion) in one or more of the following left hemisphere areas: the angular gyrus (Brodmann’s area [BA] 39), the fusiform or inferior temporal gyrus (BA 37, 20), the supramarginal gyrus (BA 40), superior temporal gyrus (BA 22), and the inferior frontal gyrus (IFG; BA 44/45). In addition, reading but not spelling deficits have been associated with lesions in occipital areas (BA 17, 18, 19), and spelling but not reading deficits have been associated with lesions to premotor areas such as BA 6 (for reviews, see Philipose et al., 2007; Hillis & Rapp, 2004; Rapcsak & Beeson, 2002, 2004; Friedman, Ween, & Albert, 1993; Roeltgen, 1993). Although some of the same neuroanatomical regions have been identified in acquired deficits of both reading and spelling, there are two important issues that limit the precision with which conclusions can be drawn. First, large lesions are usually involved, leaving open the possibility that different subregions within the same broadly defined neural areas correspond to each modality. Second, very few studies have evaluated reading and spelling in the same individuals and as a result have not directly tested the hypothesis that specific lesions affect both reading and spelling. With regard to the latter point, we review the few recent studies that have considered both tasks in the same individuals as these begin to allow for a more fine-grained analysis of the question.
Rapcsak and Beeson (2004) reported on eight individuals who, as a result of stroke, suffered damage to left hemisphere BA 37 and 20, corresponding to mid and anterior fusiform regions (sparing the angular gyrus). All exhibited both reading and spelling impairments. Consistent with these findings are the results of a study by Philipose et al. (2007), who examined reading and spelling in 69 cases of acute stroke. They carried out analyses evaluating the relationship between presence/absence of behavioral impairments in reading and/or spelling and location of tissue dysfunction (lesion and/or hypoperfusion). They reported a strong association between deficits in spelling and reading and tissue dysfunction in BA 40 and (superior) BA 37.
In addition to these reports, the detailed examination of individual patterns of behavioral impairment and lesion can be particularly useful. Tsapkini and Rapp (2010) and Gaillard et al. (2006) reported single case studies of reading and spelling in individuals with relatively circumscribed surgical lesions of the left fusiform area. The Gaillard et al. case exhibited impairment in reading but not spelling, with a lesion in the posterior portion of the left fusiform gyrus. These authors argued for a disconnection of early reading areas in the posterior fusiform from the abstract orthographic representations and processes localized in the mid-fusiform region, which they refer to as the visual word form area (VWFA). The Tsapkini and Rapp (2010) case involved a more anterior mid-fusiform lesion that was shown to specifically disrupt the mapping between orthographic representations and word meanings in both reading and spelling while sparing the processing of visual objects and auditorily presented words. The configuration of deficits and lesions in these two cases provides specific support for the conclusion that the left mid-fusiform is indeed required both for reading and for spelling (for a discussion, see Martin, 2006). Furthermore, it is encouraging that this localization is generally consistent with the results of Philipose et al. (2007) and Rapcsak and Beeson (2004).
Functional Neuroimaging Studies
Although there have been a very large number of functional neuroimaging studies of reading, only five studies have provided data concerning spelling in alphabetic systems1 (Norton, Kovelman, & Petitto, 2007; Beeson et al., 2003; Rapp & Hsieh, 2002; Menon & Desmond, 2001; Petrides, Alivisatos, & Evans, 1995). Even more problematic is the fact that there have been no functional neuroimaging studies that have considered both reading and spelling in the same subjects.
With regard to the neural substrates of reading, various reviews and meta-analyses have reported a fair amount of convergence across studies (despite disagreement regarding the specific functions assigned to these areas) for the involvement in reading of the following left hemisphere areas: superior and middle temporal gyri (posterior and middle regions), supramarginal gyrus, fusiform gyrus, inferior temporal gyrus, extrastriate occipital cortex, lingual gyrus, and left IFG (triangularis and opercularis; for reviews, see Palmer, Brown, Petersen, & Schlaggar, 2004; Jobard, Crivello, & Tzourio-Mazoyer, 2003; Mechelli, Gorno-Tempini, & Price, 2003; Turkeltaub, Eden, Jones, & Zeffiro, 2002). It is worth pointing out, however, that many of the studies that were reviewed involved oral reading or phonological judgments on written forms so that it is not straightforward to specifically identify which of the implicated areas are specifically involved in the orthographic aspects of reading and which with the planning and/or production of a spoken response.
A challenge faced by the few paradigms that have used the task of writing in the scanner is that of isolating the central components of the spelling process that might be shared by reading (see Figure 1A) from those components involved in the motor planning and execution aspects of writing. Although Menon and Desmond (2001) and Petrides et al. (1995) do not address this problem, Beeson et al. (2003) carried out a study designed to separate motor execution and planning from the more central aspects of spelling. Taking another approach, the studies of Norton et al. (2007) and Rapp and Hsieh (2002) used tasks that required access to spelling knowledge but which did not involve writing in the scanner. However, the Norton et al. study used a complex spelling verification task that recruited both spelling and reading processing, making it impossible to determine if reading and spelling actually share substrates. If we focus on the results of Beeson et al. (2003) and Rapp and Hsieh (2002), we find convergence in that they both identify spelling-specific activation in the following left hemisphere areas: the fusiform gyrus, the precentral sulcus (BA 6), the posterior inferior/middle frontal gyrus, and the supplementary motor cortex. Interestingly, although lesion studies since Dejerine’s (1982) classic study have provided evidence that lesions affecting the angular gyrus are strongly associated with disruption to both reading (e.g., Black & Behrmann, 1994; Benson, 1979) and spelling (e.g., Rapcsak & Beeson, 2002; Hillis, Kane, Barker, Beauchamp, & Wityk, 2001; Roeltgen & Heilman, 1984), the neuroimaging evidence has not provided this same strength of association. In their neuroimaging study of spelling, Beeson et al. (2003) failed to find group level effects, and in reading, Jobard et al. (2003) argued from their meta-analysis that at least some of the activations that have been reported for reading are more likely to be in posterior part of the middle temporal gyrus rather than the angular gyrus proper. In summary, if we evaluate the available neuroimaging evidence, we find that reading and spelling both recruit areas of the left fusiform gyrus and the IFG. It is noteworthy that these areas are also included among the regions identified by the lesion-deficit analyses as giving rise to impairments of reading and spelling.
Orthographic Processing: Category and Modality-specific Substrates?
Given the recency of literacy in human evolution, many have assumed that orthographic representation and processing are entirely parasitic on either visual object or spoken language processes and substrates. Contrary to these expectations, both neuroimaging and neuropsychological evidence have supported the claim that there are substrates specifically necessary for orthographic processing within the posterior and inferior temporal/fusiform area. The claim is that these substrates are specialized for the processing of written stimuli and are not required for other visual stimuli (category specificity) and also that they are necessary for written but not spoken language stimuli (domain specificity).
A number of functional neuroimaging studies have found regions of the inferior temporal lobe, including the mid-fusiform region, to be reliably more activated by written words relative to other categories of visual objects, such as faces and houses (Baker et al., 2007; Ben-Shachar, Dougherty, Deutsch, & Wandell, 2007; Gaillard et al., 2006; Hasson, Levy, Behrmann, Hendler, & Malach, 2002; Ishai, Ungerleider, Martin, Schouten, & Haxby, 1999; Puce, Allison, Asgari, Gore, & McCarthy, 1996). In addition, there have been a handful of functional neuro-imaging studies that have specifically examined both written and auditorily presented words and have found activation in this region only for written stimuli (Cohen & Dehaene, 2004; Cohen et al., 2004; Dehaene, Le Clec’H, Poline, Le Bihan, & Cohen, 2002; Binder et al., 2000). There have also been several reports of individuals with selective orthographic deficits subsequent to lesions affecting this area. These include reports of selective alexia without prosopagnosia (Feinberg, Schindler, Ochoa, Kwan, & Farah, 1994) and vice versa (Farah, Wilson, Drain, & Tanaka, 1998) and of selective visual object agnosia without alexia or prosopagnosia (Humphreys & Rumiati, 1998; Rumiati & Humphreys, 1998) and vice versa (Buxbaum, Glosser, & Coslett, 1999; De Renzi & di Pellegrino, 1998). In addition, the individuals, described by Tsapkini and Rapp (2010) and Gaillard et al. (2006), suffered lesions to the fusiform gyrus that resulted in either reading deficits alone or both reading and spelling deficits while sparing object and face processing as well as spoken word comprehension and production.
The proposal of modality- and category-specific orthographic substrates has been vigorously challenged (Devlin, Jamison, Gonnerman, & Matthews, 2006; Hillis et al., 2005; Mechelli et al., 2005; Price & Mechelli, 2005; Price & Devlin, 2003, 2004; Price et al., 2003). Generally speaking, the challenges argue that neither the activation patterns nor the deficits are as selective “as advertised.” With regard to the neuroimaging evidence, the argument is that although there may be voxels that are more responsive to orthographic than other visual stimuli, they are also responsive to other visual categories, reflecting the fact that they are involved in certain types of complex visual analysis that apply across a number of visual categories (Starrfelt & Gerlach, 2007; Martin & Chao, 2001; Moore & Price, 1999). Similarly, there are various reports of activation of the inferior temporal regions by spoken language stimuli and tasks (Vigneau, Jobard, Mazoyer, & Tzourio-Mazoyer, 2005; Price & Devlin, 2004; Jobard et al., 2003; Booth et al., 2002a, 2002b; Thompson-Schill, Aguirre, D’Esposito, & Farah, 1999; Demonet, Price, Wise, & Frackowiak, 1994). With regard to the neuropsychological evidence, it has been argued that in cases of selective impairments, other visual categories and/or spoken language are indeed affected and that the testing carried out was not sufficiently demanding of these skills.
The work we report on here will not address the question of the possible modality specificity (written vs. spoken language) of proposed written language substrates; however, our use of face and house stimuli as functional localizers will allow us to contribute to the ongoing debate regarding the proper characterization of the neural substrates that support written language processing and how they are related to object processing more generally.
METHODS
Participants
Ten individuals participated in this investigation (4 men, 6 women). They were all right-handed as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). They ranged in age from 18 to 42 years; all had a college education, were native speakers of English, and had no known history of reading or spelling disability. Furthermore, they all scored above 93% on a spelling pretest. All were paid for the participation in the research.
Tasks and Stimuli
Spelling, reading, and object processing (faces and houses) were evaluated in different tasks presented in different runs, for a total of 10 runs administered in one scanning session. The order of runs was varied across subjects. All tasks were practiced before the scanning session. Reading and face and object processing were evaluated adopting paradigms used in previous studies (reading: Cohen & Dehaene, 2004; object/face processing: Haxby et al., 2001). Using these fairly standard paradigms provided a solid basis for comparison with the spelling results that involved a relatively novel paradigm (used before only in Hsieh & Rapp, 2004; Rapp & Hsieh, 2002). Tasks were presented using E-Prime software (Schneider, Eschman, & Zuccolotto, 2002a, 2002b).
Silent reading was evaluated with three stimulus types: monomorphemic words, randomly generated consonant strings, and a black and white checkerboard rectangle. On each trial, a central fixation cross appeared for 550 msec, followed by a stimulus presented in the center of the screen for 200 msec. Participants were instructed to carefully attend to each stimulus. The words and consonant strings were three to six letters in length. All alphabetic stimuli were presented in lowercase in black font on a white background; the checkerboard rectangle had an extension comparable to that of a nine-letter word. Stimuli were presented in blocks of 28 trials, and there were 6 blocks of each stimulus type for a total of 18 blocks per run; there were a total of two runs that were identical except that blocks were presented in different pseudorandom orders. No alphabetic stimulus was repeated in a run, and the checkerboard stimulus was always the same. The repetition time (TR) was 1500, and 264 volumes were acquired in each run.
Object processing was evaluated with four stimulus types: faces, houses, and pixel-scrambled faces and houses, with 40 different stimuli of each type (from Haxby et al., 2001). Each stimulus was presented in the center of the screen for 500 msec, and participants were instructed to attend carefully. Stimuli were presented in a blocked manner according to stimulus type with 40 stimuli per block and 16 blocks per run (four of each stimulus type). Two runs were presented; they were identical except for the order of the blocks. The TR was 2000, and 169 volumes were acquired in each run.
Spelling was evaluated using two tasks: spelling probe and case verification tasks that served, respectively, as the experimental and sensory motor control tasks. The tasks involved identical sensory and motor components, varying only in the instructions given to subjects. In the spelling probe task each trial was 6 sec long, with the following sequence of events: (1) a centrally displayed task prompt (SPELLING?) for 1500 msec; (2) a central fixation cross for 300 msec, (3) an auditory word (plus a variable period of silence) for total duration of 1200 msec, (4) a single visually displayed uppercase letter for 1000 msec, and (5) a blank response screen for 2000 msec. Participants were instructed to respond yes/no (right/left hand button press) whether with the visually presented letter was in the spelling of the heard word. The trials for the case verification task were identical to those of the spelling probe task, except that that there was a different task prompt (UPPER-CASE?) and the visually displayed letter could appear in either upper- or lowercase. In this task, participants were instructed that the auditorily presented word was irrelevant and that they were simply to respond yes/no (button press) whether the visually presented letter was or was not in uppercase. Although with some limitations, the tasks were designed so that a comparison of the two tasks would identify the central components of the spelling process, without requiring written responses in the scanner. The rationale was as follows: The two tasks involved listening to a word, processing a visually presented letter, and making a yes/no decision. In addition, the spelling probe task required searching long-term memory for the spelling of a word (and/or generating it via sublexical processes) and then engaging orthographic working memory while verifying if the probe letter was contained in the spelling. Additional support for the appropriateness of the task for evaluating spelling comes from the Rapp and Kong (2002) report that individuals with acquired dysgraphia were impaired in the spelling probe task.
Across the two tasks, word stimuli were matched for length, frequency, and grammatical category. In each task, half of the stimuli were high frequency (mean = 91.2) and half were low frequency (mean = 3.7) (Francis & Kucera, 1982). Stimuli were either four or seven letters long. The majority of the words were nouns. In the spelling probe task, for four-letter words, all four-letter positions were probed, and for seven-letter words, Positions 1, 3, 5, 7 were probed. In the case verification task, the letters presented for case verification never occurred in the spelling of the heard word. Spelling probe and case verification trials were presented in a blocked manner, with six trials per block, six blocks per run, for a total of six runs. High-and low-frequency word trials formed “mini-blocks” within these larger blocks that consisted of one to four trials. The TR was 1500 msec, and a total of 176 volumes were acquired in each run.
Imaging Parameters
MRI data were acquired with a 3.0-T Phillips Intera Scanner at the F. M. Kirby Research Center for Functional Brain Imaging at the Kennedy Krieger Institute (Baltimore, MD). Whole-brain T2-weighted gradient-echo, EPIs were acquired with an eight-channel SENSE (Invivio) parallel-imaging head coil in 29 transverse slices (TR = 1500 msec, echo time = 30 msec, flip angle = 65°, field of view = 240 × 240 mm, matrix 128 × 128, slice thickness = 4 mm, gap = 1 mm). Structural images were acquired using an MP-RAGE T1-weighted sequence that yielded images with a 1-mm isotropic voxel resolution (TR = 8.06 msec, echo time = 3.8 msec, flip angle = 8°).
Data Analysis
Analysis of functional data was carried out using Brain-Voyager QX software (Maastricht, Netherlands) as well as with programs developed in MatLab (MathWorks, Natick, MA) for specific analysis purposes. With regard to preprocessing, functional images were slice time and motion corrected and then temporal high-pass filtered to remove components occurring fewer than three cycles over the course of a run. The images were normalized to Talairach coordinates, resampled to 3-mm isotropic voxels, and spatially smoothed (8-mm FWHM Gaussian kernel).
The general linear model approach (Friston et al., 1995) was used to estimate parameter values in a block design. Three general linear models separately modeled the spell, silent reading, and object processing tasks. Spelling was modeled with two regressors corresponding to the high-and low-frequency word blocks of the spelling probe task; all time points corresponding to the case verification trials were used as the baseline condition. Silent reading was modeled with three regressors corresponding to stimulus type: words, consonant strings, and fixation; all time points corresponding to checkerboard trials served as the baseline. Finally, object processing was modeled with two regressors for stimulus type: faces and houses; all time points corresponding to scrambled face and scrambled house trials served as the baseline. Regressors were created by convolving a two-parameter gamma hemodynamic response function with a boxcar function marking the temporal position of each stimulus block type. In addition to the task-specific regressors, for all tasks and each run, six motion correction regressors and an additional confound regressor (representing run number) were included in the model. Group data were subjected to random-effects analysis; for each contrast, cluster size thresholding was estimated using a plug-in implemented in BrainVoyager. This plug-in implements a Monte Carlo randomization technique to estimate a minimum cluster size that, for a given uncorrected voxelwise p value, will achieve a corrected p value < .05 (Forman et al., 1995). On this basis, for all contrasts reported, we applied a cluster size threshold corresponding to p value of <.05. In addition, the default uncorrected voxelwise p value adopted was p < .005. However, for a small number of contrasts, different uncorrected voxelwise values were adopted (between p < .01 and p < .001). This was done only on those occasions on which the default value obscured a pattern that was reliable across a range of uncorrected voxelwise values. Cluster locations are reported in terms of the location of the peak voxel of each cluster because the peak is more stable under threshold changes than the geometric center. Talairach coordinate values (Talairach & Tournoux, 1988) are used in the text, but Table 1 provides both Talairach and MNI values. Cluster sizes are reported as the number of 1-mm3 voxels; clusters smaller than 50 voxels are not reported.
Table 1.
Locations of the Activation Peaks for All Significant Clusters Found for Each of the Contrasts Carried out in Analysis 1: Talairach Coordinates Are Presented, with MNI Coordinates in Parentheses
| Object Processing
|
Reading
|
Spelling
|
|||||
|---|---|---|---|---|---|---|---|
| Face > House | House > Face | Words > Chx | Consts > Chx | Words > Consts | Spell > Case | Low > High Freq | |
| R parahippocampal g. | 27, −55, −11 (27, −56, −16) | ||||||
| L parahippocampal g. | −27, −82, 13 (−27, −85, 10) | ||||||
| R fusiform g. | 51, −46, −11 (52, −47, −16) | ||||||
| L fusiform g. | −39, −46, −5 (−39, −47, −9) | −45, −46, −11 (−45, −47, −16) | −48, −52, −11 (−48, −53, −16) | −36, −34, −14 (−36, −34, −19) | |||
| R sup temporal s./g. | 54, −43, −2 (55, −44, −5) | ||||||
| L sup temporal s./g. | −42, −49, 7 (−42, −51, 5) | −36, −43, 4 (−36, −44, 2) | −57, −10, 1 (−58, −10, 1) | ||||
| L inf frontal g/IFJ |
−36, 20, 4 (−36, 20, 5) [−39,2,28:oper] (−39, 1, 31) [−39,26,19:triang] (−39, 26, 22) [−36,20,4:triang] (−36, 20, 5) |
−42, −7, 31 (−42, −9, 33) | −24, 5, 4 (−24, 5, 5) | −42, 2, 22 (−42, 1, 24) | −27, 8, 25 (−27, 7, 28) | ||
| R inf frontal g | 45, −1, 28 (45, −2, 30) | ||||||
| L sup frontal s. | −15, 11, 37 (−15, 9, 41) | ||||||
| R mid-frontal g. | 57, −4, 34 (58, −6, 37) | ||||||
| R cingulate g. | 24, −16, 34 (24, −18, 36) | 30, −31, 4 (30, −32, 3) | 15, −37, 13 (15, −39, 12) | 27, −40, 1 (27, −41, −1) | |||
Voxelwise (uncorrected) thresholds were first applied at p < .005 for all contrasts except for Spell > Case (p < .0001) and Consts > Chx (p < .01). Voxels were then subjected to cluster size correction for multiple comparisons at p < .05, for all contrasts. Brackets indicate peak activations of the three clusters that make up the extensive Words > Chx activation region. Chx = checkerboards; Consts = consonant strings; Freq = lexical frequency; L = left hemisphere; R = right hemisphere; g = gyrus; s = sulcus; inf = inferior; sup = superior; IFJ = inferior frontal junction; oper: opercularis; triang: traingularis.
RESULTS
Analysis 1. Reading, Spelling, and Visual Object Processing: Basic Neurotopography
Silent Reading
A brain-wide evaluation of words > checkerboards (voxel-wise threshold p < .005, cluster level p < .05) yielded three large clusters: left mid-fusiform (−39, −46, −5; 6,217 voxels), left IFG (−36, 20, 4; 17,242 voxels), and the right cingu-late gyrus (24, −16, 34; 8,677 voxels) (see Figure 2 and Table 1). The right cingulate activation was not limited to the area of the peak in the anterior cingulate but rather extended almost the length of the gyrus. Further analysis revealed that the large left IFG cluster was composed of three smaller clusters (with peaks at −39, 26, 19, IFG triangularis; −36, 20, 4, IFG triangularis; and −39, 2, 28, IFG opercularis) that are reported in brackets in Table 1. The most posterior of the three was centered near an area of the left hemisphere referred to as the inferior frontal junction (IFJ) that is located at the junction of the inferior frontal sulcus and the inferior precentral sulcus. We will refer to this region as the IFG/IFJ (see Figure 2B). Both the fusiform and the IFG activations were highly left lateralized; in fact, right hemisphere fusiform activation (42, −52, −17) appeared only at an uncorrected p < .01 and right hemisphere IFG/IFJ activation did not appear at any meaningful threshold. Activation in the region of the left angular gyrus bordering on the intraparietal sulcus (IPS) was observed only at a more lenient voxelwise threshold of p < 01, a level at which activations were more diffuse and less clearly differentiated into distinct clusters.
Figure 2.
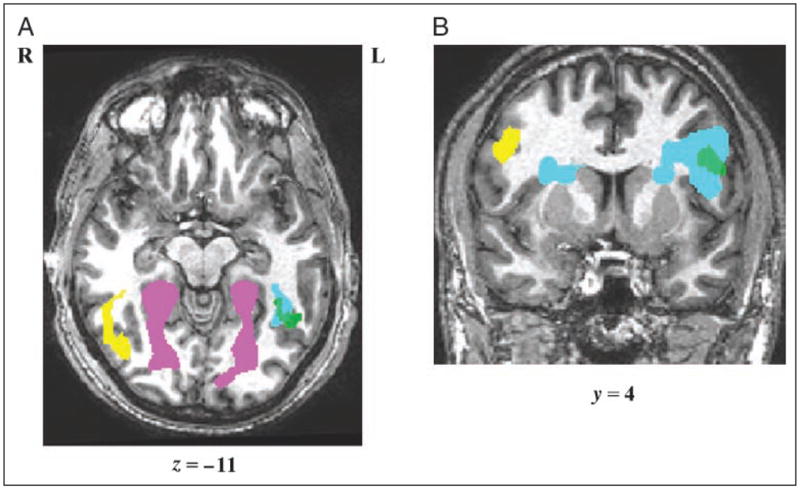
Neurotopography of orthographic and object processing. Significant clusters from Analysis 1 (vowelwise threshold p < .005–.0001, cluster level p < .05) are depicted: blue = words > checkerboards, green = spell > case, yellow = faces > houses, and pink = houses > faces. Both panels A and B depict overlapping substrates for reading and spelling and symmetrical activation for orthographic processing (left hemisphere) and face processing (right hemisphere). (A) Horizontal image at Talairach z = −11 depicts clusters for silent reading (words > checkerboards: peak = −39, −46, −5), spelling (spell > case: peak = −48, −52, −11), passive viewing of faces (faces > houses: right hemisphere peak: 51, −46, −11), and passive viewing of houses (houses > faces: right hemisphere peak: 27, −55, −11; left hemisphere peak: −27, −82, 13). (B) Coronal image at Talairach = +4 includes the bilateral IFG and IFJ and depicts significant clusters for reading (peak = −36, 20, 4), spelling (peak = −42, 2, 22), and face processing (peak: 45, −1, 28).
A brain-wide evaluation of consonants > checkerboards (voxelwise threshold p < .01, cluster level p < .05) yielded three clusters at the same left hemisphere locations as those found for the words > checkerboards contrast: left mid-fusiform (−45, −46, −11; 2,408 voxels), left IFG/IFJ (−42, −7, 31; 3,069 voxels), and right cingulate gyrus (30, −31, 4; 4,781 voxels).
A comparison of the neural response for words versus consonants (voxelwise threshold p < .005, cluster level p < .05) revealed no voxels with significantly greater activation for consonants than for words, although four clusters in which words > consonants were identified: bilateral posterior STS/middle temporal gyrus (left: 54, −43, −2; 2,405 voxels; and right: −42, −49, 7; 6,888 voxels), right posterior middle frontal gyrus (57, −4, 34; 2,397 voxels), and left IFG (−24, 5, 4; 5,955 voxels). In terms of the left fusiform specifically, words > consonants activation was apparent only at an uncorrected p < .003. We return to the comparison of words and consonants strings in Analysis 3 in which we consider targeted small volume examinations.
Spelling
Whole-brain evaluation of spell > case (voxelwise threshold p < .0001, cluster level p < .05) revealed five clusters: left mid-fusiform (−48, −52, −11; 569 voxels), left IFG/IFJ (−42, 2, 22; 604 voxels), left superior frontal sulcus (−15, 11, 37; 300 voxels), left posterior superior temporal gyrus/sulcus (−36, −43, 4; 261 voxels), and right posterior cingulate gyrus (15, −37, 13; 679 voxels) (see Figure 2 and Table 1). Only at the more lenient voxelwise thresholds of 0.01 or 0.05 was activation observed in the left intraparietal sulcus and supramarginal gyrus region; however, as was the case for the reading task, at these thresholds, activations were poorly differentiated into clusters. Overall, activation was highly left lateralized, such that the only right hemisphere activation was in the right posterior cingulate. This continued to be the case even as the threshold was lowered; in fact, at no reasonable threshold were significant spell > case activations observed in either the right fusiform or the IFG/IFJ regions.
A brain-wide evaluation of case > spell yielded no significant voxels. Only at the very lenient uncorrected p < .01 did case > spell clusters appear in the right and left hemisphere superior and middle temporal gyri (51, −58, 19; −42, −61, 22; 57, −10, −14) and the left superior frontal gyrus (−18, 50, 34).
Using only the trials from the spelling probe task, we evaluated effects of lexical frequency by comparing low-frequency words directly to a baseline of high-frequency words. Note that this direct contrast (without using the case verification task as a baseline) identifies general lexical frequency effects and not orthographic effects specifically, that is, because the spelling probe task involves both listening to a word and recovering its orthographic form from long-term memory and the case verification baseline serves to isolate the orthographic components of the task by “removing” the auditory word processing aspects. In this way we can evaluate frequency effects more broadly throughout the language system. We return to this point later. The results of the low-frequency > high-frequency contrast (voxelwise threshold p < .005, cluster level p < .05) revealed a pattern of highly left-lateralized activation with five clusters: left fusiform (−36, −34, −14; 5,257 voxels), left IFG/IFJ (−27, 8, 25; 3,219 voxels), left superior temporal gyrus (−57, −10, 1; 1,902 voxels), left posterior white matter (−27, −55, 13; 2,503 voxels), and right posterior cingulate (27, −40, 1; 2,310 voxels).
Objects
To identify brain areas that were especially responsive to faces as compared with houses and vice versa, the neural response to faces was contrasted with the response to houses, relative to a baseline condition of scrambled face and house images. Brain-wide analysis revealed two clusters for faces > houses and two clusters for houses > faces (voxelwise threshold p < .005, cluster level p < .05).
As can be seen in Figure 2, the two houses > faces clusters extended along the right and left parahippocampal/lingual gyri with peaks at 27, −55, −11 (cluster size = 19,824) and −27, −82, 13 (cluster size = 14,604). The two faces > houses clusters both appear in the right hemisphere. One is in the mid-fusiform gyrus (51, −46, −11; 9116 voxels), and the other is in the IFG/IFJ (45, −1, 28; 1561 voxels). At thresholds corrected for multiple comparisons, we observed no left hemisphere activation for faces > houses, and only at an uncorrected p < .005 did we observe a left fusiform cluster (−36, −64, −17); in addition, there was no left hemisphere IFG/IFJ activation for faces > houses at any meaningful threshold.
Summary
The results we reported for object processing are consistent with those reported in the literature for both faces and houses. For example, the activation for faces > houses includes the fusiform region most typically reported as being especially responsive to faces and sometimes referred to as the fusiform face area (39 ± 3, −40 ± 7, −16 ± 5) (Grill-Spector, Knouf, & Kanwisher, 2004). Similarly, the activation for houses > faces included the location of what is sometimes referred to as the parahippocampal place area (21 ± 5, 54 ± 7, −9 ± 4) (Aguirre, Zarahn, & D’Esposito, 1998).
The neurotopography of reading and spelling revealed by this analysis is also highly consistent with the lesion-deficit and neuroimaging research reviewed in the Introduction. Specifically, both tasks activated areas within the left fusiform and the left IFG. The apparent sharing of substrates is investigated more thoroughly in Analysis 2.
Given the prominent role played by the angular gyrus in neurological theories of written language processing, it is worth briefly commenting specifically on the results regarding this area. As indicated, we find activation in the intraparietal sulcus and angular gyrus for both reading and spelling but only at thresholds more lenient than those that clearly identified the fusiform and IFG/IFJ activations. One possibility is that these relatively weaker activations as well as the inconsistencies across imaging studies and discrepancies between neuroimaging and deficit–lesion correlation studies (described in the Introduction) may be due to greater variability in the individual recruitment of substrates within this region; certainly, this neuroanatomical area and these issues would benefit from more targeted investigation.
Analysis 2. Overlapping Substrates?
Reading and Spelling
To evaluate the relationship between the neural substrates of reading and spelling, we carried out two analyses. First, we identified areas of overlapping neural responsivity for reading and spelling, and second, we more carefully examined the identified areas by comparing the cross-modality differences in peak activations for reading and spelling to the variability observed in repeated within-modality assessments. (Note that both analyses considered only data from word stimuli, given that the spelling task only involved words.)
As depicted in Figure 3A, brain-wide evaluation of the words > checkerboards and spell > case contrasts (using the same thresholds as in Analysis 1) identified only two regions that were responsive to both modalities of orthographic processing: one in the left fusiform gyrus (−42, −48, −13; 341 voxels) and another in the left IFG/IFJ −42, 3, 24; 538 voxels). That is, spelling significantly activated voxels within two of the three regions that were found to be significantly active for reading, and reading activated voxels within two of the five regions that were active for spelling.
Figure 3.
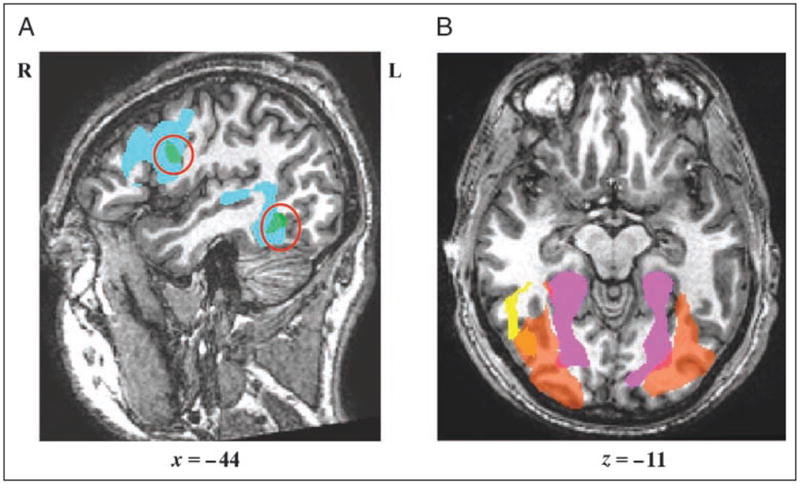
Shared substrates of reading/spelling and faces/houses. (A) Lateral view of activations produced by reading (words > checkerboards) in blue and spelling (spell > case) in green. Indicated with red circles are the regions of overlap between reading and spelling in the left mid-fusiform (−42, −48, −13; 341 voxels) and the left IFG/IFJ (−42, 3, 24; 538 voxels). Correction for multiple comparisons: for reading = voxelwise p < .005, corrected p < .05, and for spelling = voxelwise p < .0001, corrected p < .05. (B) Orange depicts shared voxels for faces > scrambled images and houses > scrambled images (right hemisphere: peak = 27, −91, −2; 18,405 voxels; left hemisphere: peak = −33, −79, −14; 13,561 voxels). Included for comparison purposes are the regions (also depicted in Figure 2A) especially sensitive to faces (yellow = faces > houses) and houses (pink = houses > faces).
Although this analysis indicates the existence of neural tissue that is jointly sensitive to reading and spelling, it does not reveal the degree of similarity in the activation “topography” within these regions for the two orthographic modalities. A key (and relatively stable) feature of the activation topography is the location of the activation peaks. For example, one possibility to be considered is that although reading and spelling coactivate voxels within the regions identified just above, their peak activations could be distinct, suggesting a potentially critical distinction between the two. In fact, the reading and spelling activation peaks in the fusiform, and the IFJ/IFG (opercularis) that were identified in Analysis 1, although geographically close, are not identical (see Table 1). To evaluate the significance of these differences, we examined whether the peak locations are reliably different across the modalities (reading and spelling) given the variability that could be expected from repeated measurements within each modality. To estimate this variability, for both the fusiform and the IFJ/IFG, we identified activation peaks for both reading and spelling on the basis of split-half samplings of the data in each modality. For spelling, we evaluated 20 samples, each consisting of three of the six run total, and for reading, we evaluated 20 samples each consisting of three of the six block total (of words and checkerboards).
The 20 values obtained for each of the x,y,z coordinates for both the fusiform and the IFG/IFJ locations were compared for reading versus spelling (see Table 2). The results of the six t test evaluations of these data sets revealed no significant differences (p values ranging from .57 to .11) between reading and spelling activation peaks, except for the x-coordinates of the IFG/IFJ clusters (p < .02). This indicates a potentially significant 2-mm difference between the spelling (mean × value = −41) and the reading (mean × value = −39) peaks. However, even this difference would not be significant if a correction for the multiple comparisons (the six t tests) was applied to this analysis.
Table 2.
Mean and SD (in Parentheses) of the Activation Peaks of Clusters in the Left Fusiform Gyrus and the Left IFG/IFJ, Obtained from 20 Split-half Data Analyses of Reading (Words > Checkerboards) and Spelling (Spell > Case)
| Coordinates | Left Mid-fusiform
|
Left IFG/IFJ
|
||
|---|---|---|---|---|
| Reading | Spelling | Reading | Spelling | |
| x | −41.4 (1.6) | −41.9 (3.3) | −39.0 (2.6) | −41.0 (2.4) |
| y | −48.9 (4.5) | −47.2 (6.2) | 2.6 (2.5) | 2.2 (2.1) |
| z | −15.5 (5.0) | −13.3 (3.6) | 25.5 (4.9) | 23.7 (2.3) |
Only the x values for the IFG/IFJ clusters differ significantly (p < .02), although not if a correction for multiple comparisons is applied. See text for more details.
Houses and Faces
The results of Analysis 1 revealed large clusters that were more responsive to houses versus faces and vice versa. The finding of nonoverlapping areas for faces and houses does not mean, of course, that voxels within these clusters are not responsive to both faces and houses when evaluated relative to some neutral baseline, such as scrambled images. To address this question directly, we carried out a brain-wide conjunction analysis of faces > scrambled images and houses > scrambled images. This analysis identifies the areas of intersection in which both faces > scrambled images and houses > scrambled images are significant. The results revealed (voxelwise threshold p < .005, cluster level p < .05) bilateral, right lateralized regions within the fusiform and the middle occipital gyrus (right hemisphere: 27, −91, −2; left hemisphere: −33, −79, −14). Figure 3B depicts the regions identified in this conjunction analysis as well as the clusters identified in Analysis 1 in which houses > faces and faces > houses. The results indicate that (when the same correction for multiple comparisons is applied) there are areas that show “selective” responsivity to houses or faces that fall outside the areas that are jointly activated by houses and faces (relative to a low-level baseline).
Objects and Orthography
No areas of intersection between objects (faces or houses) and orthography (reading or spelling) are seen when brain-wide activations specific to faces, houses, reading, and spelling (at a voxelwise threshold p < .005, cluster level p < .05; or more stringent) are superimposed on one another. It is only when the voxelwise threshold is lowered beyond this level and when activations become large and ill defined that we begin to see overlapping areas, and these increase in extent as the threshold is lowered. We address the question of the relationship between object and orthographic substrates more systematically in Analysis 3.
Summary
With regard to the relationship between reading and spelling, the analyses clearly reveal that not only is there neural tissue that is jointly sensitive to reading and spelling in the left mid-fusiform and the IFG/IFJ but that the locations of the peak activity in the two modalities are statistically indistinguishable. With regard to object processing and the relationship between object and orthographic processing, we find regions of cortex within the fusiform and occipital lobes that are differentially sensitive to the categories of objects, faces, and written words. Although we cannot rule out that these categories may also activate common substrates, it is quite clear that there are distinctive activation distributions for stimulus processing in these categories.
Analysis 3. Small Volume Investigation of the Fusiform, Occipital, and IFG/IFJ Regions
Targeted small volume analyses were carried out in the fusiform gyrus, the occipital lobe, and the IFG/IFJ region. Seven locations were selected bilaterally (for a total of fourteen 1-cm3 volumes), and t tests were used to examine the average responsiveness of the voxels within each volume (using the BrainVoyager’s VOI analysis procedure; see the red squares in Figure 4A–C for VOI locations).
Figure 4.
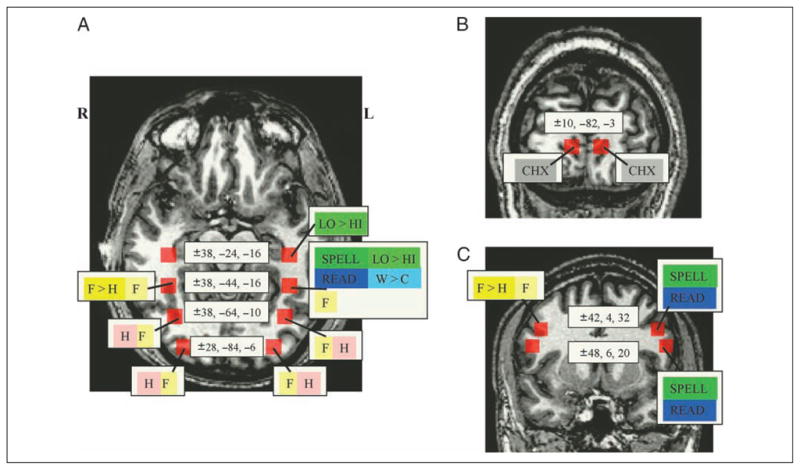
Results of bilateral VOI analyses. Fourteen 1-cm3 VOIs (depicted in red) were selected in the fusiform (A) and occipital gyri (A and B) and the IFJ (C). Each VOI was subjected to eight contrasts; significant contrasts are listed for each VOI (evaluated at Bonferroni-corrected p < .0063) and are depicted in the following colors: gray = checkerboards > words or consonant strings; pale yellow = faces > scrambled images; pale pink = houses > scrambled images; yellow: faces > houses; dark blue: words or consonants > checkerboards; light blue: words > consonants; dark green: spell > case; light green: low-frequency > high-frequency words.
A mid-fusiform volume was selected at coordinates commonly reported in the literature for the VWFA (±38, −44, −16), and then anterior (±38, −24, −16) and posterior (±38, −64, −10) volumes were selected to be equidistant from the mid-fusiform volume (while remaining within the neuroanatomical confines of the gyrus). Two occipital locations were examined bilaterally, one in the middle occipital gyrus, just posterior to the fusiform gyrus (±28, −84, −6), and another in the lingual gyrus (±10, −82, −3), the latter permitting an evaluation of early visual processes. Two bilateral posterior frontal locations were selected: bilateral IFJ volumes were created centered on the average coordinates of IFJ activations reported in the recent literature (±42, 3.5, 32; Brass & von Cramon, 2002, 2004; Derrfuss, Brass, & von Cramon, 2004) and located at the junction between the inferior and middle frontal gyri and the sulcus of the precentral gyrus; in addition, bilateral IFG volumes were created so as to be fully contained within the posterior IFG (opercularis; ±48, 6, 20).
At each volume, eight contrasts were examined: spell/case, low/high frequency (spelling), words/checkerboards (reading), consonants/checkerboards (reading), words/consonants (reading), faces/houses, houses/scrambled, and faces/scrambled. To correct for the fact that each volume was subjected to eight comparisons, a Bonferroni-corrected value of p < .0063 was applied to determine statistical significance of the contrasts at each volume. The results are depicted in Figure 4A–C.
With regard to the fusiform (see Figure 4A), the bilateral posterior fusiform was responsive only to houses (p < .00008) and faces (p < .0003) relative to scrambled images. The bilateral mid-fusiform was sensitive to the general contrast of face > scrambled (right: p < .0005 left: p < .0002). In addition, the right mid-fusiform was significantly sensitive to the specific contrast of faces > houses (p < .002), and the left mid-fusiform showed a very strong trend toward significance for this contrast (p < .008). The mid-fusiform VOIs exhibited a markedly asymmetric responsiveness to all of the orthographic contrasts, with only the left mid-fusiform exhibiting significant sensitivity to the following: in spelling, spell > case (p < .0002) as well as low-frequency > high-frequency words (p < .004); in reading, both words > checkerboards and consonants > checkerboards were significant (words: p < .002; consonants: p < .006) as was the comparison of words > consonants (p < .003). In the anterior fusiform, the only significant effect was in the left anterior fusiform, which exhibited sensitivity to low-frequency > high-frequency words (p < .002).
In the occipital lobe (see Figure 4A and B), the middle occipital gyrus exhibited bilateral effects of house > scrambled images and face > scrambled images (houses: p < .00002; faces: p < .005), whereas the bilateral lingual VOIs were the only ones to show an effect of checkerboards > words (p < .005) or consonant strings (p < .003). In addition, the right lingual gyrus exhibited an effect of scrambled images > faces (p < .004).
The IFJ and the IFG volumes had highly similar patterns of responsivity. They exhibited markedly asymmetric responses (Figure 4C) such that in the left hemisphere, both the IFJ and the IFG exhibited sensitivity only to orthographic conditions: spell > case (p < .0003), words > checkerboards (p < .00001), and consonants > checkerboards (p < .005); in the right hemisphere, the right IFJ exhibited significant effects only for faces, specifically faces > houses (p < .004) and faces > scrambled images (p < .005), and the right IFG exhibited similar sensitivity to faces although the effect did not quite pass the Bonferroni threshold faces > houses (p < .007).
Summary
The small volume analyses2 confirm the results of the brain-wide analyses reported in Analyses 1 and 2 and reveal a highly differentiated pattern of responsivity across the posterior brain and in the IFG/IFJ for both orthographic and object stimuli. The primary axes of this differentiation are posterior to anterior and right versus left hemispheres. We discuss these findings in more detail in the General discussion.
GENERAL DISCUSSION
In this article, we report on an fMRI investigation evaluating the brain’s response to the tasks of silent reading, spelling, and the passive viewing of faces and houses. The objectives were to further our understanding of the relationship between orthographic comprehension and production (reading and spelling) and, in turn, their relationship to visual object processing. Whole-brain and small volume analyses converge on the following empirical findings. (1) Neural tissue in the left hemisphere mid-fusiform gyrus and the IFG (including the IFJ) are responsive to both reading and spelling. (2) The left mid-fusiform region, in addition to its general responsiveness to orthographic processing, exhibits sensitivity to lexical factors, namely, greater responsivity to words relative to consonant strings, and to low- relative to high-frequency words. (3) In contrast, the anterior portion of the left fusiform gyrus is responsive to differences in lexical frequency but not to orthographic processing (either reading or spelling). (4) Within the inferior temporal lobes, we find bilateral regions that are responsive to both faces and houses as well as additional regions that are more strongly activated by faces compared with houses or vice versa. (5) Substrates for objects (faces and houses) and orthographic processing (reading and spelling) are largely nonoverlapping, except for a strong trend in the left mid-fusiform for responsivity to both faces and orthography. In fact, orthography (reading and spelling) and face processing activate generally complementary homologous areas in the fusiform and IFG, with activations that are lateralized to the left and right hemispheres, respectively.
Reading and Spelling: Shared Substrates?
This study evaluated both reading and spelling in the same individuals, providing a strong test of the hypothesis of shared components for reading and spelling. Both whole-brain and small volume analyses revealed highly reliable areas of overlapping activation for reading and spelling in both the left mid-fusiform gyrus and the left IFG/IFJ. Furthermore, the activation peaks for reading and spelling in these two areas are neuroanatomically close and statistically indistinguishable. In this way, the results provide perhaps the strongest evidence to date that reading and spelling share at least some cognitive machinery (see Figure 1A).
As would be expected, reading and spelling also show regions of nonoverlapping activation; for reading, this lies primarily in the triangularis area of the IFG; for spelling, non-overlapping regions include the left superior temporal and superior frontal sulci. These modality-specific activations are certainly of interest and may indicate that some processes are not shared between reading and spelling. However, the absence of activation overlap in these regions presents the usual interpretative challenges. Namely, the landscape of nonoverlapping regions is likely to change as thresholds are relaxed and corrections for multiple comparisons are reduced. Furthermore, the likely increased task demands—attentional, temporal, and otherwise—for the spelling probe task as compared with the silent reading task may result in more and larger clusters reaching statistical significance in one task compared with the other. For these reasons, we focus this discussion on the highly reliable regions of overlap between the two tasks and discuss what these results reveal about the shared machinery of reading and spelling.
The Left Fusiform Gyrus
With regard to the left fusiform, the area of shared activation for reading and spelling (Figure 3A) clearly coincides with the region observed in a large number of neuroimaging studies of reading and referred to as the VWFA (Cohen & Dehaene, 2004). It also falls within the region implicated in lesion studies of spelling (Rapcsak & Beeson, 2004) and is consistent with the sparse neuroimaging data on spelling that is available (Beeson et al., 2003; Rapp & Hsieh, 2002). The natural next question is which shared orthographic processing components (Figure 1A) make use of the left mid-fusiform area? There are various aspects of the results that are relevant in answering this question. First, the mid-fusiform area is responsive to both words and consonant strings relative to checkerboards, indicating a role in orthographic processing. Second, the area is more sensitive to words than consonant strings and more sensitive to low-than high-frequency words. This sensitivity to lexical factors indicates that the region is not merely sensitive to orthography in general but to lexical orthography in particular. Third, we can rule out that the sensitivity to both orthographic and lexical factors is attributable to attentional factors that can be expected to affect the hemodynamic response more broadly, because the pattern observed in mid-fusiform stands in clear contrast with that observed in the anterior fusiform. This combination of findings leads to the conclusion that the mid-fusiform region is specifically involved in the representation of (or access to/from) orthographic word forms. In other words, it contributes to the retrieval or processing of the long-term memory representations of the spellings of words. This function, often referred to as the orthographic lexicon, mediates between letter forms and meaning (see Figure 1A). This characterization of the function of the left mid-fusiform is consistent with the proposals of a number of researchers regarding reading (Glezer, Jiang, & Riesenhuber, 2009; Proverbio, Zani, & Adorni, 2008; Vinckier et al., 2007; Hillis et al., 2001; Samuelsson, 2000; but see Jobard et al., 2003; Mechelli et al., 2003; Howard et al., 1992) and also spelling (e.g., Tsapkini & Rapp, 2010; Rapcsak & Beeson, 2004). That is, the results support the view that reading and spelling share an orthographic lexicon. Also noteworthy is the posterior–anterior transition in sensitivity from both lexical and orthographic factors (in the mid-fusiform) to only lexical frequency (in the anterior fusiform). This finding is consistent with proposals in which the posterior–anterior axis of the left fusiform is characterized as instantiating increasingly abstract, complex, and eventually modality-independent lexical processing (Vinckier et al., 2007; Binder, Medler, Westbury, Liebenthal, & Buchanan, 2006; Dehaene, Cohen, Sigman, & Vinckier, 2005). Along these lines, a number of results have supported the claim that anterior region of the left fusiform is an amodal language area representing the abstract word representations that mediate between orthographic, phonemic, and semantic information (among others, see Jobard, Vigneau, Mazoyer, & Tzourio-Mazoyer, 2006; Hillis et al., 2005; Cohen et al., 2004; Jobard et al., 2003; Damasio, 1989). Furthermore, this claim is also consistent with the evidence from semantic dementia and other sources that underscores the role of the anterior temporal lobe in the representation and processing of word meaning (e.g., Mummery et al., 1999). Finally, the finding of substrates sensitive to non-orthographic lexical factors adjacent to those that appear to be specifically involved in orthographic processing is consistent with a number of reports reviewed in the Introduction, indicating activation in the fusiform region for spoken language lexical retrieval (for reviews, see Price & Devlin, 2003, 2004). It should be evident that considerable work is still required to understand the precise neural underpinnings of these various intimately related cognitive operations that apparently depend on left inferior temporal substrates.
The Left IFG/IFJ
The finding of shared substrates for reading and spelling within the left IFG/IFJ region is generally consistent with results reported in the neuroimaging and neuropsychological literature for reading (Bolger, Perfetti, & Schneider, 2005; Jobard et al., 2003; Mechelli et al., 2003; Turkeltaub et al., 2002; Paulesu et al., 2000; Price, 2000; Fiez & Petersen, 1998). It is worth noting, however, that the precise location of activations within the posterior IFG region for reading is quite variable across neuroimaging studies (x = −32 to −61; y = −5 to +20; x = 10 to 30), and the tasks used to evaluate reading are also quite disparate. For spelling, it has been reported that dysgraphia can also result from lesions to this general region (see Hillis et al., 2002 for a review).3
The attribution of cognitive functions to the left posterior IFG/IFJ is not straightforward because, although activation in the opercular IFG is often reported in neuroimaging studies of reading, it has received markedly less attention than has the fusiform. Furthermore, the cognitive functions that have been attributed to the posterior IFG are extremely diverse and include lexical semantics (Bolger et al., 2005), grapheme–phonology conversion (Jobard et al., 2003), lexical retrieval (Price, 2000), phonological processing (Pugh et al., 1996), and the orthographic lexicon (Hillis et al., 2002). The results of our VOI analyses do not strongly support any one of these proposals. The results reveal a highly similar pattern of responsiveness in both the left IFJ and the IFG, with both showing significant sensitivity to reading and spelling. In both areas, the effects of lexical factors (frequency, lexical status) did not meet the Bonferroni-corrected thresholds, although in all cases the p values for the contrasts of low-frequency > high-frequency and words > consonants were less than .05. In sum, in terms of orthographic processing, the pattern of responsivity of the left IFG/IFJ region was quite similar to that of the left mid-fusiform, except that sensitivity to lexical factors was weaker.
In addition to possible language-specific functions of the posterior IFG/IFJ mentioned just above, other types of functions have been proposed. In recent articles, Derrfuss, Brass, Neumann, and von Cramon (2005) and Brass and von Cramon (2002, 2004) have identified the IFJ as a functional area that is independent of the middorsolateral pFC, and they have proposed that it is involved in cognitive control, with the specific function of updating task representations in situations where task and response demands are changing. In a meta-analysis, Derrfuss et al. found bilateral IFJ activation for experimental paradigms that required updating task representation (e.g., set switching, task switching, S-R reversal tasks) and also left IFJ activation for various Stroop tasks. However, although the paradigm we used for evaluating spelling certainly involved updating task representations (between the spell and case tasks), the silent reading task did not. The reading task was a passive viewing task, and although the stimuli did switch between blocks of words, consonant strings, and checkerboards, the task remained constant throughout (to simply attend carefully to the stimuli). Similarly, the right IFJ sensitivity to faces > houses that we have reported was also the product of a passive viewing task that involved stimulus set switching (houses, faces, and scrambled images) but not task changes. Given this, the function of updating representations does not provide a satisfactory candidate for the processes that are shared by reading and spelling in this area.
Another direction for thinking about the functionality of this region comes from the literature on mirror neurons. It has been argued that this system, in which the same neurons are responsive to both seeing an action performed and performing the action, is likely to play a key role in action imitation and/or in forming the basis of action understanding (for a review, see Rizzolatti & Craighero, 2004). In the monkey, premotor area F5 has been identified as a key component of the motor neuron system. Intriguingly, the human homologue of area F5 is considered to be the opercularis region of the IFG (Petrides & Pandya, 1997). fMRI studies in humans have supported this localization, reporting responsiveness in this area in a number of critical conditions (e.g., Buccino et al., 2001; Iacoboni et al., 1999). However, it is not straightforward to derive specific conclusions regarding the functionality of this region for written language, especially given the broader claim that spoken language may have its roots in gestural communication and the motor neuron system (Rizzolatti & Arbib, 1998). At this point, this remains an avenue that merits additional scrutiny.
In sum, we find clear evidence of shared substrates for reading and spelling. The results specifically provide strong support for a shared lexical orthographic function in reading and spelling in the left mid-fusiform region. With regard to the IFG/IFJ, the results reveal a common recruitment of the posterior inferior frontal area by both reading and spelling, although the function of this area for written language remains unclear. One concern that should be discussed is the possibility that the spelling probe task recruits shared substrates with reading, although these substrates would not normally be recruited in spontaneous spelling or spelling to dictation. Although this cannot be ruled out, there are good reasons to think that this is unlikely. First, as we have noted, there is considerable lesion evidence indicating that left fusiform and IFG lesions are associated with acquired dysgraphia. Second, previous neuroimaging studies of spelling reported activation in these same regions. Thus, the neural substrates identified in this work are not unexpected, what the research does is (a) provide converging evidence of spelling substrates from a different spelling task and (b) allow for a strong test of the shared components hypothesis by examining both reading and spelling in the same individuals.
Objects and Orthography in the Literate Brain
With regard to object processing, we found bilateral regions of the inferior temporal/occipital lobes that were jointly responsive to both faces and houses as well as regions of special sensitivity to houses versus faces and vice versa (Figure 3B). These results support the claims of considerable differentiation in the neural substrates that support the processing/representation of different object categories (e.g., Haxby et al., 2001). Also consistent with the literature is our report of left–right and anterior–posterior asymmetries for object processing (e.g., Lerner, Hendler, Ben-Bashat, Harel, & Malach, 2001). Activation is right lateralized for both faces and houses, and we see an increasing specificity of response along a posterior–anterior axis from the lingual gyrus, through the middle occipital and posterior fusiform gyri to the mid-fusiform and parahippocampal areas. However, this study was not designed to evaluate whether the category-specific response differences we have reported are indeed category specific or if, instead, they represent some aspect of visual object processing that is accentuated in these categories but not limited to them.
With regard to the relationship between objects and orthography, brain-wide analyses revealed no areas of intersection between objects (faces or houses) and orthography (reading or spelling) at the various thresholds that showed clear activation patterns for each of these tasks (Figure 2A and B). These results indicate, as has been proposed by other researchers (e.g., Puce et al., 1996), that orthographic and object stimuli produce distinctive patterns of neural activity. When considering these patterns of activation, one is struck by the relationship between the activations produced by faces and orthography in both the mid-fusiform and the IFG/IFJ. In both regions, activation is highly symmetrical for the two categories but with complementary lateralization.
With respect to the IFG/IFJ area, the recruitment of right and left IFG/IFJ by face and orthographic processing, respectively, indicates a lateralized category specificity in the frontal operculum that has not been highlighted in the literature. Interestingly, Derrfuss et al. (2005) in their meta-analysis reported (but did not discuss) differences in the IFJ lateralization of activations for the two sets of studies they analyzed. The set of studies that consisted of task-switching paradigms produced bilateral IFJ activation, and the set that included Stroop tasks yielded primarily left hemisphere IFJ activation. This asymmetry could be consistent with our findings as it may be based on stimulus category differences. The two sets of tasks Derrfuss et al. and Brass and von Cramon (2002, 2004) used also differed in stimulus types, with the left-lateralized Stroop set involving words and the bilateral task-switching involving objects, figures, numbers, or faces (in addition to words or letters).
The observed spatial symmetry between faces and orthography suggests a fundamental relationship between the two. Given the recent introduction of written language into the human repertoire, one possibility is that written language makes use of (perhaps redundant) substrates that were selected for face processing over the course of evolution. In line with this idea, Dehaene and Cohen (2007) recently proposed that the parts of human cortex that are specialized for cultural domains (such as reading or arithmetic) are the product of “cultural recycling of cortical maps.” They argue that cultural skills recruit or “invade” preexisting neural circuits that carry out computational functions that are similar to those required by the cultural skill and that are also sufficiently plastic so that they can adapt to the specific demands of the newly acquired skill. At least for the mid-fusiform area, one can speculate that the original functionality should be something that distinguishes face processing from house processing but is shared by both face and written word processing. There may be a common computational demand for the accurate perception of the spatial characteristics and positioning of the complex internal elements (letters/facial features) that define objects in categories whose members are “wholistically” very similar (Kleinschmidt & Cohen, 2006). In addition, Dehaene and Cohen argue that the “prior organization is never entirely erased” and suggest that, as a consequence, we would expect the original functionality of the substrates to influence processing within the newly acquired cultural domain. Along these lines, we might also expect to see activation evidence of the original functionality in the culturally “requisitioned” substrates. This would provide a nice account of why the left hemisphere mid-fusiform area continues to be (weakly) responsive to faces.
Conclusions
Since the early work on mirror neurons (Rizzolatti, Fadiga, Fogassi, & Gallese, 1996; Di Pellegrino, Fadiga, Fogassi, Gallese, & Rizzolatti, 1992), it has become increasingly clear that, across a range of cognitive domains, there is an intimate relationship between perception and action. Although the purpose of this relationship continues to be debated, our finding of shared substrates for written language comprehension and production indicates that literacy may also be structured according to these basic principles of neurocognitive organization.
Acknowledgments
The authors thank James Haxby for graciously allowing us to use the face and house stimuli from Haxby et al. (2001). We are grateful to Brian Glucroft, Manuel Vindiola, and Li Hsieh for their many important contributions to this project as well as to Susan Courtney for her invaluable advice. The support of NIDCD grant DC006740 to the first author made this work possible.
Footnotes
We note that there have been a number of excellent studies in nonalphabetic scripts such as Chinese or Japanese kanji. We do not include them here because the difference in orthographic systems introduces a factor that could add variability to the findings (see Bolger et al., 2005).
We carried out the same small volume analyses with un-smoothed functional data. The results were highly similar with the following two relatively minor differences: significant effects for spell > case extended more posteriorly, including both mid and posterior left fusiform VOIs; also, lexical effects in the left IFG (but not IFJ) were attenuated for both reading and spelling tasks.
Exner, in 1881, reported that a region of the posterior, middle frontal gyrus was critical for writing. Since then, a number of studies have referred to this region, and disruption to this area has been associated with spelling and reading deficits as well as reading and writing epilepsy (for a review, see Matsuo et al., 2003). Although Matsuo et al. (2003) identified Exner’s area with coordinates (−46, 3, 27) that are extremely close to the region of shared activation for reading and spelling (−41, 3, 24), most others have identified Exner’s area with more superior premotor regions of the posterior middle and even superior frontal gyri (e.g., Roux, Dufor, Giussani, Draper, & Démonet, 2009).
References
- Aguirre GK, Zarahn E, D’Esposito M. An area within human ventral cortex sensitive to “building” stimuli: Evidence and implications. Neuron. 1998;21:373–383. doi: 10.1016/s0896-6273(00)80546-2. [DOI] [PubMed] [Google Scholar]
- Allport DA, Funnell E. Components of the mental lexicon. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 1981;295:397–410. [Google Scholar]
- Baker CI, Liu J, Wald LL, Kwong KK, Benner T, Kanwisher N. Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proceedings of the National Academy of Sciences, USA. 2007;104:9087–9092. doi: 10.1073/pnas.0703300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvois MF, Dérouesné J. Lexical or orthographic agraphia. Brain. 1981;104:21–49. doi: 10.1093/brain/104.1.21. [DOI] [PubMed] [Google Scholar]
- Beeson PM, Rapcsak SZ, Plante E, Chargualaf J, Chung A, Johnson S, et al. The neural substrates of writing: A functional magnetic resonance imaging study. Aphasiology. 2003;17:647–665. [Google Scholar]
- Behrmann M, Bub D. Surface dyslexia and dysgraphia: Dual routes, single lexicon. Cognitive Neuropsychology. 1992;9:209–251. [Google Scholar]
- Ben-Shachar M, Dougherty RF, Deutsch GK, Wandell BA. Differential sensitivity to words and shapes in ventral occipito-temporal cortex. Cerebral Cortex. 2007;17:1604–1611. doi: 10.1093/cercor/bhl071. [DOI] [PubMed] [Google Scholar]
- Benson DF. Aphasia, alexia, and agraphia. New York: Churchill Livingstone; 1979. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, et al. Human temporal lobe activation by speech and nonspeech sounds. Cerebral Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, Buchanan L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage. 2006;33:739–748. doi: 10.1016/j.neuroimage.2006.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S, Behrmann M. Localization in alexia. In: Kertesz A, editor. Localization and neuroimaging in neuropsychology. San Diego, CA: Academic Press; 1994. [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: Universal structures plus writing system variation. Human Brain Mapping. 2005;25:92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. Functional anatomy of intra- and cross-modal lexical tasks. Neuroimage. 2002a;16:7–22. doi: 10.1006/nimg.2002.1081. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TR, Mesulam MM. Modality independence of word comprehension. Human Brain Mapping. 2002b;16:251–261. doi: 10.1002/hbm.10054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. The role of the frontal cortex in task preparation. Cerebral Cortex. 2002;12:908–914. doi: 10.1093/cercor/12.9.908. [DOI] [PubMed] [Google Scholar]
- Brass M, von Cramon DY. Decomposing components of task perception with functional magnetic resonance imaging. Journal of Cognitive Neuroscience. 2004;16:609–620. doi: 10.1162/089892904323057335. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, et al. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study. European Journal of Neuroscience. 2001;13:400–404. [PubMed] [Google Scholar]
- Burt JS, Tate H. Does a reading lexicon provide orthographic representations for spelling? Journal of Memory and Language. 2002;46:518–543. [Google Scholar]
- Buxbaum LJ, Glosser G, Coslett HB. Impaired face and word recognition without object agnosia. Neuropsychologia. 1999;37:41–50. doi: 10.1016/s0028-3932(98)00048-7. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Capasso R, Miceli G. The role of the graphemic buffer in spelling. Cognitive Neuropsychology. 1996;13:673–698. [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: The case for the visual word form area. Neuroimage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L, Henry C, Dehaene S, Martinaud O, Lehericy S, Lemer C, et al. The pathophysiology of letter-by-letter reading. Neuropsychologia. 2004;42:1768–1780. doi: 10.1016/j.neuropsychologia.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Funnell E. Reading and writing: One lexicon or two? In: Allport A, MacKay DG, Prinz W, Sheerer E, editors. Language perception and production: Shared mechanisms in listening, speaking, reading and writing. London: Academic Press; 1987. pp. 313–339. [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: A systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33:25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- De Renzi E, di Pellegrino G. Prosopagnosia and alexia without object agnosia. Cortex. 1998;34:403–415. doi: 10.1016/s0010-9452(08)70763-9. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. Cultural recycling of cortical maps. Neuron. 2007;56:384–398. doi: 10.1016/j.neuron.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: A proposal. Trends in Cognitive Sciences. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec’H G, Poline JB, Le Bihan D, Cohen L. The visual word form area: A prelexical representation of visual words in the fusiform gyrus. NeuroReport. 2002;13:321–325. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Contribution a l’etude anatomoclinique et clinique des differentes varietes de cecite verbale. Memoires de la Societé de Biologié. 1982;4:61–90. [Google Scholar]
- Demonet JF, Price C, Wise R, Frackowiak RSJ. A PET study of cognitive strategies in normal subjects during language tasks: Influence of phonetic ambiguity and sequence processing on phoneme monitoring. Brain. 1994;117:671–682. doi: 10.1093/brain/117.4.671. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: Meta-analyses of switching and Stroop studies. Human Brain Mapping. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon DY. Cognitive control in the posterior frontolateral cortex: Evidence from common activations in task coordination, interference control, and working memory. Neuroimage. 2004;23:604–612. doi: 10.1016/j.neuroimage.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Gonnerman LM, Matthews PM. The role of the posterior fusiform gyrus in reading. Journal of Cognitive Neuroscience. 2006;18:911–922. doi: 10.1162/jocn.2006.18.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: A neurophysiological study. Experimental Brain Research. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychological Review. 1998;105:482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- Feinberg TE, Schindler RJ, Ochoa E, Kwan PC, Farah MJ. Associative visual agnosia and alexia without prosopagnosia. Cortex. 1994;30:395–411. doi: 10.1016/s0010-9452(13)80337-1. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proceedings of the National Academy of Sciences, USA. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency analysis of English usage. Boston, MA: Houghton Mifflin Company; 1982. [Google Scholar]
- Friedman RB, Hadley JA. Letter-by-letter surface alexia. Cognitive Neuropsychology. 1992;9:185–208. [Google Scholar]
- Friedman RF, Ween JE, Albert ML. Alexia. In: Heilman KM, Valenstein E, editors. Clinical neuropsychology. 3. New York: Oxford University Press; 1993. pp. 37–62. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Gaillard R, Naccache L, Pinel P, Clémenceau S, Volle E, Hasboun D, et al. Direct intracranial, fMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron. 2006;50:191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Glezer LS, Jiang X, Riesenhuber M. Evidence for highly selective neuronal tuning to whole words in the “visual word form area. Neuron. 2009;62:199–204. doi: 10.1016/j.neuron.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nature Neuroscience. 2004;7:555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Hasson U, Levy I, Behrmann M, Hendler T, Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron. 2002;34:479–490. doi: 10.1016/s0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Hillis A, Rapp B. Cognitive and neural substrates of written language comprehension and production. In: Gazzaniga M, editor. The new cognitive neurosciences. 3. Cambridge, MA: MIT Press; 2004. pp. 775–788. [Google Scholar]
- Hillis AE. The role of models of language processing in rehabilitation of language impairments. Aphasiology. 1993;7:5–26. [Google Scholar]
- Hillis AE, Kane A, Barker P, Beauchamp NJ, Wityk RJ. Neural substrates of the cognitive processes underlying reading: Evidence from magnetic resonance perfusion imaging in hyperacute stroke. Aphasiology. 2001;15:919–931. [Google Scholar]
- Hillis AE, Kane A, Tuffiash E, Beauchamp NJ, Barker PB, Jacobs MA, et al. Neural substrates of the cognitive processes underlying spelling: Evidence from MR diffusion and perfusion imaging. Aphasiology. 2002;16:425–438. [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker P, Herskovits E, Degaonkar M. The roles of the “visual word form area” in reading. Neuroimage. 2005;24:548–559. doi: 10.1016/j.neuroimage.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Holmes VM, Carruthers J. The relation between reading and spelling in skilled adult readers. Journal of Memory and Language. 1998;39:264–289. [Google Scholar]
- Howard D, Patterson K, Wise R, Brown WD, Friston K, Weiller C, et al. The cortical localization of the lexicons. Positron emission tomography evidence. Brain. 1992;115:1769–1782. doi: 10.1093/brain/115.6.1769. [DOI] [PubMed] [Google Scholar]
- Hsieh L, Rapp B. Functional magnetic resonance imaging of the cognitive components of the spelling process. Brain and Language. 2004;91:40–41. [Google Scholar]
- Humphreys GW, Rumiati RI. Agnosia without prosopagnosia or alexia: Evidence for stored visual memories specific to objects. Cognitive Neuropsychology. 1998;15:243–277. doi: 10.1080/026432998381177. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzollati G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proceedings of the National Academy of Sciences, USA. 1999;96:9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Jobard G, Vigneau M, Mazoyer B, Tzourio-Mazoyer N. Impact of modality and linguistic complexity during reading and listening tasks. Neuroimage. 2006;34:784–800. doi: 10.1016/j.neuroimage.2006.06.067. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Cohen L. The neural bases of prosopagnosia and pure alexia: Recent insights from functional neuroimaging. Current Opinion in Neurology. 2006;19:386–391. doi: 10.1097/01.wco.0000236619.89710.ee. [DOI] [PubMed] [Google Scholar]
- Lerner Y, Hendler T, Ben-Bashat D, Harel M, Malach R. A hierarchical axis of object processing stages in the human visual cortex. Cerebral Cortex. 2001;11:287–297. doi: 10.1093/cercor/11.4.287. [DOI] [PubMed] [Google Scholar]
- Martin A. Shades of Déjerine–Forging a causal link between the visual word form area and reading. Neuron. 2006;50:173–175. doi: 10.1016/j.neuron.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: Structure and processes. Current Opinion in Neurobiology. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Kato C, Sumiyoshi C, Toma K, Duy Thuy DH, Moriya T, et al. Discrimination of Exner’s area and the frontal eye field in humans—Functional magnetic resonance imaging during language and saccade tasks. Neuroscience Letters. 2003;340:13–16. doi: 10.1016/s0304-3940(03)00050-8. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Lambon-Ralph MA, Patterson K, et al. Dissociating reading processes on the basis of neuronal interactions. Journal of Cognitive Neuroscience. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: A comparison of PET and fMRI. Journal of Cognitive Neuroscience. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Menon V, Desmond JE. Left superior parietal cortex involvement in writing: Integrating fMRI with lesion evidence. Cognitive Brain Research. 2001;12:337–340. doi: 10.1016/s0926-6410(01)00063-5. [DOI] [PubMed] [Google Scholar]
- Monsell S. Nonvisual orthographic processing and the orthographic input lexicon. In: Coltheart M, editor. Attention and performance XII. Hillsdale, NJ: Lawrence Erlbaum; 1987. pp. 299–323. [Google Scholar]
- Moore CJ, Price CJ. Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage. 1999;10:181–192. doi: 10.1006/nimg.1999.0450. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Wise RJ, Vandenberghe R, Price CJ, Hodges JR. Disrupted temporal lobe connections in semantic dementia. Brain. 1999;122:61–73. doi: 10.1093/brain/122.1.61. [DOI] [PubMed] [Google Scholar]
- Norton ES, Kovelman I, Petitto L. Are there separate neural systems for spelling? New insights into the role of rules and memory in spelling from functional magnetic resonance imaging. Mind, Brain and Education. 2007;1:48–59. doi: 10.1111/j.1751-228X.2007.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;23:223–232. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Palmer ED, Brown TT, Petersen SE, Schlaggar BL. Investigation of the functional neuroanatomy of single word reading and its development. Scientific Studies of Reading. 2004;8:203–223. [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, et al. A cultural effect on brain function. Nature Neuroscience. 2000;3:91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC. Functional activation of the human ventrolateral frontal cortex during mnemonic retrieval of verbal information. Proceedings of the National Academy of Sciences, USA. 1995;92:5803–5807. doi: 10.1073/pnas.92.13.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative architectonic analysis of the human and the macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of neuropsychology. New York: Elsevier; 1997. pp. 17–58. [Google Scholar]
- Philipose LE, Gottesman RF, Newhart M, Kleinman JT, Herskovits EH, Pawlak MA, et al. Neural regions essential for reading and spelling of words and pseudowords. Annals of Neurology. 2007;62:481–492. doi: 10.1002/ana.21182. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: Contributions from functional neuroimaging. Journal of Anatomy. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word for area. Neuroimage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The pro and cons of labelling a left occipitotemporal region: “The visual word form area. Neuroimage. 2004;22:477–479. doi: 10.1016/j.neuroimage.2004.01.018. [DOI] [PubMed] [Google Scholar]
- Price CJ, Gorno-Tempini ML, Graham KS, Biggio N, Mechelli A, Patterson K, et al. Normal and pathological reading: Converging data from lesion and imaging studies. Neuroimage. 2003;20:30–41. doi: 10.1016/j.neuroimage.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Price CJ, Mechelli A. Reading and reading disturbance. Current Opinion in Neurobiology. 2005;15:231–238. doi: 10.1016/j.conb.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Zani A, Adorni R. The left fusiform area is affected by written frequency of words. Neuropsychologia. 2008;46:2292–2299. doi: 10.1016/j.neuropsychologia.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: A functional magnetic resonance imaging study. Journal of Neuroscience. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz GA, Shaywitz SE, Constable RT, Skudlarski P, Fulbright RK, et al. Cerebral organization of component processes in reading. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Beeson PM. Neuropsychology, neurology, and rehabilitation. Philadelphia: Psychology Press; 2002. Neuroanatomical correlates of spelling and writing; pp. 71–99. [Google Scholar]
- Rapcsak SZ, Beeson PM. The role of left posterior inferior temporal cortex in spelling. Neurology. 2004;62:2221–2229. doi: 10.1212/01.wnl.0000130169.60752.c5. [DOI] [PubMed] [Google Scholar]
- Rapp B, Caramazza A. Letter processing in reading and spelling: Some dissociations. Reading and Writing. 1989;1:13–33. [Google Scholar]
- Rapp B, Caramazza A. From graphemes to abstract letter shapes: Levels of representation in written spelling. Journal of Experimental Psychology: Human Perception and Performance. 1997;23:1130–1152. doi: 10.1037//0096-1523.23.4.1130. [DOI] [PubMed] [Google Scholar]
- Rapp B, Hsieh L. Functional magnetic resonance imaging of the cognitive components of the spelling process. Poster presented at the Cognitive Neuroscience Society Meeting; San Francisco, CA. 2002. [Google Scholar]
- Rapp B, Kong D. Revealing the component functions of the graphemic buffer. Brain and Language. 2002;83:112–114. [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp Trends in Neurosciences. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review of Neuroscience. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Fogassi L, Gallese V. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Roeltgen DP. Agraphia. In: Heilman KM, Valenstein E, editors. Clinical neuropsychology. New York: Oxford University Press; 1993. pp. 63–89. [Google Scholar]
- Roeltgen DP, Heilman KM. Lexical agraphia further support for the two-system hypothesis of linguistic agraphia. Brain. 1984;107:811–827. doi: 10.1093/brain/107.3.811. [DOI] [PubMed] [Google Scholar]
- Roux FE, Dufor O, Giussani C, Draper L, Démonet JF. The graphemic/motor frontal area (GMFA): Exner’s area revisited. Annals of Neurology. 2009;66:537–545. doi: 10.1002/ana.21804. [DOI] [PubMed] [Google Scholar]
- Rumiati RI, Humphreys GW. Recognition by action: Dissociating visual and semantic routes to action in normal observers. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:631–647. doi: 10.1037//0096-1523.24.2.631. [DOI] [PubMed] [Google Scholar]
- Samuelsson S. Converging evidence for the role of occipital regions in orthographic processing: A case of developmental surface dyslexia. Neuropsychologia. 2000;38:351–362. doi: 10.1016/s0028-3932(99)00096-2. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime reference guide. Pittsburgh: Psychology Software Tools, Inc; 2002a. [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime user’s guide. Pittsburgh: Psychology Software Tools, Inc; 2002b. [Google Scholar]
- Starrfelt R, Gerlach C. The visual what for area: Words and pictures in the left fusiform gyrus. Neuroimage. 2007;35:334–342. doi: 10.1016/j.neuroimage.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Tainturier MJ, Rapp B. The spelling process. In: Rapp B, editor. What deficits reveal about the human mind/brain: A handbook of cognitive neuropsychology. Philadelphia: Psychology Press; 2001. [Google Scholar]
- Tainturier MJ, Rapp B. Is a single graphemic buffer used in reading and spelling? Aphasiology. 2003;17:537–562. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Thompson-Schill SL, Aguirre GK, D’Esposito M, Farah MJ. A neural basis for category and modality specific of semantic knowledge. Neuropsychologia. 1999;37:671–676. doi: 10.1016/s0028-3932(98)00126-2. [DOI] [PubMed] [Google Scholar]
- Tsapkini K, Rapp B. The orthography-specific functions of the left fusiform gyrus: Evidence of modality and category specificity. Cortex. 2010;46:185–205. doi: 10.1016/j.cortex.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: Method and variation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Jobard G, Mazoyer B, Tzourio-Mazoyer N. Word and non-word reading: What role for the visual word form area? Neuroimage. 2005;27:694–705. doi: 10.1016/j.neuroimage.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: Dissecting the inner organization of the visual word-form system. Neuron. 2007;55:143–156. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Weekes B, Coltheart M. Surface dyslexia and surface dysgraphia: Treatment studies and their theoretical implications. Cognitive Neuropsychology. 1996;13:277–315. [Google Scholar]