Summary
In Gram-negative bacteria, a variety of high molecular weight ‘exoproteins’ are translocated across the outer membrane (OM) via the two-partner secretion (TPS) pathway by interacting with a dedicated transporter. It is unclear, however, whether the translocation of exoproteins across the OM is coupled to their translocation across the inner membrane (IM). To address this question, we separated the production of an Escherichia coli O157:H7 exoprotein (OtpA) and its transporter (OtpB) temporally by placing otpA and otpB under the control of distinct regulatable promoters. We found that when both full-length and truncated forms of OtpA were expressed prior to OtpB, a significant fraction of the exoprotein was secreted. The results indicate that OtpA can be translocated into the periplasm and briefly remain secretion-competent. Furthermore, by engineering cysteine residues into OtpA and using disulphide bond formation as a reporter of periplasmic localization, we obtained additional evidence that the C-terminus of OtpA enters the periplasm before the N-terminus is translocated across the OM even when OtpA and OtpB are expressed simultaneously. Taken together, our results demonstrate that the translocation of a TPS exoprotein across the OM can occur independently from its translocation across the IM.
Introduction
Many Gram-negative bacteria utilize the two-partner secretion (TPS) pathway to transport proteins into the extracellular space (Jacob-Dubuisson et al., 2001). As the name implies, the TPS system is comprised of two components, a secreted ‘exoprotein’ (TpsA) that is typically > 100 kDa and a ∼60 kDa outer membrane (OM) protein (TpsB) that appears to be a translocation channel dedicated to the secretion of TpsA. TpsA and TpsB molecules are generally encoded in adjacent genes located in a single operon (Jacob-Dubuisson et al., 2001). Although all TpsA molecules contain a conserved N-terminal ∼250 amino acid segment (TPS domain), they are otherwise highly variable in size and sequence (Henderson et al., 2004; Jacob-Dubuisson et al., 2004; Choi et al., 2007). The function of TpsA molecules is also very diverse; while the best characterized exoproteins are haemolysins/cytolysins (such as Serratia marcescens ShlA) or adhesins (such as Bordetella pertussis FHA and Haemophilus influenzae HMW1), exoproteins that mediate contact-dependent growth inhibition, proteolytic reactions and biofilm formation have recently been described (Aoki et al., 2005; Kida et al., 2008; Neil and Apicella, 2009). Despite their sequence and functional diversity, most exoproteins are predicted to fold into an extended β-helix (Jacob-Dubuisson et al., 2004; Kajava and Steven, 2006). TpsB molecules are essential for TpsA secretion, but typically promote the secretion of only their cognate TpsA molecule and closely related exoproteins (Jacob-Dubuisson et al., 1997). The fact that TpsB molecules also exhibit considerable sequence diversity (Yen et al., 2002; Choi et al., 2007) suggests that each TpsA/TpsB pair evolved to function together. The C-terminal domain of one TpsB protein, FhaC, has been shown to form a β barrel structure that is typical of OM transporters, and most other TpsB molecules are predicted to have a similar architecture (Clantin et al., 2004). Furthermore, consistent with their putative function as protein-conducting channels, TpsB proteins belong to a superfamily of proteins that have been implicated in protein transport reactions in bacteria, chloroplasts and mitochondria (Gentle et al., 2005).
Many aspects of the mechanism by which TpsB molecules promote TpsA secretion are still poorly understood. The TPS domain of the exoprotein is clearly necessary for secretion, and it is probably also sufficient because truncated exoproteins containing little more than the TPS domain are secreted efficiently (Schonherr et al., 1993; Renauld-Mongenie et al., 1996; Grass and St Geme, 2000). This segment has been shown to form a β-helix that, at least in principle, might nucleate the folding of the rest of the molecule (Clantin et al., 2004). Available evidence indicates that the TPS domain encodes an extremely extended signal that targets TpsA molecules in an unfolded conformation to the TpsB transporter (Guedin et al., 1998; Hodak et al., 2006), but it is unclear whether correct targeting or any subsequent steps require that TpsA and TpsB be co-ordinately synthesized. The targeting signal of FHA (and presumably other TpsA proteins as well) is recognized by two polypeptide-transport-associated (POTRA) domains that are found at the N-terminus of TpsB (Guedin et al., 2000; Clantin et al., 2007). The interaction of TpsA with the POTRA domains presumably catalyses the initiation of secretion, but the details of this process are unknown. FHA has been proposed to traverse the channel formed by the FhaC β barrel (Meli et al., 2006; Clantin et al., 2007; Hodak and Jacob-Dubuisson, 2007). The crystal structure of FhaC shows that the β barrel is plugged by both an extracellular loop and an α-helical segment, however, and exoprotein transport through the β barrel pore would require displacement of at least the loop (Clantin et al., 2007). Likewise, an examination of the HMW1 transporter (HMW1B) by cryo-EM revealed an occluded β barrel pore and suggested that HMW1B undergoes a large conformational change prior to the secretion of its substrate (Li et al., 2007). Based on work with FHA, it has also been proposed that the N-terminus of an exoprotein remains bound to the POTRA domains while the rest of the protein is threaded through the transporter in an N- to C-terminal direction, and is secreted only after the C-terminus of the protein has crossed the OM (Mazar and Cotter, 2006). This model implies that the bulk of the exoprotein is secreted in a hairpin conformation. Both the maximum diameter of the FhaC channel (16 Å) and the observation that folded domains cannot be secreted through TpsB strongly suggests that the exoprotein lacks significant tertiary structure during secretion (Guedin et al., 1998; Clantin et al., 2007). Interestingly, even though exoprotein secretion presumably involves a complex series of events, secretion is extremely fast (Choi et al., 2007).
The energy source for protein secretion through the TPS pathway has remained especially enigmatic. Although considerable energy must be required to translocate exoproteins from the periplasm to the extracellular space, the periplasm is devoid of ATP. One possible solution to this paradox would involve co-ordinated transport of exoproteins across the inner membrane (IM) and OM. If transport across the two membranes is coupled, then translocation across the OM might be driven by the cytoplasmic SecA protein, which uses the energy of ATP hydrolysis to promote translocation across the IM through the SecYEG complex. To date, however, no direct evidence for a co-ordinated transport reaction in any TPS system has been reported. The coupled translocation model was originally proposed to explain the observation that an 80 kDa N-terminal fragment of FHA (Fha44) is not secreted when the expression of FhaC is delayed (Guedin et al., 1998). While the results are consistent with the coupled translocation model, they are also compatible with the possibility that Fha44 is first transported into the periplasm, but loses secretion competence rapidly if no FhaC is available. Alternatively, the energy for TpsA secretion might be derived from the progressive folding of the protein in the extracellular space (Hodak and Jacob-Dubuisson, 2007). While the observation that ShlA and HMW1 can be detected in the periplasm is consistent with the folding model (Schiebel et al., 1989; St Geme and Grass, 1998), it is not clear if these molecules are secretion intermediates or a subpopulation that remains orphaned inside the cell because of a loss of secretion competence.
To gain further insight into the mechanism and energetics of secretion through the TPS pathway, we revisited the coupled secretion hypothesis. We used the Escherichia coli O157:H7 OtpA/OtpB proteins that we recently characterized as a model TPS system in our experiments (Choi et al., 2007). While the sequence of OtpA diverges from that of other TpsA proteins and OtpA does not function as a haemolysin or adhesin, the otpA/otpB genes are contained within an O-island found in serotypes that are highly associated with haemolytic uraemic syndrome (Shen et al., 2005). By using a two-plasmid system that enabled us to temporally uncouple the expression of otpA and otpB, we obtained evidence that both truncated and full-length versions of OtpA can be completely translocated into the periplasm and remain secretion competent briefly. Furthermore, experiments in which cysteine residues were introduced into the C-terminus of OtpA showed that disulphide bond formation (which occurs only in the periplasm in E. coli) precedes the onset of secretion and provided additional evidence that the entire protein resides transiently in the periplasmic space. Taken together, our results strongly suggest that the translocation of OtpA across the IM and OM can occur in two separable steps and thereby imply that the energy for secretion is not derived from IM or cytoplasmic factors.
Results
OtpA secretion does not require co-ordinated synthesis of OtpB
Although previous experiments have demonstrated that TPS exoproteins can be secreted when tpsA and tpsB are expressed under the control of two separate promoters (Guedin et al., 1998; Choi et al., 2007), it is unclear whether exoproteins can be secreted when the expression of the two genes is temporally uncoupled. To assess the functionality of OtpB molecules that are assembled before otpA is expressed, we transformed HDB114 with two plasmids, one harbouring otpB under the control of a tightly regulated araBAD promoter (pPC11) and one harbouring otpA under the control of the IPTG-inducible trc promoter (pPC20). Initially, cells were grown in minimal medium and otpB expression was induced by the addition of arabinose. Aliquots were removed 10–30 min later and separated into cell and culture medium (supernatant) fractions, and the fractions were analysed by Western blot with an anti-OtpB antiserum. As expected, OtpB was found in cells that were incubated with arabinose, but not in control cells (Fig. 1A). In subsequent experiments, otpB expression was induced for 30 min and cells were washed extensively to remove the arabinose. OtpA expression was then induced for various times by the addition of IPTG. Western blot analysis using an antiserum directed against an OtpA N-terminal peptide showed that OtpA was secreted from cells that were initially incubated with arabinose, but remained inside control cells that had never expressed otpB (Fig. 1B). The observation that the amount of OtpA in the supernatant fraction increased over a 30 min time period despite the removal of arabinose from the medium provided the first indication that OtpB synthesized before OtpA might remain functional.
Fig. 1.
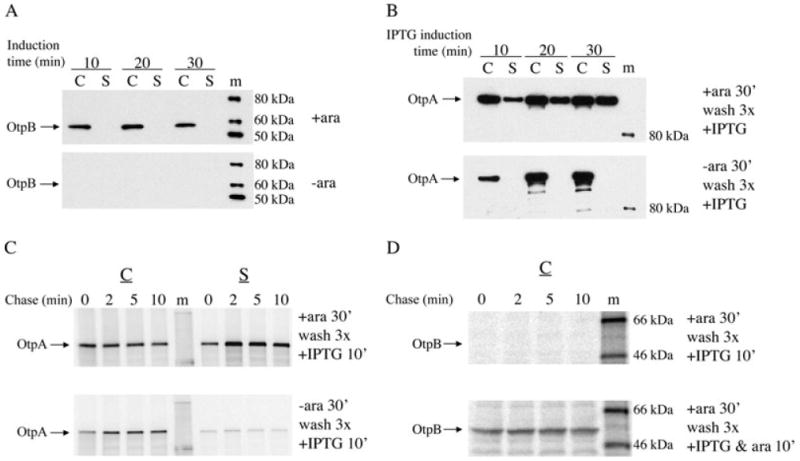
Pre-assembled OtpB promotes OtpA secretion. HDB114 transformed with pPC11 (PBAD-otpB) and pPC20 (Ptrc-otpA) were grown in minimal medium.
A. Cells were incubated with or without arabinose (ara) for the indicated time and cell (C) and secreted (S) fractions were analysed by Western blot using anti-OtpB.
B. Cells were incubated with or without arabinose for 30 min, washed three times and then incubated with IPTG for the indicated time. Cell and supernatant fractions were analysed by Western blot using anti-OtpA.
C. Cells were incubated with or without arabinose for 30 min, washed three times, incubated with IPTG for 10 min, and then subjected to pulse-chase labelling. OtpA was subsequently immunoprecipitated from cell and supernatant fractions.
D. Cells were incubated with arabinose for 30 min, washed three times, incubated with either IPTG or IPTG plus arabinose for 10 min, and then subjected to pulse-chase labelling. OtpB was subsequently immunoprecipitated from the cell fraction. m, molecular weight markers.
To clarify the relative timing of expression of otpB and otpA in these experiments, HDB114 transformed with pPC11 (PBAD-otpB) and pPC20 (Ptrc-otpA) were incubated in the presence or absence of arabinose, washed and incubated with IPTG for 10 min. Cells were then subjected to pulse-chase labelling and separated from the culture medium. OtpA and OtpB synthesized during the pulse-labelling period were then isolated by immunoprecipitation. Consistent with the results described above, OtpA was secreted only when the expression of otpB was pre-induced (Fig. 1C). Interestingly, OtpB was no longer synthesized at the time the radiolabelled OtpA was secreted (Fig. 1D, top panel). This observation indicates that the araBAD promoter was silenced rapidly after the removal of the inducer and that OtpA was secreted by previously assembled OtpB molecules. The observation that OtpB could be immunoprecipitated from the cell extract if arabinose was added together with the IPTG in the second stage of the experiment confirmed that the absence of an OtpB signal was not due to a technical problem (Fig. 1D, bottom panel). Taken together, these results demonstrate that OtpA secretion does not require simultaneous synthesis of OtpB and strongly suggest that the two proteins interact only after OtpB is integrated into the OM.
OtpA remains secretion-competent following its translocation across the IM
Having obtained evidence that OtpB synthesized independently from OtpA is functional, we next wished to determine if OtpA molecules that are translocated across the IM in the absence of OtpB can be secreted when otpB is subsequently expressed. In initial experiments we examined the secretion of an N-terminal ∼31 kDa fragment of OtpA that contains a C-terminal HA tag [OtpA(N′)-HA] (Fig. 2A). We used a truncated form of OtpA in part because we surmised that it might be less likely to fold into a secretion-incompetent conformation in the periplasm in the absence of OtpB than the full-length protein. In addition, translocation of OtpA(N′)-HA across the IM is easier to monitor because the precursor and mature forms can be separated by SDS-PAGE, whereas the two forms of the full-length protein are difficult to resolve. To examine the secretion of OtpA(N′)-HA when it is synthesized co-ordinately with OtpB, HDB114 transformed with pPC11 (PBAD-otpB) and pPC110, a plasmid in which the expression of otpA(N′)-HA is under the control of a trc promoter, were incubated in the presence of both IPTG and arabinose. Cells were subjected to pulse-chase labelling, separated from the culture medium, and divided into two aliquots. One aliquot was treated with proteinase K to degrade newly synthesized OtpA that was secreted but not yet released from the cell surface. OtpA was then immunoprecipitated from all of the samples. Consistent with previous results (Choi et al., 2007), we found that OtpA(N′)-HA was secreted extremely quickly. Most of the pulse-labelled protein that was translocated across the IM (i.e. that was converted from pre-OtpA(N′)-HA to OtpA(N′)-HA) was located either in the culture medium or on the cell surface (Fig. 2B, lanes 1–3). Almost all of the protein was secreted by 2 min, but the relatively slow conversion of pre-OtpA(N′)-HA to mature OtpA(N′)-HA implied that the rate of secretion was limited by the transport of the protein across the IM (Fig. 2B, lanes 4–9).
Fig. 2.
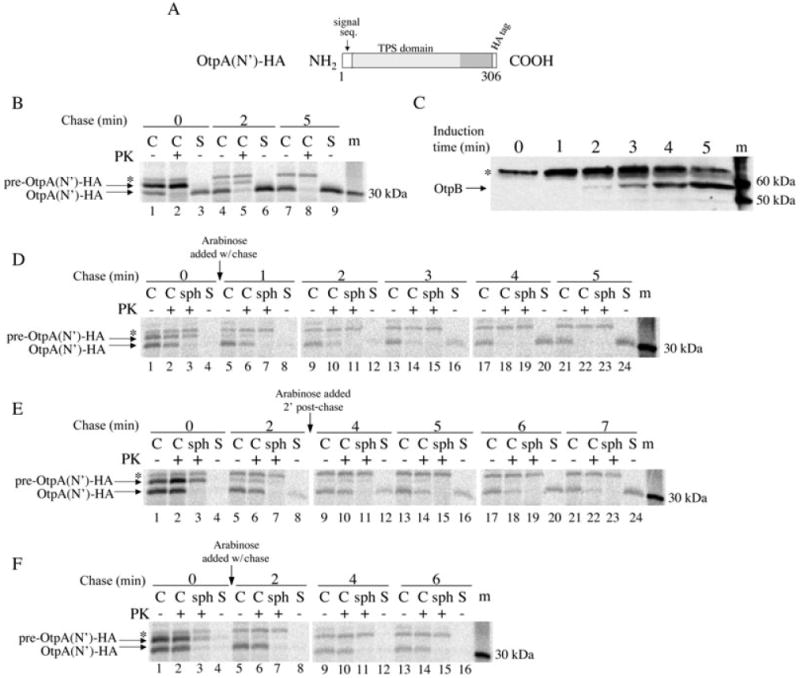
OtpA(N′)-HA can be secreted following its translocation into the periplasm.
A. The OtpA(N′)-HA construct is illustrated.
B. HDB114 transformed with pPC11 (PBAD-otpB) and pPC110 (Ptrc-otpA(N′)-HA) were incubated with arabinose and IPTG and then subjected to pulse-chase labelling. Cultures were divided into cell (C) and secreted (S) fractions and half of the cells were treated with proteinase K (PK). OtpA was then immunoprecipitated from each sample.
C. HDB114 transformed with pPC11 and pPC110 were incubated with arabinose and harvested at the indicated times. Cell extracts were then analysed by Western blot with anti-OtpB.
D–E. The experiment described in part A was repeated except that arabinose was added at the time of the chase (D) or after a 2 min chase (E) and the cell fraction was divided into three portions. One aliquot was untreated, the second was treated with PK, and the third was converted to a spheroplast suspension prior to PK treatment (sph).
F. The experiment in part D was repeated except that cells harboured pPC117 (PBAD-otpB G469P). Because the entire secreted fraction was used in the immunoprecipitations, the S samples were derived from twice as much culture volume (part B) or three times as much culture volume (parts D-F) as each C and sph sample. Cellular proteins that cross-react with the anti-OtpA and anti-OtpB antisera are indicated by the asterisk. m, molecular weight markers.
Experiments in which the expression of otpB was delayed provided clear evidence that the translocation of OtpA(N′)-HA across the OM can be temporally uncoupled from its translocation across the IM. In a preliminary experiment, the lag between the addition of inducer and the expression of otpB was examined by incubating HDB114 transformed with pPC11 (PBAD-otpB) and pPC110 (Ptrc-otpA(N′)-HA) with arabinose and measuring the level of OtpB by Western blot at various time points. The data indicate that OtpB protein becomes detectable ∼2 min after the addition of the inducer (Fig. 2C). With this result in mind, we next incubated HDB114 transformed with pPC11 and pPC110 with IPTG to pre-induce otpA(N′)-HA expression, subjected the cells to pulse-chase labelling, and then added arabinose either at the time of the chase or 2 min later. Cells were then separated from the culture medium and divided into three aliquots. The first aliquot was untreated, the second was treated with proteinase K, and the third was treated with lysozyme/EDTA to permeabilize the OM (and to create a spheroplast suspension) before proteinase K treatment. Permeabilization of the OM was expected to expose OtpA(N′)-HA that resided in the periplasm to the protease. OtpA(N′)-HA was then immunoprecipitated from each aliquot as well as the culture medium. As expected, no pulse-labelled OtpA(N′)-HA was observed either in the medium or on the cell surface (Fig. 2D and E, lanes 1–4). When arabinose was added at the time of the chase, increasing amounts of OtpA(N′)-HA were observed first on the cell surface and then in the medium at later time points; by 4–5 min, essentially all of the protein was secreted (Fig. 2D, lanes 5–24). The same pattern was observed when arabinose was added after a 2 min chase, except that secretion started later and was not complete until ∼7 min (Fig. 2E, lanes 5–24). The data indicate that significant OtpA(N′)-HA secretion occurs only upon synthesis of OtpB. Because translocation of OtpA(N′)-HA across the IM was complete by ∼1–2 min (i.e. all of the pre-OtpA(N′)-HA was converted to OtpA(N′)-HA) but secretion across the OM continued for at least several minutes longer, the substrate presumably resided in the periplasm before it was transported into the extracellular space. This conclusion was corroborated by the observation that all of the mature OtpA(N′)-HA was sensitive to proteinase K when cells were converted to spheroplasts (Fig. 2D and E, lanes 3, 7, 11, 15, 19, 23). The efficiency of OtpA(N′)-HA secretion appeared to decline progressively when the synthesis of OtpB was delayed even further (and no secretion was detected when arabinose was added after a 10 min chase), but interpretation of the data was complicated by the gradual degradation of the exoprotein in the periplasm (data not shown).
We performed several control experiments to confirm that the delayed appearance of OtpA(N′)-HA in the culture medium was due to OtpB-dependent secretion of a periplasmic intermediate. First, we repeated the experiment described above in which otpA(N′)-HA expression was pre-induced, cells were subjected to pulse-chase labelling, and arabinose was added at the time of the chase to induce otpB expression, except that we used cells transformed with pPC117, a plasmid that encodes the otpB G496P mutant. The G496P mutation abolishes the secretion activity of OtpB but does not affect OtpB stability (Fig. S1). When the OtpB mutant was produced, no OtpA(N′)-HA was detected on the cell surface or in the medium (Fig. 2F). Second, we examined the fate of endogenous periplasmic, IM and OM proteins in an experiment in which secretion of OtpA(N′)-HA was delayed (Fig. 3, top panel). Essentially, none of the periplasmic protein DsbA was observed in the culture medium at any time point (Fig. 3, second panel). Thus, the secretion of OtpA(N′)-HA was not due to non-specific leakage of the protein into the extracellular milieu. Consistent with previous results, DsbA was resistant to proteinase K digestion even when cells were converted to spheroplasts (Shimohata et al., 2005). The cleavage of the IM protein YidC at a periplasmically oriented site (Sääf et al., 1998) confirmed that the OM was effectively permeabilized (Fig. 3, third panel, lanes 3, 7, 11 and 15). We also found that the OM protein OmpA remained cell-associated during the entire time course (Fig. 3, bottom panel). This result shows that OtpA(N′)-HA does not reach the extracellular space through a vesicle budding mechanism. Finally, to obtain direct evidence that the C-terminus of OtpA(N′)-HA enters the periplasm prior to the transport of the protein across the OM, we pre-induced otpA(N′)-HA expression, pulse-labelled cells, added arabinose at the time of the chase, and harvested the cells 2 min later. Cells were then converted into spheroplasts, and the spheroplasts were separated from the periplasm. OtpA(N′)-HA was then immunoprecipitated from each fraction using an anti-HA antiserum. As expected, OtpA(N′)-HA was located predominantly in the periplasmic fraction whereas the IM protein SecY was in the spheroplast fraction (Fig. 4). This finding excludes the formal possibility that the C-terminus of OtpA(N′)-HA remains associated with the Sec complex after most of the protein is transferred across the IM.
Fig. 3.
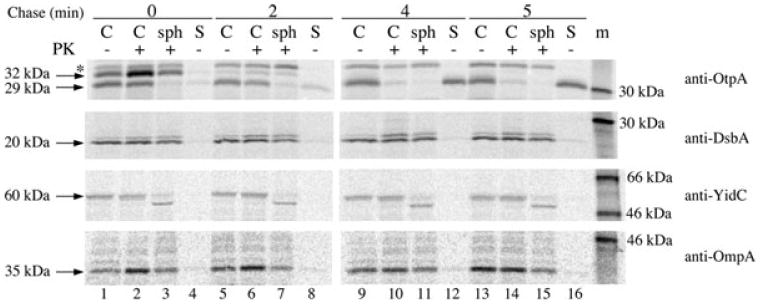
Periplasmic OtpA(N′)-HA is not secreted by an OtpB-independent mechanism. HDB114 transformed with pPC11 (PBAD-otpB) and pPC110 [Ptrc-otpA(N′)-HA] were incubated with IPTG and then subjected to pulse-chase labelling. Arabinose was added at the beginning of the chase. Cultures were divided into cell (C) and secreted (S) fractions, and the cell fraction was divided into three portions. One aliquot was untreated, the second was treated with proteinase K (PK), and the third was converted to a spheroplast suspension prior to PK treatment (sph). Immunoprecipitations were conducted with the indicated antisera. The S samples were derived from three times as much culture volume as each C and sph sample. m, molecular weight markers.
Fig. 4.
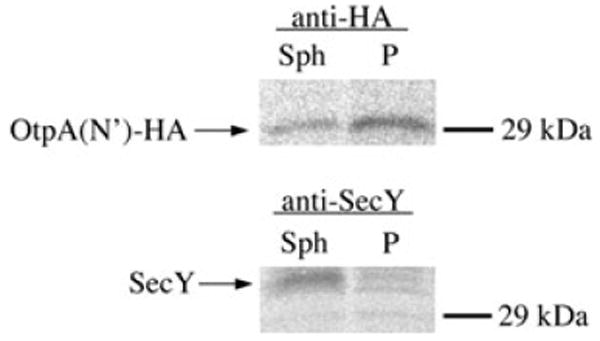
OtpA(N′)-HA resides entirely in the periplasm prior to secretion. HDB114 transformed with pPC11 (PBAD-otpB) and pPC110 [Ptrc-otpA(N′)-HA] were incubated with IPTG, pulse-labelled, and subjected to a 2 min chase. Arabinose was added at the beginning of the chase. Cells were isolated and the OM was permeabilized. Permeabilized cells were then divided into spheroplast (Sph) and periplasmic (P) fractions. Proteins were then immmunoprecipitated from each fraction using the indicated antisera.
We next wished to determine whether the transport of full-length OtpA across the IM could be uncoupled from its transport across the OM. To gauge the kinetics of OtpA translocation across the IM, HDB114 transformed with pPC20 (Ptrc-otpA) were incubated with IPTG, and cells were subjected to pulse-chase labelling. Because the precursor and mature forms of OtpA are difficult to resolve by SDS-PAGE, we assessed the transport of OtpA across the IM by permeabilizing the OM and treating the spheroplast suspension with proteinase K. Most of the pulse-labelled OtpA was digested by the protease (Fig. 5A, lane 1). Thus, the full-length protein appears to be translocated across the IM considerably faster than OtpA(N′)-HA. For reasons that are unclear, a small fraction of the protein remained insensitive to proteinase K digestion (presumably because it folded into a protease-resistant conformation or was trapped in the cytoplasm) even after a prolonged chase (Fig. 5A, lanes 2–6). To determine whether OtpA is secretion-competent after it is transported into the periplasm, HDB114 were transformed with both pPC11 (PBAD-otpB) and pPC20, otpA expression was induced by the addition of IPTG, cells were subjected to pulse-chase labelling, and otpB expression was induced by the addition of arabinose at the time of the chase. At the time of OtpB induction (0 min), no OtpA was present in the medium or exposed to proteinase K on the cell surface (Fig. 5B, lanes 1–4). However, by 2 min after the addition of arabinose (at which time synthesis of OtpB presumably would have been detected), some of the OtpA was exposed on the cell surface or released into the medium (Fig. 5B, lanes 5–8). Increasing amounts of OtpA were secreted at later time points, although even after 4 min a significant fraction of the protein remained resistant to protease digestion and appeared to be intracellular (Fig. 5B, lanes 9–16; compare with Fig. 8C and with Fig. 6A in Choi et al., 2007). These results strongly suggest that OtpA can be secreted following its translocation across the IM, although less efficiently than OtpA(N′)-HA. The relative inefficiency of secretion is consistent with our hypothesis that the secretion-competence of the full-length protein in the periplasm is very limited.
Fig. 5.
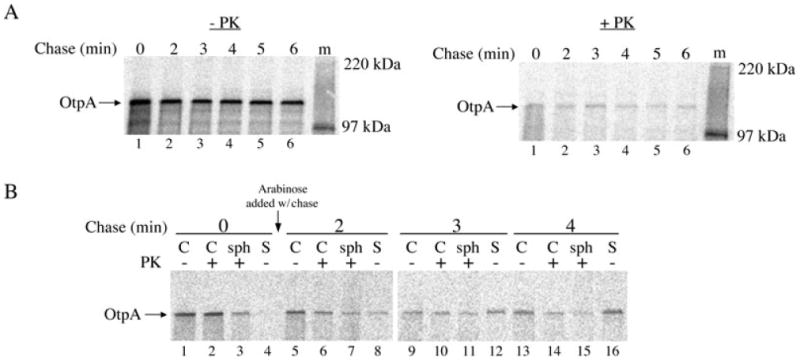
Secretion-competence of full-length OtpA in the periplasm.
A. HDB114 transformed with pPC20 (Ptrc-otpA) were incubated with IPTG and subjected to pulse-chase labelling. After cells were converted to a spheroplast suspension, half of the spheroplast suspension was untreated while the other half was treated with proteinase K (PK).
B. HDB114 transformed with pPC11 (PBAD-otpB) and pPC20 were incubated with IPTG and then subjected to pulse-chase labelling. Arabinose was added at the time of the chase. Cultures were divided into cell (C) and secreted (S) fractions, and the cell fraction was divided into three portions. One aliquot was untreated, the second was treated with PK, and the third was converted to a spheroplast suspension prior to PK treatment (sph). OtpA was immunoprecipitated from all samples. In part B, the S samples were derived from three times as much culture volume as each C and sph sample. m, molecular weight markers.
Fig. 8.
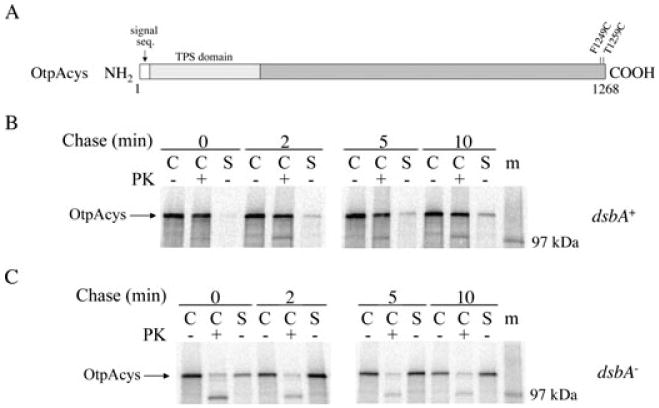
A C-terminal disulphide bond impairs the secretion of full-length OtpA.
A. The location of the cysteine residues in OtpAcys is shown.
B–C. HDB114 (B) or PC97 (C) transformed with pPC11 (PBAD-otpB) and pPC105 (Ptrc-otpAcys) were incubated with IPTG and arabinose and then subjected to pulse-chase labelling. Cultures were divided into cell (C) and secreted (S) fractions and half of the cells were treated with proteinase K (PK). OtpA was immunoprecipitated from all samples. The S samples were derived from twice as much culture volume as each C sample. m, molecular weight markers.
A C-terminal disulphide bond reduces the exposure of the OtpA N-terminus on the cell surface
Experiments that were originally devised to examine the ability of OtpB to promote the secretion of partially folded substrates fortuitously yielded additional evidence that the translocation of OtpA across the OM is independent from its translocation across the IM. Previous work has shown that the formation of a disulphide bond at the C-terminus of HMW1A (which, unlike OtpA, contains two native cysteine residues) prevents secretion of the last few residues and anchors the protein to the cell surface (Buscher et al., 2006). We wished to determine whether an equivalent structure in which the cysteine residues were spaced the same distance apart would exert a similar effect on OtpA secretion. To this end we introduced two cysteine residues at the C-terminus of OtpA(N), a truncated form of OtpA that is slightly larger than OtpA(N′), to create OtpA(N)cys (Fig. 6A). When OtpA(N) is synthesized together with OtpB, it is secreted with the same efficiency and kinetics as OtpA(N′) (Choi et al., 2007). HDB114 and PC97, a dsbA– derivative of HDB114 that cannot form disulphide bonds in the periplasm, were transformed with pPC11 (PBAD-otpB) and pPC91, a plasmid that encodes otpA(N)cys under the control of a trc promoter. Simultaneous expression of both TPS components was induced by the addition of arabinose and IPTG, and cells were subjected to pulse-chase labelling. OtpA(N)cys was secreted from the dsbA+ strain relatively slowly and inefficiently; even after 10 min, more than half of the protein was resistant to proteinase K digestion (Fig. 6B). The observation that this fraction of the protein was completely degraded when proteinase K was added following permeabilization of the OM confirmed that it was trapped in the periplasm (Fig. S2). In contrast, OtpA(N)cys was secreted as rapidly and efficiently from the dsbA– strain as the unmodified protein was secreted from the dsbA+ strain (compare Fig. 2B and Fig. 6C). These results indicate that the formation of a C-terminal disulphide bond impedes OtpA(N)cys secretion. The observation that the N-terminus of most of the OtpA(N)cys produced in HDB114 was resistant to proteinase K digestion, however, indicates that the disulphide bond did not simply tether the last few residues to the cell surface. Instead, the formation of a C-terminal disulphide bond appeared to affect the secretion-competence of the entire protein. This interpretation implies that the C-terminus of the protein entered the periplasm before OtpB could initiate secretion of the N-terminus.
Fig. 6.
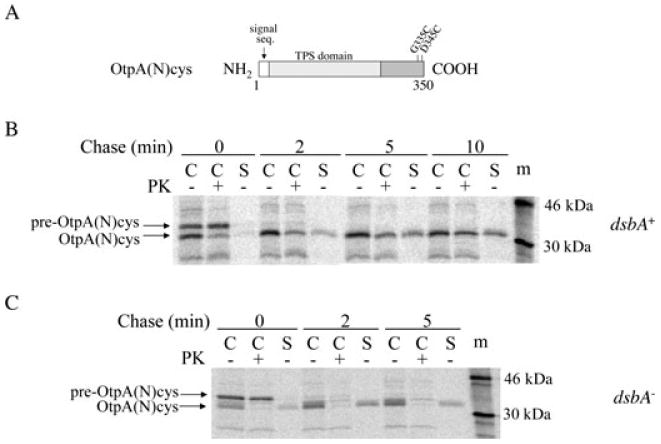
A C-terminal disulphide bond reduces the efficiency of OtpA(N) secretion.
A. The location of the cysteine residues in OtpA(N)cys is shown.
B–C. HDB114 (B) or PC97 (C) transformed with pPC11 (PBAD-otpB) and pPC91 [Ptrc-otpA(N)cys] were incubated with IPTG and arabinose and then subjected to pulse-chase labelling. Cultures were divided into cell (C) and secreted (S) fractions and half of the cells were treated with proteinase K (PK). OtpA was immunoprecipitated from all samples. The S samples were derived from twice as much culture volume as each C sample. m, molecular weight markers.
We next wished to determine whether the OtpA(N)cys that was secreted from HDB114 represented a discrete population of molecules that failed to undergo disulphide bond formation, perhaps as a result of co-ordinated transport across both membranes. To test this possibility, we repeated the experiment described above and treated both cell and supernatant fractions with maleimide-PEO2-biotin, a membrane-permeable biotinylation reagent that reacts only with free thiol groups. OtpA derivatives were immunoprecipitated from each sample with the anti-OtpA antiserum, and half of each sample was then incubated with streptavidin beads to isolate biotinylated OtpA(N)cys. As expected, a significant fraction of the pre-OtpA(N)cys (which is located in the cytoplasm and therefore contains free cysteines) was biotinylated in HDB114 (Fig. 7A, lanes 2, 6 and 10). A tiny fraction of the cell-associated mature OtpA(N)cys (which was presumably localized in the periplasm) was also biotinylated. All of the secreted protein, however, was resistant to modification and therefore contained a disulphide bond (Fig. 7A, lanes 4, 8 and 12). In contrast, a significant fraction of the OtpA(N)cys secreted from PC97 (as well as the protein that remained cell-associated) reacted with the cysteine-modifying reagent (Fig. 7B). These results strongly suggest that in wild-type cells all of the OtpA(N)cys entered the periplasm and rapidly underwent disulphide bond formation. Thus it is very unlikely that the fraction of OtpA(N)cys that was secreted was co-ordinately transported across the IM and OM. Presumably the protein that was retained in the periplasm became secretion-incompetent as a result of aberrant intramolecular or intermolecular interactions between the disulphide-bonded C-terminus and more proximal segments, not as a result of disulphide bond formation per se. Furthermore, the data indicate that secretion of OtpA(N)cys (unlike the secretion of HMW1A) is compatible with the presence of at least limited tertiary structure.
Fig. 7.
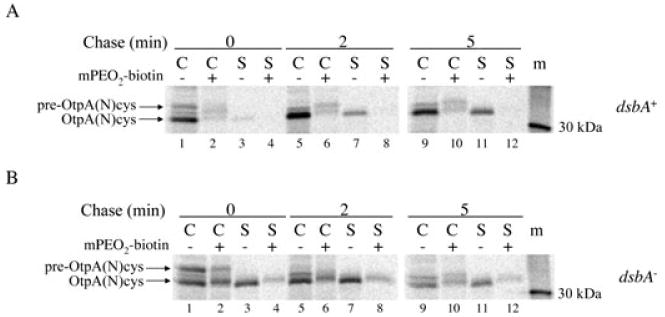
Secreted OtpA(N)cys contains a disulphide bond. HDB114 (A) or PC97 (B) transformed with pPC11 (PBAD-otpB) and pPC91 [Ptrc-otpA(N)cys] were incubated with IPTG and arabinose and subjected to pulse-chase labelling. Cell (C) and secreted (S) fractions were then divided in half, and one half was untreated while the other half was incubated with maleimide-PEO2-biotin (mPEO2-biotin). Biotinylated proteins were bound to and eluted from avidin agarose beads, and OtpA was subsequently immunoprecipitated from all samples. m, molecular weight markers.
Finally, to examine the effect of a C-terminal disulphide bond on the secretion of full-length OtpA, we introduced two cysteine residues into the C-terminus of OtpA to create OtpAcys (Fig. 8A). HDB114 and PC97 transformed with pPC11 (PBAD-otpB) and a plasmid encoding otpAcys under the control of a trc promoter were grown in the presence of arabinose and IPTG, and cells were subjected to pulse-chase labelling. A small amount of OtpAcys was gradually secreted from HDB114 over a 10 min period, but most of the protein remained cell-associated and resistant to proteinase K digestion (Fig. 8B). The majority of the cell-associated OtpAcys was degraded after the OM was permeabilized and therefore resided in the periplasm (Fig. S3). In contrast, most of the OtpAcys produced in the dsbA– strain was either secreted or exposed on the cell surface within the pulse-labelling period (Fig. 8C). Taken together, these results strongly suggest that the presence of a C-terminal disulphide bond impairs the secretion of full-length OtpA. Given that disulphide bonds typically form very quickly, the slow increase in the level of OtpAcys in the culture medium of HDB114 cells suggests that OtpB can secrete the disulphide bonded form of the protein, although inefficiently. Presumably, the disulphide bond in OtpAcys (like that in OtpA(N)cys) caused a secretion defect by grossly altering the conformation or folding of the protein. More significantly, the observation that most of the OtpAcys produced by the dsbA+ strain was completely insensitive to protease treatment provides clear evidence that translocation of the protein across the IM and OM occurs in two sequential steps. If translocation across both membranes were coupled, we would have expected the OtpAcys N-terminus to be translocated across the OM (and therefore susceptible to proteolysis) before the C-terminal cysteine residues entered the periplasm and formed a disulphide bond.
Discussion
In this study, we present evidence that a TPS exoprotein can be translocated across the IM and OM in two discrete steps. We first demonstrated that OtpA secretion does not require the simultaneous production of OtpB. The data strongly suggest that secretion does not require an interaction between the two partners prior to or during the assembly of the transporter, but rather that the exoprotein is targeted to fully assembled transporter molecules. With this observation in mind, we next examined the consequences of producing OtpB only after OtpAis translocated across the IM. We found that both truncated and full-length versions of OtpA remain secretion-competent in the periplasm, although very briefly. Given that the secretion-competence of periplasmic OtpA is short-lived and that each exoprotein probably has slightly different folding properties, it is not surprising that Fha44 was trapped in the periplasm when it was produced prior to FhaC (Guedin et al., 1998). Finally, we found that the presence of a C-terminal disulphide bond reduced the exposure of the N-terminus of OtpA on the cell surface when the two partners were synthesized simultaneously (i.e. under conditions in which wild-type OtpA is secreted efficiently). The observation that a C-terminal disulphide bond inhibits OtpA secretion implies that the C-terminus enters the periplasm before the N-terminus is transported across the OM and thereby demonstrates that the IM and OM transport steps are not obligately coupled. Presumably the disulphide bond blocks the initiation of secretion by leading to the formation of non-productive intramolecular or intermolecular interactions that reduce secretion-competence.
While our data indicate that the translocation of OtpA across the OM is not obligately coupled to its transport across the IM, they by no means rule out the possibility that exoproteins cross both membranes in a concerted fashion at least occasionally. If fully extended, all of the naturally occurring exoproteins that have been characterized to date would be long enough to span the periplasm, and it is conceivable that the TPS domain interacts with TpsB while the exoprotein is still engaged by the Sec machinery. Indeed from a theoretical standpoint, coupled translocation would be advantageous because it would obviate the need to maintain exoproteins in a secretion-competent conformation in the periplasm and would thereby reduce constraints on exoprotein sequence. Coupled translocation across both membranes, however, would require exquisite temporal and spatial co-ordination of IM and OM transport processes. Unless the transport of exoproteins through the Sec complex is a rate-limiting step that is slower than both the diffusion of the N-terminus through the periplasm (which includes passage across the cell wall) and the binding of the TPS domain to TpsB, then coupled translocation would not be possible. In this regard it is interesting to note that a subset of TPS exoproteins (including FHA) contain unusually long signal peptides that are distinguished by a conserved sequence motif (Jacob-Dubuisson et al., 2001). Homologous signal peptides associated with bacterial autotransporters appear to influence the rate of protein transport through or release from the Sec machinery (Szabady et al., 2005).
Our results imply that the Sec complex plays a minor, if any role in providing the energy required for the transport of TPS exoproteins across the OM. Because there is no obvious physical link between exoproteins and any other IM proteins, it is also unlikely that the proton motive force is harnessed to drive secretion. Thus in all probability secretion is driven by exoprotein folding. One possibility is that gradual folding of the exoprotein as it is extruded across the OM acts as a ‘Brownian ratchet’ that prevents back sliding. Although the folded state of TPS exoproteins in the periplasm is unknown, the small pore size of FhaC as well as the observation that a Fha44 chimera that contained a folded domain (the cholera toxin B submit) was not secreted suggests that exoproteins traverse the OM in a largely unfolded conformation and only fold in the extracellular space (Guedin et al., 1998; Clantin et al., 2007). Alternatively, if the exoprotein does not traverse the channel formed by a monomeric TpsB β barrel, then it is conceivable that the folding of the exoprotein in the periplasm might generate a structure that drives translocation by a novel mechanism.
Finally, our study demonstrates that the TPS pathway can tolerate at least limited elements of tertiary structure. Because previous studies on HMW1A showed that a naturally occurring disulphide bond serves as a ‘stop-transfer’ sequence that anchors the protein to the cell surface (Buscher et al., 2006), the observation that a significant amount of OtpA(N)cys was secreted was somewhat unexpected. The apparent discrepancy in the data, however, might be explained by the divergence of the sequences of the cognate TpsB proteins, HMW1B and OtpB (Choi et al., 2007). Given the considerable evolutionary distance between the two transporters, it is entirely plausible that OtpB forms a slightly larger pore than HMW1B and can therefore secrete a substrate that contains a small hairpin structure more readily. Furthermore, our observation that the presence of a disulphide bond in OtpA(N)cys and OtpAcys impedes secretion prior to the transport of the N-terminus of the protein across the OM raises an important cautionary note. Presumably a fraction of both OtpA(N)cys and OtpAcys was completely resistant to proteinase K digestion because it misfolded or aggregated in the periplasm. This interpretation of the results implies that modified or non-native globular segments associated with TPS exoproteins do not necessarily behave as independent domains, but can perturb the folding of distant segments of the protein through intramolecular or intermolecular interactions. Thus defects in the secretion of modified exoproteins or exoprotein chimeras into the extracellular space cannot be attributed to a ‘clogging’ of the translocation pore at the site of the modification (or the site at which the globular segment is attached) without a more detailed analysis of the fate of the protein.
Experimental procedures
Antisera, bacterial strains and media
Polyclonal rabbit antisera raised against peptides derived from the N-terminus of OtpA, OtpB and SecY were previously described (Arkowitz and Wickner, 1994; Choi et al., 2007). A polyclonal rabbit antiserum was generated against a YidC C-terminal peptide (NH2-CYRGLEKRGLEKRGLHSREKKKSCOOH). Polyclonal rabbit antisera raised against the influenza hemagglutinin HA.11 epitope and DsbA were obtained from Santa Cruz Biotechnology and Stressgen respectively. An antiserum against OmpA was kindly provided by P.C. Tai. The strains used in this study were MC4100 (F- araD139 Δ(argF-lac)U169 rpsL150 relA1 thi fib5301 deoC1 ptsF25 rbsR), AD 202 (MC4100 ompT∷kan), HDB114 (AD 202 ara+) and PC97 (HDB114 ΔdsbA). In all experiments bacteria were grown at 37°C in M9 medium containing 0.2% glycerol and 40 mg ml−1 of all of the L-amino acids except cysteine and methionine. Media were supplemented as needed with ampicillin (100 μg ml−1) and/or chloramphenicol (30 μg ml−1).
Plasmid construction
Plasmids pPC11 (otpB cloned into pBAD33), pPC20 (otpA cloned into pTRC99a) and pPC69 (otpA(N) cloned into pTRC99a) have been described previously (Choi et al., 2007). Plasmid pPC110, which encodes otpA(N′)HA, was generated by first amplifying a fragment of otpA by PCR using the oligonucleotides PC7 (5′-GCGGATCCATCAATAAGGAAAACGTTAG-3′) and PC72 (5′-GCGGATCCTTAGTAATCTGGAACATCGTACGGATAACCGGTGGTGTTATCCAGAGTAC-3′) and plasmid pPC20 as a template. The resulting PCR product was then digested with BamHI and cloned into pTrc99A. Plasmids pPC91, pPC105 and pPC117 were created by introducing G335C and D345C mutations into pPC69, F1249C and T1259C mutations into pPC20, and a G496P mutation into pPC11 respectively, using the QuikChange Mutagenesis kit (Stratagene).
Analysis of OtpA secretion
Overnight cultures were washed and diluted into fresh medium at OD550 = 0.04. For experiments in which otpB was pre-expressed, 0.2% arabinose was added when cultures reached OD550 = 0.2. After 30 min, cells were washed three times at 4°C in medium lacking arabinose and resuspended in the original volume. OtpA expression was then induced by the addition of 100 μM IPTG. At various time points cells were chilled and separated from the culture medium by centrifugation (20 800 g, 5 min, 4°C). Residual cells were removed from the supernatant by repeating the centrifugation step. The cell pellet and final supernatant fractions were then TCA precipitated, and the level of OtpA and OtpB in each fraction was analysed by Western blot. As part of the same experiment, aliquots were removed from cultures 10 min after the addition of IPTG and pulse-chase labelling was performed as previously described (Ulbrandt et al., 1997).The pulse-labelling period was 30 s. For experiments in which otpA and otpB were expressed co-ordinately, 100 μM IPTG and 0.2% arabinose were added when cultures reached OD550 = 0.2. After 30 min, pulse-chase labelling was performed. For experiments in which otpA was expressed before otpB, otpA expression was induced by the addition of 10 μM or 100 μM IPTG when cultures reached OD550 = 0.2. After 30 min, pulse-chase labelling was performed and 0.2% arabinose was added at the time of the chase or at the indicated time post-chase.
In all pulse-chase experiments, cells were poured over ice and harvested by centrifugation (20 800 g, 5 min, 4°C). Proteins in the culture medium were then TCA precipitated, and cell pellets were resuspended in 1 ml of growth medium. In experiments in which the exposure of OtpA on the cell surface was analysed, the cell suspensions were divided in half. One half was untreated and the other half was treated with proteinase K as previously described (Ulbrandt et al., 1997). Proteins were then collected from all samples by TCA precipitation. In experiments in which the localization of OtpA in the periplasm was also analysed, cell suspensions were divided in thirds. One aliquot was untreated, and another was treated with proteinase K. Cells in the last aliquot were pelleted again, resuspended in 33 mM Tris pH 8.0/40% sucrose and converted to spheroplasts as previously described (Choi et al., 2007). After the addition of 10 mM MgSO4, the spheroplast suspension was treated with proteinase K. Proteins were collected from all samples by TCA precipitation. TCA precipitates were solubilized and immunoprecipitations were conducted as previously described (Newitt and Bernstein, 1998). To examine the presence of free sulfhydryl groups in OtpA derivatives, radiolabelled cells were collected as described above and resuspended in 1 ml of growth medium. Half of the cells and half of the culture medium were subject to TCA precipitation, and the remainder of each sample was incubated with 400 μM EZ-Link® Maleimide-PEO2-Biotin (Pierce) for 1 h at 25°C. OtpA derivatives were then immunoprecitated from all samples, and protein that was subjected to biotinylation was eluted from protein A-sepharose beads by boiling for 5 min in 50 mM Tris base/2% SDS. The beads were removed by centrifugation (20 800 g, 1 min, 4°C), and the supernatant was diluted 1:20 in RIPA buffer (Harlow and Lane, 1999) and incubated with ImmunoPure Immobilzed Avidin beads (Pierce) for 30 min at 25°C. After the beads were washed in RIPA buffer, protein was eluted and analysed by SDS-PAGE.
SDS-PAGE and Western blot analysis
Protein samples were resolved by SDS-PAGE on 8–16% minigels (Invitrogen) and radioactive bands were visualized using a Fuji BAS-2500 phosphorimager. For Western blots, antigen–antibody complexes were detected using the Super-Signal West Pico Chemiluminscent kit (Pierce).
Supplementary Material
Acknowledgments
We thank Janine Peterson for providing outstanding technical assistance, Rob Boulianne for generating the anti-YidC antiserum and Yihong Ye for providing helpful comments on the manuscript. This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Supporting information: Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Aoki SK, Pamma R, Hernday AD, Bickham JE, Braaten BA, Low DA. Contact-dependent inhibition of growth in Escherichia coli. Science. 2005;309:1245–1248. doi: 10.1126/science.1115109. [DOI] [PubMed] [Google Scholar]
- Arkowitz RA, Wickner W. SecD and SecF are required for the proton electrochemical gradient stimulation of preprotein translocation. EMBO J. 1994;13:954–963. doi: 10.1002/j.1460-2075.1994.tb06340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscher AZ, Grass S, Heuser J, Roth R, St Geme JW., 3rd Surface anchoring of a bacterial adhesin secreted by the two-partner secretion pathway. Mol Microbiol. 2006;61:470–483. doi: 10.1111/j.1365-2958.2006.05236.x. [DOI] [PubMed] [Google Scholar]
- Choi PS, Dawson AJ, Bernstein HD. Characterization of a novel two-partner secretion system in Escherichia coli O157:H7. J Bacteriol. 2007;189:3452–3461. doi: 10.1128/JB.01751-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clantin B, Hodak H, Willery E, Locht C, Jacob-Dubuisson F, Villeret V. The crystal structure of filamentous hemagglutinin secretion domain and its implications for the two-partner secretion pathway. Proc Natl Acad Sci USA. 2004;101:6194–6199. doi: 10.1073/pnas.0400291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clantin B, Delattre AS, Rucktooa P, Saint N, Meli AC, Locht C, et al. Structure of the membrane protein FhaC: a member of the Omp85-TpsB transporter superfamily. Science. 2007;317:957–961. doi: 10.1126/science.1143860. [DOI] [PubMed] [Google Scholar]
- Gentle IE, Burri L, Lithgow T. Molecular architecture and function of the Omp85 family of proteins. Mol Microbiol. 2005;58:1216–1225. doi: 10.1111/j.1365-2958.2005.04906.x. [DOI] [PubMed] [Google Scholar]
- Grass S, St Geme JW., 3rd Maturation and secretion of the non-typable Haemophilus influenzae HMW1 adhesin: roles of the N-terminal and C-terminal domains. Mol Microbiol. 2000;36:55–67. doi: 10.1046/j.1365-2958.2000.01812.x. [DOI] [PubMed] [Google Scholar]
- Guedin S, Willery E, Locht C, Jacob-Dubuisson F. Evidence that a globular conformation is not compatible with FhaC-mediated secretion of the Bordetella pertussis filamentous haemagglutinin. Mol Microbiol. 1998;29:763–774. doi: 10.1046/j.1365-2958.1998.00970.x. [DOI] [PubMed] [Google Scholar]
- Guedin S, Willery E, Tommassen J, Fort E, Drobecq H, Locht C, Jacob-Dubuisson F. Novel topological features of FhaC, the outer membrane transporter involved in the secretion of the Bordetella pertussis filamentous hemagglutinin. J Biol Chem. 2000;275:30202–30210. doi: 10.1074/jbc.M005515200. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Using Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala'Aldeen D. Type V protein secretion pathway: the autotransporter story. Microbiol Mol Biol Rev. 2004;68:692–744. doi: 10.1128/MMBR.68.4.692-744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodak H, Jacob-Dubuisson F. Current challenges in autotransport and two-partner protein secretion pathways. Res Microbiol. 2007;158:631–637. doi: 10.1016/j.resmic.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Hodak H, Clantin B, Willery E, Villeret V, Locht C, Jacob-Dubuisson F. Secretion signal of the filamentous haemagglutinin, a model two-partner secretion substrate. Mol Microbiol. 2006;61:368–382. doi: 10.1111/j.1365-2958.2006.05242.x. [DOI] [PubMed] [Google Scholar]
- Jacob-Dubuisson F, Buisine C, Willery E, Renauld-Mongenie G, Locht C. Lack of functional complementation between Bordetella pertussis filamentous hemagglutinin and Proteus mirabilis HpmA hemolysin secretion machineries. J Bacteriol. 1997;179:775–783. doi: 10.1128/jb.179.3.775-783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob-Dubuisson F, Locht C, Antoine R. Two-partner secretion in Gram-negative bacteria: a thrifty, specific pathway for large virulence proteins. Mol Microbiol. 2001;40:306–313. doi: 10.1046/j.1365-2958.2001.02278.x. [DOI] [PubMed] [Google Scholar]
- Jacob-Dubuisson F, Fernandez R, Coutte L. Protein secretion through autotransporter and two-partner pathways. Biochim Biophys Acta. 2004;1694:235–257. doi: 10.1016/j.bbamcr.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kajava AV, Steven AC. The turn of the screw: variations of the abundant beta-solenoid motif in passenger domains of Type V secretory proteins. J Struct Biol. 2006;155:306–315. doi: 10.1016/j.jsb.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Kida Y, Higashimoto Y, Inoue H, Shimizu T, Kuwano K. A novel secreted protease from Pseudomonas aeruginosa activates NF-kappaB through protease-activated receptors. Cell Microbiol. 2008;10:1491–1504. doi: 10.1111/j.1462-5822.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- Li H, Grass S, Wang T, Liu T, St Geme JW., III Structure of the Haemophilus influenzae HMW1B translocator protein: evidence for a twin pore. J Bacteriol. 2007;189:7497–7502. doi: 10.1128/JB.00541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazar J, Cotter PA. Topology and maturation of filamentous haemagglutinin suggest a new model for two-partner secretion. Mol Microbiol. 2006;62:641–654. doi: 10.1111/j.1365-2958.2006.05392.x. [DOI] [PubMed] [Google Scholar]
- Meli AC, Hodak H, Clantin B, Locht C, Molle G, Jacob-Dubuisson F, Saint N. Channel properties of TpsB transporter FhaC point to two functional domains with a C-terminal protein-conducting pore. J Biol Chem. 2006;281:158–166. doi: 10.1074/jbc.M508524200. [DOI] [PubMed] [Google Scholar]
- Neil RB, Apicella MA. Role of HrpA in biofilm formation of Neisseria meningitidis and regulation of the hrpBAS transcripts. Infect Immun. 2009;77:2285–2293. doi: 10.1128/IAI.01502-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newitt JA, Bernstein HD. A mutation in the Escherichia coli secY gene that produces distinct effects on inner membrane protein insertion and protein export. J Biol Chem. 1998;273:12451–12456. doi: 10.1074/jbc.273.20.12451. [DOI] [PubMed] [Google Scholar]
- Renauld-Mongenie G, Cornette J, Mielcarek N, Menozzi FD, Locht C. Distinct roles of the N-terminal and C-terminal precursor domains in the biogenesis of the Bordetella pertussis filamentous hemagglutinin. J Bacteriol. 1996;178:1053–1060. doi: 10.1128/jb.178.4.1053-1060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sääf A, Monné M, de Gier JW, von Heijne G. Membrane topology of the 60-kDa Oxa1p homologue from Escherichia coli. J Biol Chem. 1998;273:30415–30418. doi: 10.1074/jbc.273.46.30415. [DOI] [PubMed] [Google Scholar]
- Schiebel E, Schwarz H, Braun V. Subcellular location and unique secretion of the hemolysin of Serratia marcescens. J Biol Chem. 1989;264:16311–16320. [PubMed] [Google Scholar]
- Schonherr R, Tsolis R, Focareta T, Braun V. Amino acid replacements in the Serratia marcescens haemolysin ShIA define sites involved in activation and secretion. Mol Microbiol. 1993;9:1229–1237. doi: 10.1111/j.1365-2958.1993.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Shen S, Mascarenhas M, Morgan R, Rahn K, Karmali MA. Identification of four fimbria-encoding genomic islands that are highly specific for verocytotoxin-producing Escherichia coli serotype O157 strains. J Clin Microbiol. 2005;43:3840–3850. doi: 10.1128/JCM.43.8.3840-3850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimohata N, Akiyama Y, Ito K. Peculiar properties of DsbA in its export across the Escherichia coli cytoplasmic membrane. J Bacteriol. 2005;187:3997–4004. doi: 10.1128/JB.187.12.3997-4004.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Geme JW, 3rd, Grass S. Secretion of the Haemophilus influenzae HMW1 and HMW2 adhesins involves a periplasmic intermediate and requires the HMWB and HMWC proteins. Mol Microbiol. 1998;27:617–630. doi: 10.1046/j.1365-2958.1998.00711.x. [DOI] [PubMed] [Google Scholar]
- Szabady RL, Peterson JH, Skillman KM, Bernstein HD. An unusual signal peptide facilitates late steps in the biogenesis of a bacterial autotransporter. Proc Natl Acad Sci USA. 2005;102:221–226. doi: 10.1073/pnas.0406055102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrandt ND, Newitt JA, Bernstein HD. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell. 1997;88:187–196. doi: 10.1016/s0092-8674(00)81839-5. [DOI] [PubMed] [Google Scholar]
- Yen MR, Peabody CR, Partovi SM, Zhai Y, Tseng YH, Saier MH. Protein-translocating outer membrane porins of Gram-negative bacteria. Biochim Biophys Acta. 2002;1562:6–31. doi: 10.1016/s0005-2736(02)00359-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


